Abstract
Many studies of vertebral artery (VA) blood flow changes related to cervical spine rotation have been published, but the findings are controversial and the evidence unconvincing. Recent Doppler measurements suggest that contralateral VA blood flow is compromised on full rotation in both healthy subjects and patients. More rigorous research is needed, and it was the aim of this study to conduct a meta-analysis of published data to inform professional practice. A systematic literature search, including only Doppler studies of VA blood flow velocity associated with cervical spine rotation in adults, yielded nine reports with published data. Using weighted means of the pooled data, the magnitude of the effect size (Cohen's d) was calculated for differences between patients and subjects, sitting or lying supine for testing, the parts of the VA insonated, and the changes recorded after cervical spine rotation. From this meta-analysis, VA blood flow velocity was found to be compromised more in patients than healthy individuals, on contralateral rotation, with the subject sitting, and more in the intracranial compared to the cervical part of the VA. Possible reasons for these findings are suggested, and it is advised that sustained end-of-range rotation and quick-thrust rotational manipulations be avoided until there is a stronger evidence base for clinical practice.
KEYWORDS: Blood Flow, Cervical Spine Rotation, Physical Therapy, Vertebral Artery
The clinical importance and relevance of changes in vertebral artery (VA) blood flow associated with cervical spine rotation, as used by manual therapists in the treatment of patients, have been the focus of considerable research over the past 50 years. The findings of early cadaver studies1–5, suggesting a significant decrease of VA blood flow on the side contralateral to the direction of cervical spine rotation, led to in vivo measurements of VA blood flow using flowmetry, angiography, magnetic resonance imaging6–13 and, most commonly, Doppler insonation10,14–45. Despite the many studies published to date, the findings remain controversial, and the evidence base for clinical practice is not strongly convincing.
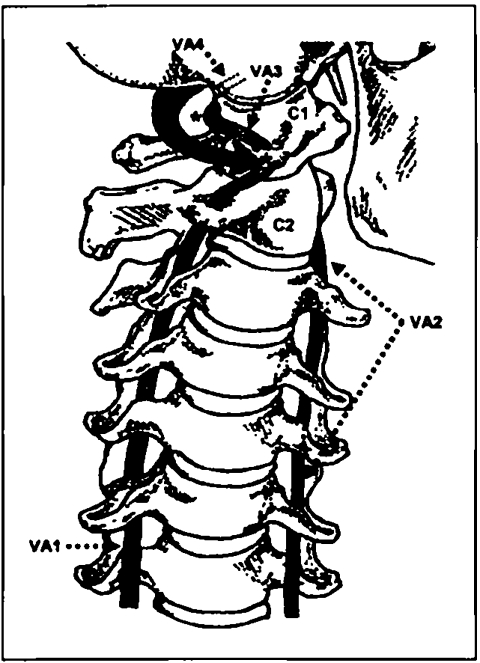
The majority of these studies report compromised contralateral VA blood flow15,16,19,22,25,27–29,39,42,43. However, some authors found no associated change in VA blood flow10,18,21,32,35,38,41, while a few reports indicated an increase, in some cases, in contralateral15,30,40,44 and in ipsilateral25 blood flow. These contradictory findings are most likely because of differences in methodology and procedures for measuring VA blood flow changes across the various studies. For instance, healthy persons10,16,18,19,22,24,25,29,39–43 as well as patients15,23,30–32,35,38, some exhibiting signs and symptoms of vertebrobasilar insufficiency or ischemia (VBI)21,23,29–32,38, were used as subjects. The individuals were variously positioned in sitting16,22,30,43,44,46, supine lying25,29,40,42, or prone lying39. Different instrumentation was used by the researchers for Doppler insonation of the VA, including continuous wave18,23,30,31 and pulsed-wave 10,15,16,19,21,22,25,26,28,29,32,35,38,44,46 Doppler, with and without color flow imaging. Lastly, different parts of the VA were studied, such as the pre-vertebral (VA1)10,25,26,32,38, cervical (VA2)15,18,19,21,23,24,29–31,35–37,40–42, suboccipital (VA3)44,45, or intracranial (VA4)16,22,28,39,43 parts (Figure 1). All of these differences are possible sources of bias and, ideally, should be standardized in future research projects.
FIGURE 1.
Anterior view of the cervical spine and vertebral arteries. The head is fully rotated to the left. The right vertebral artery, divided into its four parts (VA1–4), is illustrated. Note the possible torsion, during cervical spine rotation, on VA3, as it passes through the transverse foramen, along the posterior arch, or around the lateral mass of the atlas vertebra (C1) (marked with ∗). Key: C1 = atlas, C2 = axis
It has been suggested in the literature that the part of the VA insonated is an important factor in measurements of blood flow changes associated with cervical spine rotation47,48. This view relates to the belief that the most vulnerable part of the VA to distortion is that part between the axis and atlas vertebrae, where most cervical spine rotation takes place15,29,35,40–42,49–52. However, it has been proposed also that the natural tortuosity of the VA between the first and second vertebrae should prevent much of this apparent distortion of the vessel35,51,53. In addition, mechanical compression and/ or stretching of the VA is more likely to occur to its suboccipital part, on full-range cervical spine rotation, as it passes through the transverse foramen and along the posterior arch47,48,54–60, or around the bony lateral mass of the atlas vertebra39,43,48,49,51,59–63, where it is bound down by connective tissue16,22,35,39,43,59,64 (Figure 1).
Considering these anatomical factors and the hemodynamics of normal blood flow in medium-sized muscular vessels such as the VA65, some researchers have put forward the idea that VA blood flow changes associated with rotation would be more accurately measured down-stream from or distal to the possible point of distortion, that is, in the suboccipital or intracranial parts of the artery41,47,48. According to Bernoulli's Principle65, there is an increase in blood flow velocity at and/or immediately beyond the point of constriction of a vessel, because of either compression or stretching. At this point, there is a reduction in the diameter of the vessel, which causes an increase in flow velocity (Poiseuille's Law: Flow is proportional to the fourth power of the radius of the vessel)65. This may manifest as a “spurting” of blood at this point or turbulence of flow in the artery immediately down-stream from the region of distortion. This results in a decrease in blood flow velocity a short distance distal to this point in the artery, and blood flow usually returns to normal laminar flow in the more distal parts of the vessel65. Therefore, it seems logical to measure blood flow in that part of the VA distal to these regions of disturbed hemodynamics as it is likely that the part of the VA measured for changes in blood flow associated with cervical spine rotation may affect the outcomes.
Most blood flow studies conducted have measured changes in blood flow velocity associated with cervical spine rotation. Although blood flow volume is the main parameter affecting brain perfusion65, relative changes in velocity may be used to reflect changes in volume47,66,67. These assumptions are based on the low pulsatility index of the VA and the fact that its internal diameter (and mean cross-sectional area) does not change during normal laminar blood flow. It may be expected, therefore, that blood flow velocity (cm/s, i.e., speed of blood flow) and blood flow volume (cm3/s, i.e., taking the speed of flow and the cross-sectional area of the vessel into account) will vary proportionally66,67. Thus, a measure of changes in blood flow velocity, associated with movements of the cervical spine, can be considered a good indicator of related changes in blood flow volume.
Several narrative or descriptive critical reviews of available reports have been conducted47,48,59,68, and all concluded that more controlled research is needed. However, to the best knowledge of this author, an analysis of the data of these studies has not been done. In this context, a quantitative analysis, such as a meta-analysis of published data, may be of value. A meta-analysis is an accepted method of quantitatively comparing and summarizing the results of previous research across a range of studies. It does not depend on sample size as do traditional statistical tests such as the t- and F-tests, and it uses an estimate of the measure of the magnitude of the effect of an intervention. The most common estimate of effect size in current meta-analyses is Cohen's d, which is calculated as the standardized difference between two means, that is, by dividing the difference of the means by the pooled standard deviations for the pre- and post-intervention data reported in the studies.
As no meta-analyses of VA blood flow data have been published to date, it was the aim of this study to critically review the current literature and quantitatively analyze the reported findings of VA blood flow velocity changes related to cervical spine rotation. From this analysis, unreliable and invalid findings can be disregarded, and key messages based on stronger evidence can be put forward to inform professional practice.
Methods
A systematic literature search was conducted of all available reports, related to VA blood flow changes associated with cervical spine rotation, in manual therapy, physical therapy, chiropractic, and allied medical journals over the past 50 years. The search engines AMED, CI-NAhL, Cochrane Library, Embase, Medline (PubMed), and Pedro were used, and previous searches47,48,68 were refined to include only search terms such as vertebral artery, vertebrobasilar insufficiency/ischemia, cervical spine movements/rotation/extension, blood flow volume/velocity/measurement, and Doppler/ultrasound/insonation. Unpublished reports; papers written in foreign languages without English abstracts; human cadaver, animal, and in vitro studies; and single case studies were excluded. Reports of adult subjects and patients who may or may not have been exhibiting signs and symptoms of VBI, any or all parts of the VA measured, studies of multiple cases, descriptive studies, and controlled trials were included. The search was refined further by selecting only reports of Doppler insonation or measurement of VA blood flow velocity.
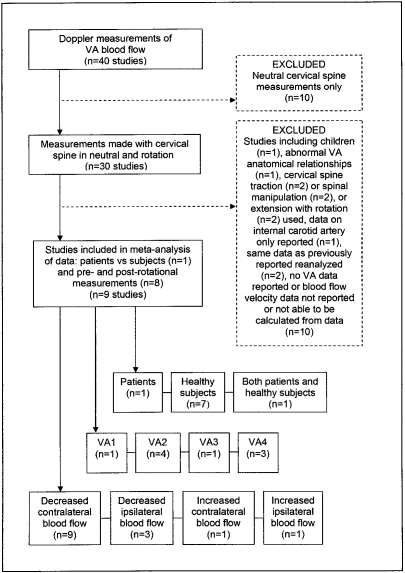
From this literature search, 40 articles were retrieved, of which 10 were reports of VA blood flow measurements in a neutral cervical spine position only, and 30 involved rotation of the cervical spine and pre- and post-rotational measurements of VA blood flow velocity. Of these 30 articles, nine were excluded initially in an effort to minimize the influence of confounding variables when comparing research findings across several studies. These either involved subjects under 18 years of age23; abnormal VA anatomical relationships such as hypoplasia or aplasia45; use of cervical spine traction18,24, spinal manipulation10,21,26, or extension28 as opposed to normal, full-range rotation; and data on the internal carotid artery only reported38. A further 12 articles were rejected finally: those reporting re-analysis of previously published data22,35 and blood flow velocity data not given or able to be calculated from the documented measurements19,30–32,37,41,46,69–71.
Of the remaining nine reports, one article compared two groups of individuals: subjects not showing signs and symptoms of VBI on the standard test, and those positive for VBI on testing29, while eight studies involved pre- and post-rotational (-interventional) measurements of blood flow velocity, each subject acting as his or her own control15,16,25,39,40,42–44 (Figure 2). None of these studies could be classified strictly as controlled trials, however.
FIGURE 2.
Flow chart illustrating the process of selection of research reports for the meta-analysis of published data.
In conducting a meta-analysis of the data given in these reports, the articles were reviewed according to the following factors:
Type of Doppler used
Patient or healthy subject measured
Current signs and symptoms of VBI noted
Measurement position of subject used
Part of VA insonated
Changes in VA blood flow recorded
To standardize the calculation of blood flow velocity across the studies that provided the required data, the following equation was used: BFvel = [(PS−ED)/3] + ED where BFvel = blood flow velocity, PS = peak systolic velocity, and ED = end diastolic velocity. This equation was derived from that used for mean arterial pressure, which equals a third of the pulse pressure (i.e., the difference between systolic and diastolic pressure) plus the diastolic pressure65. The standard t-test was used to test for statistical differences between the VA blood flow means across the various studies, using the weighted means and a significance level of p ≤ 0.05. To compare the measured blood flow velocities and changes of VA blood flow associated with cervical spine rotation as a standardized mean across these studies, in order to calculate the magnitude of the effect of the intervention, the following equation was used: d = mean1 − mean2 / √ [(sd12 + sd22) / 2] where d = Cohen's d, the descriptive measure of effect size, and sd = standard deviation. Values for d of more than or equal to 0.8, 0.5, and 0.2 were taken to represent large, medium, and small effect sizes, respectively, with 0.0 indicating that there is a 50% overlap of the one mean value with the other mean value or that there is no difference in the distribution of scores between the two categories being compared (Table 1).
TABLE 1.
Cohen's effect sizes and corresponding percentages
| Effect size value d | Percentage of score distribution overlap | Effect size strength |
|---|---|---|
| 0.0 | 50% | |
| 0.1 | 54% | |
| 0.2 | 58% | Small |
| 0.3 | 62% | |
| 0.4 | 66% | |
| 0.5 | 69% | Moderate |
| 0.6 | 73% | |
| 0.7 | 76% | |
| 0.8 | 79% | Large |
| 0.9 | 82% | |
| 1.0 | 84% | |
| 1.2 | 88% | |
| 1.4 | 92% | |
| 1.6 | 95% | |
| 1.8 | 96% | |
| ≥ 2.0 | 98+% |
d = Cohen's d (measure of effect size)
Results
Doppler Method and Measurement Position
All nine studies reviewed and analyzed15,16,25,29,39,40,42–44 used pulsed-wave Doppler and imaging of the vessel in insonating extracranial15,25,29,40,42,44 and intracranial16,39,43 VA blood flow changes (Table 2). Blood flow velocities were given or could be calculated from the peak systolic velocities in four studies15,29,40,42, and the remaining five reports16,25,39,43,44 documented the data as blood flow velocities. Thus, it was possible to make comparisons of the blood flow data in this analysis.
TABLE 2.
Description of studies of vertebral artery blood flow measurements associated with cervical spine rotation
| Mean blood flow measurements (cm/s) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Subjects | VBI | Doppler method | VA | change | neutral | ipsi | difference | contra | difference |
| Arnold et al42 | 22s-supine | nil | PW+C | VA2 | ↓ contra | 31.40 | 32.97 | +1.57 | 27.17 | −4.23 |
| Licht et al25 | 20s-supine | nil | PW+C | VA1 | ↓ contra | 68.00 | 72.00 | +4.00 | 61.00 | −7.00 |
| ↑ ipsi | ||||||||||
| Mitchell39 | 120s-prone | nil | TCD | VA4 | ↓ contra | 36.78 | 33.56 | −3.22 | 29.74 | −7.04 |
| ↑ ipsi | ||||||||||
| Mitchell et al43 | 30s-sitting | nil | TCD | VA4 | ↓ contra | 36.85 | 33.83 | −3.02 | 33.53 | −3.32 |
| ↑ ipsi | ||||||||||
| Mitchell & Kraumschuster44 | 35s-sitting | nil | PW+C | VA3 | ↓ ipsi | 24.93 | 24.67 | −0.26 | 25.18 | 0.25 |
| ↑contra | ||||||||||
| Rivett et al29 | 10p-supine | VBI nil | PW+C | VA2 | ↓ contra | 30.78 | 24.33 | −6.45 | ||
| 10c-supine | ↓ contra | 27.70 | 21.17 | −6.53 | ||||||
| Rivett et al40 | 20s-supine | nil | PW+C | VA2 | ↓ contra | 21.25 | 20.05 | −1.20 | ||
| Rossitti et al16 | 30s-sitting | nil | TCD | VA4 | ↓ contra | 34.00 | 29.00 | −5.00 | 30.10 | −3.90 |
| Stevens15 | 7p | — | PW+C | VA2 | ↓ contra | 14.83 | 8.33 | −6.50 | ||
c = control group, C = Doppler with color flow imaging, change = VA blood flow change from neutral to rotated cervical spine position, contra = contralateral rotation/blood flow, difference = difference in blood flow measurements between the neutral and rotated positions, ipsi = ipsilateral rotation/blood flow, neutral = no cervical spine rotation, p = patients or subjects with VBI signs and symptoms, PW = pulsed-wave Doppler, s = healthy subjects with no VBI signs and symptoms, TCD = transcranial Doppler (PW+C), VA = vertebral artery (VA1–4 = parts 1, 2, 3, 4), VBI = vertebrobasilar insufficiency, 2 = decreased blood flow, 1 = increased blood flow
With the exception of one study that did not record the patient's position15, and one that positioned the subjects in prone lying39, the measurements were carried out with the individuals in either sitting16,43,44 or supine lying25,29,40,42 (Table 2). On statistical analysis of the data, using the weighted means and the t-test, no significant difference between supine lying and sitting was found in any of the blood flow measurements recorded. However, again using the weighted means, when comparing the pooled blood flow data with the subjects sitting and lying supine, VA blood flow was found to be higher in supine lying for all measurements (Table 3). The effect size was large in the neutral cervical spine position (d = 1.45 [total group], d = 1.39 [subjects only]), with contralateral rotation (d = 0.90 [total group], d = 1.01 [subjects only]), and for the calculated mean change in blood flow across all studies (d = 4.03 [total group], d = 3.27 [subjects only]) (Table 4). On analyzing the data across the few studies that provided ipsilateral blood flow measurements16,25,39,42,44, a small effect size (d = 0.10, [total group]) was found for changes with ipsilateral rotation.
TABLE 3.
Weighted mean vertebral artery blood flow velocity measurements (cm/s)
| VA1–4 neutral contra change | VA2 neutral contra change | VA3 neutral contra change | VA4 neutral contra change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood flow velocity: pooled data | ||||||||||||
| Total group | n=10 | n=5 | ||||||||||
| mean | 34.79 | 29.90 | −4.89 | 27.43 | 23.45 | −3.98 | ||||||
| sd | 4.10 | 3.32 | 0.82 | 3.04 | 2.85 | 0.46 | ||||||
| Subjects | n=8 | n=3 | n=1 | n=3 | ||||||||
| mean | 35.42 | 30.62 | −4.79 | 26.78 | 23.28 | −3.51 | 24.93 | 25.18 | 0.25 | 36.33 | 30.43 | −5.90 |
| sd | 4.59 | 3.66 | 0.96 | 4.03 | 3.71 | 0.67 | 1.58 | 1.50 | 0.25 | 10.75 | 8.39 | 2.36 |
| Patients | n=2 | n=2 | ||||||||||
| mean | 24.21 | 17.74 | −6.47 | 24.21 | 17.74 | −6.47 | ||||||
| sd | 8.49 | 7.70 | 0.79 | 8.49 | 7.70 | 0.79 | ||||||
| Supine-lying | n=5 | |||||||||||
| Total group | ||||||||||||
| Mean | 37.32 | 32.61 | −4.72 | |||||||||
| sd | 5.47 | 5.03 | 0.52 | |||||||||
| subjects | n=3 | 38.23 | 33.75 | −4.48 | ||||||||
| mean | 24.21 | 17.74 | −6.47 | 24.21 | 17.74 | −6.47 | ||||||
| sd | 6.66 | 6.08 | 0.68 | |||||||||
| Patients | n=1 | |||||||||||
| mean sd | 30.78 | 24.33 | −6.45 | |||||||||
| sd | ||||||||||||
| Sitting | ||||||||||||
| Total group | n=3 | |||||||||||
| Mean | 31.56 | 29.37 | −2.19 | |||||||||
| sd | 1.24 | 0.70 | 0.72 | |||||||||
| Subjects | n=3 | |||||||||||
| mean | 31.56 | 29.37 | −2.19 | |||||||||
| sd | 1.24 | 0.70 | 0.72 | |||||||||
| Patients | n=0 | |||||||||||
change = VA blood flow change from neutral to rotated cervical spine position, contra = contralateral rotation/blood flow. n = number of groups of subjects or patients/pooled measurements, neutral = no cervical spine rotation, VA = vertebral artery (VA1–4 = parts 1, 2, 3, 4).
TABLE 4.
Effect size (Cohen's d) of comparable data
| VA1–4 neutral contra change | VA2 neutral contra change | VA3 neutral contra change | VA4 neutral contra change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d (neutral/rotation) | ||||||||||
| total group | 1.31 | 1.35 | ||||||||
| subjects | 1.16 | 0.90 | 0.16 | 0.61 | ||||||
| patients | 0.80 | 0.80 | ||||||||
| d (sitting/supine) | ||||||||||
| total group | 1.45 | 0.90 | 4.03 | |||||||
| subjects | 1.39 | 1.01 | 3.27 | |||||||
| patients | ||||||||||
| d (subjects/patients) | 1.64 | 2.14 | 1.91 | 0.39 | 0.92 | 4.04 | ||||
| d (VA2/VA3) | ||||||||||
| subjects | 0.60 | 0.67 | 7.44 | |||||||
| d (VA 2/VA 4) | ||||||||||
| subjects | 1.18 | 1.10 | 1.38 | |||||||
| d (VA 3/VA 4) | ||||||||||
| subjects | 1.48 | 0.87 | 3.66 | |||||||
change = VA blood flow change from neutral to rotated cervical spine position, contra = contralateral rotation/blood flow, d = Cohen's d (measure of effect size), neutral = no cervical spine rotation, VA = vertebral artery (VA1–4 = parts 1, 2, 3, 4).
Subjects and VBI
One study15 used patients only and did not report whether signs and symptoms of VBI were experienced during or as a result of the measurement procedure. A further report involved both healthy subjects and patients29, some of whom had a history of VBI. Healthy persons, with no manifestations of VBI, were the subjects in the remaining seven articles16,25,39,40,42–44 (Table 2).
The mean VA blood flow values after pooling the data across all the studies indicated that patients had considerably lower blood flow velocities than healthy individuals, both in the neutral cervical spine position and with contralateral rotation (Table 3). Although this was the expected result, statistical analysis with the t-test and using the weighted means showed no significant differences between the two groups of subjects, either for VA1–4, or in VA2 alone, for the neutral position or with contralateral rotation. Values for ipsilateral rotation were not given for patients; hence, it was not possible to compare outcomes for this type of rotation in these two groups of individuals.
On further comparison of blood flow data between healthy subjects and patients, across all the studies, a large effect size was found for VA blood flow in the neutral cervical spine position (d = 1.64), with contralateral rotation (d = 2.14), and for the change in blood flow (d = 1.91) for VA1–4, indicating a lower blood flow and a greater reduction in velocity in the patients in the pre- and post-intervention measurements (i.e., in the neutral and rotated cervical spine positions). On using data for VA2 only, a large effect size was found for contralateral rotation (d = 0.92) only, and a larger change in blood flow (d = 4.04) (Table 4). A comparison between healthy subjects and patients for VA4 blood flow could not be done as no data was provided for patients in the studies reviewed.
Changes in VA Blood Flow
All nine studies reviewed described a change in VA blood flow velocity associated with cervical spine rotation (Tables 2 and 3). Overwhelmingly, this change was related to contralateral rotation regardless of whether healthy subjects or patients were measured.
Large effect sizes were calculated for VA1–4 (d = 1.31) and for VA2 (d = 1.35), comparing VA blood flow values in the neutral position with contralateral rotation of the cervical spine across the studies (Table 4). Interestingly, VA2 blood flow was not more effectively compromised in patients than healthy subjects (d = 0.8 and 0.9, respectively) (Table 4). The VA3 blood flow changes associated with cervical spine rotation only showed a small effect size (d = 0.16) (Table 4), and there was a similarly small effect size for ipsilateral rotation (d = 0.16).
Part of the VA Insonated
In the nine articles reviewed, one reported measurement of blood flow in the first or pre-cervical part of the artery (VA1)25, one in the suboccipital region (VA3)44, three in the intracranial area (VA4)16,39,43, and four in the mid- to upper cervical part of the artery (VA2)15,29,40,42 (Tables 2 and 3). Further quantitative analysis comparing VA2, VA3, and VA4 blood flow velocities across the studies revealed that in the neutral cervical spine position, although the weighted means are similar, VA2 blood flow was effectively greater than that in VA3 (d = 0.60) but less than that in VA4 (d = 1.48) (Table 4). On contralateral rotation, VA blood flow was more effectively reduced in VA4 when compared to that in VA2 (d = 1.10) or VA3 (d = 0.87), while there was less of a reduction effect (d = 0.67) seen on comparing VA2 and VA3 blood flow.
Discussion
Doppler Method and Measurement Position
The method of insonation was not regarded as a differentiating factor for the purpose of this analysis as all nine studies reviewed used pulsed-wave Doppler and imaging to insonate the VA.
Both sitting and supine lying, as used in the reports analyzed, are the common postures for the standard VBI test in clinical practice. Although prone lying may allow free access to VA3 and VA4, it is not the most comfortable position for many older individuals. Supine lying offers the most direct access to VA2 but does not enable unhindered insonation of VA3 or VA4, especially in the neutral position of the cervical spine. Although there was no significant difference shown between the measurements in the two positions in this analysis, the sitting position seems optimal for insonating all four parts of the VA.
However, the higher blood flow velocities recorded in supine lying and the larger effect sizes calculated on comparing the positions, for the neutral cervical spine position and contralateral rotation, indicate that the choice of the position used for measuring the subjects could effectively influence the outcome measures (i.e., VA blood flow). These differences are difficult to explain in terms of current hemodynamic theory, especially in the group of healthy, young adults. In the studies reviewed, different parts of the VA were measured in the two positions: VA4 in sitting and VA2 in supine lying. The position of the subject may have influenced the ease and accuracy of insonation and, hence, the blood flow measurements. The possibility of the various parts of the VA having different blood flow velocities is discussed further in the section below entitled Part of the VA Insonated.
Subjects and VBI
This meta-analysis of data suggests that both the pre- and post-intervention blood flow values are greater in healthy subjects and effectively compromised by contralateral rotation more so in the patient group, particularly in VA2. This may be a result of a number of factors, including an inadequate collateral vertebrobasilar circulation or vascular pathology (e.g., atherosclerosis) compromising VA blood flow in this patient group. This is partly supported by the fact that some of the patients manifested signs and symptoms of VBI. However, this was not discussed by any of the authors and remains a tentative suggestion as none of the studies reported evidence of turbulent blood flow, narrowing of vessels, or other reasons for compromised blood flow on insonation of the VAs.
Changes in VA Blood Flow
This review indicates that it is contralateral rotation that is associated with VA blood flow velocity changes with cervical spine movements. An explanation for this may be that blood flow in the VA is likely to be reduced because of compression or stretching distortion forces by osteophytes in the cervical region46,72–78, between the atlas and axis vertebrae29,35,41,42,49–51, or along its course related to the atlas vertebra39,43,47–49,51,54–63. Where the VA was insonated is likely to be an influencing factor, therefore, and this is discussed in the following section.
In addition, Rivett et al40 reported a decreased contralateral blood flow in the region of the second to third cervical vertebrae but an increased contralateral VA blood flow at the atlas-axis vertebral level. The authors gave no explanation for this difference in outcome. Similarly, the increase in VA2 blood flow found in 20% of cases on contralateral rotation by Stevens15, when 60% of subjects demonstrated decreased contralateral blood flow, may be a result of a number of confounding factors such as the small sample of seven patients used; a possible history or signs and symptoms of VBI, which were not discussed; the subject's position for blood flow measurement, which was not given; and the possibility of inaccurate insonation of the artery. On the other hand, Licht et al25 reported an associated increase in blood flow on ipsilateral rotation in some subjects. However, these authors measured blood flow and documented average values only in VA1, which precludes further quantitative analysis of the data. Lastly, in a recent study of VA344, a significant decrease in VA blood flow was found on ipsilateral rotation, possibly a result of compression of the vessel as discussed in the Introduction above, and a measurable but non-significant increase on contralateral rotation. Again, the part of the VA measured may have influenced the outcome.
Despite these differences in the results in some of the studies, the overall evidence indicates significantly compromised VA blood flow on contralateral rotation of the cervical spine. The large effect sizes found for VA1–4 and for VA2, when comparing VA blood flow values in the neutral position with contralateral rotation of the cervical spine across the studies, support this (Table 4). Moreover, the lack of a more effectively compromised VA2 blood flow velocity in patients than in healthy subjects concurs with the findings of Rivett et al29 of similar changes in VA2 blood flow in their patients and healthy subjects (Table 2). This suggests that the signs and symptoms of VBI on sustained cervical spine rotation reported by the patients in their study were more likely a result of an inadequate collateral vertebrobasilar circulation or of compromised blood flow because of vascular pathology, for example, in the more distal parts of the VA. No evidence was provided in that report to support this supposition, however, and it must be noted that false positive (and false negative) findings are possible in blood flow measurements. Nevertheless, the less effectively reduced blood flow in VA4, when compared with VA2, on contralateral rotation in the healthy subjects measured16,39,43 may be a function of the part of the VA insonated, as discussed in the Introduction above and Part of the VA Insonated below.
The small effect size calculated in this analysis for VA3 blood flow changes associated with cervical spine rotation concurs with the previous finding of a non-significant change in VA3 blood flow with contralateral rotation44. However, the small effect size found for ipsilateral rotation is difficult to explain, considering the statistically significant change in blood flow velocities in healthy, young females44. It is likely that these results may have been confounded by the small sample size (n=30) in the earlier, single study44, and more evidence of such changes is necessary to establish the validity of the results.
Part of the VA Insonated
The majority of all studies surveyed insonated VA2, which is the most easily accessible part of the vessel. However, neither VA2 nor VA1 fulfill the criteria for the optimal point for measurement of blood flow described in the Introduction, i.e., being upstream and often far removed from the region of cervical spine rotation. Although there is a lack of evidence for this, it seems logical that VA blood flow will vary below and above points of mechanical distortion associated with rotation of the cervical spine, with an increase in velocity because of the “spurting effect” at or immediately beyond the point of constriction of the artery65. Furthermore, the mean diameter of the artery changes along its course79,80, and because blood flow is a function of the cross-sectional area of a vessel65, the blood flow velocity would be expected to differ in the various parts of the VA, being greatest in the narrowest part (i.e., VA4). This is supported by the greater weighted mean blood flow values for VA4, compared to those for VA2 and VA3 (Table 3). In other words, the slower VA3 blood flow velocity to that of either VA2 or VA4, in the neutral cervical spine position, concurs with the hemodynamic theories related to blood flow velocities and size of vessels65.
However, on contralateral rotation, a greater reduction in VA4 blood flow velocity than in the more proximal VA2 and VA3 was found (Table 3). This is difficult to explain if one considers the hypothesis that rotation causes mechanical distortion of the VA at the suboccipital level and it would be expected that both VA3 and VA4 blood flow would be compromised. This was not shown in the recent study of VA3 blood flow44, when an insignificant reduction in contralateral but a significant increase in ipsilateral blood flow was reported. A possible explanation for the finding in the present analysis, shown as a small effect regardless of the direction of rotation, is that the compressive forces on VA3 associated with ipsilateral cervical spine rotation were as effective as any stretching of the vessel on contralateral rotation, despite the blood flow being measurably more reduced in the former. It must be noted, however, that there is no proof of this to date. Nevertheless, the results of this quantitative meta-analysis support the suggestion that VA blood flow will vary according to the part insonated, and this should be taken into account in clinical practice.
Limitations of the Meta-Analysis
Because these findings across all the studies reviewed apply to small groups of subjects and patients tested, they may be biased and the external validity or generalizability of the meta-analysis may be limited. Despite the claim that meta-analyses do not depend on sample sizes, there is always the possibility of both Type-I and Type-II errors occurring in individual studies such as these because of small sample sizes. Furthermore, the validity of a meta-analysis is dependent largely on the quality of the systematic review of reports. Ideally, both published and non-published studies should be analyzed, but in this analysis, only those reports published in English and with English abstracts were included. This means that some valuable data in unpublished or foreign reports may have been overlooked. Lastly, although an attempt was made to avoid some bias by using strict exclusion/inclusion criteria, the selection of studies to review may have confounded the findings in the meta-analysis of data. Such limitations should be taken into account in future analyses of this kind.
Conclusions and Implications for Professional Practice
It is evident from this literature review that there is a paucity of rigorously controlled research trials to measure VA blood flow velocity changes associated with cervical spine rotation. More evidence of change in blood flow is needed, in larger homogeneous groups of both healthy persons (i.e., base-line values) and patients with a variety of neuro-musculoskeletal conditions, across several discrete adult age groups, in males and in females. Therefore, it must be emphasized that a stronger evidence base is necessary before any conclusions can be applied to the general patient population.
From the review of the literature, it is suggested that pulsed-wave Doppler insonation with color flow imaging is preferable in investigating VA blood flow, as this method ensures accurate visualization of the VA, and any pathology or unusual flow patterns during measurement of blood flow. It is advisable to use the sitting position for the subject, which is optimal for access to all parts of the VA, and for control of cervical spine rotation during insonation of the artery.
Despite there being only a small number of published reports with well-documented VA blood flow velocity data associated with rotation of the cervical spine, it was concluded from this meta-analysis that VA blood flow is compromised, particularly in VA4, with full or sustained contralateral rotation, with the subject measured in sitting, and in patients more so than in healthy, young individuals. This finding is of particular concern in patients or older persons who are more likely to have vascular pathology, such as atherosclerosis.
Although sustained, full-range rotation of the cervical spine, as used in the pre-treatment VBI test, can indicate only the functional state of an individual's vertebrobasilar and collateral circulations, it will allow suspect cases of vascular pathology to be referred for further investigation of the vascular system. This test cannot claim to indicate the safety of high-velocity, low-amplitude manipulative therapy thrusts in all patients, however, and more evidence of the effect of such cervical spine movements on the VA and its blood flow is needed, despite the many difficulties in carrying out such research.
Based on this meta-analysis of available evidence, the following key messages may be of value to manual therapists in clinical and professional practice:
A thorough and complete pretreatment history, including full subjective and objective assessments, must be taken for each patient. Where abnormal anatomical vascular patterns or vascular pathology are suspected, Doppler insonation of vessels, preferably using pulsed-wave Doppler and color flow imaging to ensure accuracy of VA insonation, should be carried out.
The same position of the individual should be adopted for both pre-manipulative tests, such as the VBI test, and the subsequent treatment of each patient, as the large effect size for the comparison of sitting and supine lying suggests that this may influence results. Cognizance should be taken of the fact that false positive and false negative results of these tests are possible, and repeated measurements are advisable.
Patients may have reduced VA blood flow compared to healthy, young subjects, as shown by the large effect size on comparing the two groups, in the neutral position and with rotation of the cervical spine. This result is of particular importance in older persons, regardless of whether they exhibit signs and symptoms of VBI, because this group is more likely to have associated risk factors for neurovascular incidents post-manual therapy, such as previous micro-trauma of the VA, atherosclerosis, loss of elasticity, increase in collagen in arterial walls concomitant with normal aging, or osteoarthritis.
The large effect size found on comparing VA blood flow in the neutral cervical spine position and with contralateral rotation strongly supports the results of most studies of a significant decrease in blood flow on contralateral rotation. The fact that this finding relates to sustained, full rotation of the cervical spine indicates that it would be judicious to avoid such movements, particularly full-range rotation held for a minute or longer. In addition, it has been suggested in the literature that there is a danger of repeated thrusting rotational cervical spine movements causing micro-trauma to vessels such as the VA. Therefore, in the absence of strong evidence to support or refute this, it is strongly advised that such high-velocity manipulative thrusts at the end-of-range of rotation should be avoided in clinical practice.
REFERENCES
- 1.De Kleyn A, Nieuwenhuyse AC. Vertigo and nystagmus with various head positions. Acta Otolaryngol. 1927;11:155–157. [Google Scholar]
- 2.Tissington-Tatlow WF, Brammer HG. Syndrome of vertebral artery compression. Neurology. 1957;7:331–340. doi: 10.1212/wnl.7.5.331. [DOI] [PubMed] [Google Scholar]
- 3.Toole JF, Tucker SH. Influence of head position upon cerebral circulation. Arch Neurol. 1960;2:616–623. doi: 10.1001/archneur.1960.03840120022003. [DOI] [PubMed] [Google Scholar]
- 4.Brown BSTJ, Tissington-Tatlow WF. Radiographic studies of the vertebral arteries in cadavers. Radiology. 1963;81:80–88. doi: 10.1148/81.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Selecki BR. The effects of rotation of the atlas on the axis: Experimental work. Med J Aust. 1969;1:1012–1015. [PubMed] [Google Scholar]
- 6.Faris AA, Poser CM, Wilmore DW, Agnew CH. Radiologic visualization of neck vessels in healthy men. Neurology. 1963;13:386–396. doi: 10.1212/wnl.13.5.386. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi I, Kaneko S, Asaoka K, Harada T. Angiographic examination of the vertebral artery at the atlantoaxial joint during head rotation. No Shinkei Geka. 1994;22:749–753. [PubMed] [Google Scholar]
- 8.Bartels E, Flugel KA. Evaluation of extracranial vertebral artery dissection with duplex colour-flow imaging. Stroke. 1996;27:290–295. doi: 10.1161/01.str.27.2.290. [DOI] [PubMed] [Google Scholar]
- 9.Kuether TA, Nesbit GM, Clark WM, Barnwell SL. Rotational vertebral artery occlusion: A mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41:427–432. doi: 10.1097/00006123-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Licht PB, Christensen HW, Hoilund-Carlsen PF. Vertebral artery volume flow in human beings. J Manipulative Physiol Ther. 1999;22:363–367. doi: 10.1016/s0161-4754(99)70080-1. [DOI] [PubMed] [Google Scholar]
- 11.Nicolau C, Gilabert R, Chamorro A, Vazquez F, Bargallo N, Bru C. Doppler sonography of the intertransverse segment of the vertebral artery. J Ultrasound Med. 2000;19:47–53. doi: 10.7863/jum.2000.19.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Nicolau C, Gilabert R, Garcia A, Blasco J, Chamorro A, Bru C. Effect of internal carotid artery occlusion on vertebral artery blood flow: A duplex ultrasonographic evaluation. J Ultrasound Med. 2001;20:105–111. doi: 10.7863/jum.2001.20.2.105. [DOI] [PubMed] [Google Scholar]
- 13.Saito K, Kimura K, Nagatsuka K, et al. Vertebral artery occlusion in duplex color-coded ultrasonography. Stroke. 2004;35:1068–1072. doi: 10.1161/01.STR.0000125857.63427.59. [DOI] [PubMed] [Google Scholar]
- 14.Trattnig S, Hubsch P, Schuster H, Polzleitner D. Color-coded Doppler imaging of normal vertebral arteries. Stroke. 1990;21:1222–1225. doi: 10.1161/01.str.21.8.1222. [DOI] [PubMed] [Google Scholar]
- 15.Stevens A. Functional Doppler sonography of the vertebral artery and some considerations about manual techniques. J Man Med. 1991;6:102–105. [Google Scholar]
- 16.Rossitti S, Volkmann R, Lofgren J. Changes of blood flow velocity in the vertebro-basilar circulation during rotation of the head in the normal human. Biomech Sem. 1992;6:92–99. [Google Scholar]
- 17.Schoning M, Walter J. Evaluation of the vertebrobasilar-posterior system by transcranial color duplex sonography in adults. Stroke. 1992;23:1280–1286. doi: 10.1161/01.str.23.9.1280. [DOI] [PubMed] [Google Scholar]
- 18.Weingart JR, Bischoff HP. Doppler-sonographische Untersuchung der A. Vertebralis unter Berucksichtigung chirotherapeutisch relevanter Kopfpositionen. J Man Med. 1992;30:62–65. [Google Scholar]
- 19.Refshauge KM. Rotation: A valid pre-manipulative dizziness test? Does it predict safe manipulation? J Manipulative Physiol Ther. 1994;17:15–19. [PubMed] [Google Scholar]
- 20.Schoning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994;25:17–22. doi: 10.1161/01.str.25.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Thiel H, Wallace K, Donat J, Yong-Hing K. Effects of various head and neck positions on vertebral artery blood flow. Clin Biomech. 1994;9:105–110. doi: 10.1016/0268-0033(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Rossitti S, Volkmann R. Changes of blood flow velocity indicating mechanical compression of the vertebral arteries during rotation of the head in the normal human measured with transcranial Doppler sonography. Arq Neuropsiquiatr. 1995;53:26–33. doi: 10.1590/s0004-282x1995000100005. [DOI] [PubMed] [Google Scholar]
- 23.Haynes MJ. Doppler studies comparing the effects of cervical rotation and lateral flexion on vertebral artery blood flow. J Manipulative Physiol Ther. 1996;19:378–384. [PubMed] [Google Scholar]
- 24.Weingart JR, Bischoff HP. Farbcodierte Duplexsonographie der A. Vertebralis: Abhangigkeit von Rotation und Traktion des Kopfes. J Man Med. 1997;35:254–257. [Google Scholar]
- 25.Licht PB, Christensen HW, Hojgaard P, Hoilund-Carlsen PF. Triplex ultrasound of vertebral artery flow during cervical rotation. J Manipulative Physiol Ther. 1998;21:27–31. [PubMed] [Google Scholar]
- 26.Licht PB, Christensen HW, Hojgaard P, Marving J. Vertebral artery flow and spinal manipulation: A randomized, controlled and observer-blinded study. J Manipulative Physiol Ther. 1998;21:141–144. [PubMed] [Google Scholar]
- 27.Rivett DA, Milburn PD, Chapple C. Negative pre-manipulative vertebral artery testing despite occlusion: A case of false negativity? Man Ther. 1998;3:102–107. [Google Scholar]
- 28.Li YK, Zhang YK, Lu CM, Zhong SZ. Changes and implications of blood flow velocity of the vertebral artery during rotation and extension of the head. J Manipulative Physiol Ther. 1999;22:91–95. doi: 10.1016/s0161-4754(99)70113-2. [DOI] [PubMed] [Google Scholar]
- 29.Rivett DA, Sharples KJ, Milburn PD. Effect of pre-manipulative tests on vertebral artery and internal carotid artery blood flow: A pilot study. J Manipulative Physiol Ther. 1999;22:368–375. doi: 10.1016/s0161-4754(99)70081-3. [DOI] [PubMed] [Google Scholar]
- 30.Haynes MJ. Vertebral arteries and neck rotation: Doppler velocimeter and duplex results compared. Ultrasound Med Biol. 2000;26:57–62. doi: 10.1016/s0301-5629(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 31.Haynes M, Hart R, McGeachie J. Vertebral arteries and neck rotation: Doppler velocimeter interexaminer reliability. Ultrasound Med Biol. 2000;26:1363–1367. doi: 10.1016/s0301-5629(00)00303-3. [DOI] [PubMed] [Google Scholar]
- 32.Licht PB, Christensen HW, Hoilund-Carlsen PF. Is there a role for pre-manipulative testing before cervical manipulation? J Manipulative Physiol Ther. 2000;23:175–179. doi: 10.1016/s0161-4754(00)90247-1. [DOI] [PubMed] [Google Scholar]
- 33.Scheel P, Ruge C, Schoning M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: Reference data and the effects of age. Ultrasound Med Biol. 2000;26:1261–1266. doi: 10.1016/s0301-5629(00)00293-3. [DOI] [PubMed] [Google Scholar]
- 34.Scheel P, Ruge C, Petruch UR, Schoning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31:147–150. doi: 10.1161/01.str.31.1.147. [DOI] [PubMed] [Google Scholar]
- 35.Haynes M, Milne N. Color duplex sonographic findings in human vertebral arteries during cervical rotation. J Clin Ultrasound. 2001;29:14–24. doi: 10.1002/1097-0096(200101)29:1<14::aid-jcu3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Haynes MJ. Vertebral arteries and cervical movement: Doppler ultrasound velocimetry for screening before manipulation. J Manipulative Physiol Ther. 2002;25:556–567. doi: 10.1067/mmt.2002.127077. [DOI] [PubMed] [Google Scholar]
- 37.Haynes MJ, Cala LA, Melsom A, Mastaglia FL, Milne N, McGeachie K. Vertebral arteries and cervical rotation: Modeling and magnetic resonance angiography studies. J Manipulative Physiol Ther. 2002;25:370–383. doi: 10.1067/mmt.2002.126130. [DOI] [PubMed] [Google Scholar]
- 38.Licht PB, Christensen HW, Hoilund-Carlsen PF. Carotid artery blood flow during premanipulative testing. J Manipulative Physiol Ther. 2002;25:568–572. doi: 10.1067/mmt.2002.128367. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell J. Changes in vertebral artery blood flow following rotation of the cervical spine. J Manipulative Physiol Ther. 2003;26:347–351. doi: 10.1016/S0161-4754(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 40.Rivett DA, Sharples KJ, Milburn PD. Reliability of ultrasonograpahic measurement of vertebral artery blood flow. NZ J Physiother. 2003;31:119–128. [Google Scholar]
- 41.Zaina C, Grant R, Johnson C, Dansie B, Taylor J, Spyropolous P. The effect of cervical rotation on blood flow in the contralateral vertebral artery. Man Ther. 2003;8:103–109. doi: 10.1016/s1356-689x(02)00155-8. [DOI] [PubMed] [Google Scholar]
- 42.Arnold C, Bourassa T, Langer T, Stoneham G. Doppler studies evaluating the effect of a physical therapy screening protocol on vertebral artery blood flow. Man Ther. 2004;9:13–21. doi: 10.1016/s1356-689x(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell J, Keene D, Dyson C, Harvey L, Pruvey C, Phillips R. Is cervical spine rotation, as used in the standard vertebrobasilar insuffciency test, associated with a measurable change in intracranial vertebral artery blood flow? Man Ther. 2004;9:220–227. doi: 10.1016/j.math.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell J, Kramschuster K. Real-time ultrasound measurements of changes in suboccipital vertebral artery diameter and blood flow velocity associated with cervical spine rotation. Physiother Res Internat. 2009;13:241–254. doi: 10.1002/pri.400. DOI:10.1002/pri.400. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J, Kramschuster K. Atypical suboccipital vertebral artery blood flow in healthy subjects: Case studies using real-time ultrasound. Physiother Theory Pract. 2009 doi: 10.1080/09593980902776647. In Press. [DOI] [PubMed] [Google Scholar]
- 46.Ozdemir H, Cihangiroglu M, Berilgen S, Bulut S. Effects of cervical rotation on hemodynamics in vertebral arteries. J Diagnostic Med Sonography. 2005;21:384–391. [Google Scholar]
- 47.Mitchell J. Doppler insonation of vertebral artery blood flow changes associated with cervical spine rotation: Implications for manual therapists. Physiother Theory Pract. 2007;23:303–313. doi: 10.1080/09593980701593771. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell J. Is mechanical deformation of the suboccipital vertebral artery during cervical spine rotation responsible for vertebrobasilar insuffciency? Physiother Res Internat. 2008;13:53–66. doi: 10.1002/pri.370. DOI:10.1002/pri.370. [DOI] [PubMed] [Google Scholar]
- 49.Parkin PJ, Wallis WE, Wilson JL. Vertebral artery occlusion following manipulation of the neck. NZ Med J. 1978;88:441–443. [PubMed] [Google Scholar]
- 50.Mas JL, Henin D, Bousser MG, Chain F, Hauw JJ. Dissecting aneurysm of the vertebral artery and cervical manipulation: A case report with autopsy. Neurology. 1989;39:512–515. doi: 10.1212/wnl.39.4.512. [DOI] [PubMed] [Google Scholar]
- 51.Thiel HW. Gross morphology and pathoanatomy of the vertebral arteries. J Manipulative Physiol Ther. 1991;14:133–141. [PubMed] [Google Scholar]
- 52.Kuether TA, Nesbit GM, Clark WM, Barnwell SL. Rotational vertebral artery occlusion: A mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41:427–432. doi: 10.1097/00006123-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Johnson CP, Scraggs M, How T, Burns J. A necropsy and histomorphometric study of abnormalities in the course of the vertebral artery associated with ossified stylohyoid ligaments. J Clin Pathol. 1995;48:637–640. doi: 10.1136/jcp.48.7.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hufnagel A, Hammers A, Schonle PW, Bohm KD, Leonhardt G. Stroke following chiropractic manipulation of the cervical spine. J Neurol. 1999;246:683–688. doi: 10.1007/s004150050432. [DOI] [PubMed] [Google Scholar]
- 55.Sheth TN, Winslow JL, Mikulis DJ. Rotational changes in the morphology of the vertebral artery at a common site of arterial dissection. Can Assoc Radiol J. 2001;52:236–241. [PubMed] [Google Scholar]
- 56.Michaud TC. Uneventful upper cervical manipulation in the presence of a damaged vertebral artery. J Manipulative Physiol Ther. 2002;25:472–483. doi: 10.1067/mmt.2002.126468. [DOI] [PubMed] [Google Scholar]
- 57.Reddy M, Reddy B, Schoggl A, Saringer W, Matula C. The complexity of trauma to the cranio-cervical junction: Correlation of clinical presentation with Doppler flow velocities in the V3-segment of the vertebral arteries. Acta Neurochir. 2002;144:575–580. doi: 10.1007/s007010200078. [DOI] [PubMed] [Google Scholar]
- 58.Herzog W. Letters to the editor. J Manipulative Physiol Ther. 2003;26:339–340. [Google Scholar]
- 59.Mitchell J. The vertebral artery: A review of anatomical, histopathological and functional factors influencing blood flow to the hindbrain. Physiother Theory Pract. 2005;21:23–36. doi: 10.1080/09593980590911570. [DOI] [PubMed] [Google Scholar]
- 60.Kawchuk GN, Jhangri GS, Hurwitz EL, Wynd S, Haldeman S, Hill MD. The relation between the spatial distribution of vertebral artery compromise and exposure to cervical manipulation. J Neurol. 2008;255:371–377. doi: 10.1007/s00415-008-0667-3. Epub 2008 Jan 15. [DOI] [PubMed] [Google Scholar]
- 61.Krueger BR, Okazaki H. Vertebral-basilar distribution infarction following chiropractic cervical manipulation. Mayo Clin Proc. 1980;55:322–332. [PubMed] [Google Scholar]
- 62.Schellhas KP, Latchaw RE, Wendling LR, Gold LHA. Vertebrobasilar injuries following cervical manipulation. JAMA. 1980;244:1450–1453. [PubMed] [Google Scholar]
- 63.Bolton PS, Stick PE, Lord RSA. Failure of clinical tests to predict cerebral ischemia before neck manipulation. J Manipulative Physiol Ther. 1989;12:304–307. [PubMed] [Google Scholar]
- 64.Mann T, Refshauge KM. Causes of complications from cervical spine manipulation. Austr J Physiother. 2001;47:255–266. doi: 10.1016/s0004-9514(14)60273-7. [DOI] [PubMed] [Google Scholar]
- 65.Ganong WF. Review of Medical Physiology, 22nd ed. New York: McGraw-Hill; 2005. [Google Scholar]
- 66.Aaslid R. Transcranial Doppler Sonography. Vienna, Austria: Springer-Verlag; 1986. [Google Scholar]
- 67.Newell DW, Aaslid R. Transcranial Doppler. New York: Raven Press; 1992. [Google Scholar]
- 68.Kerry R, Taylor AJ, Mitchell J, McCarthy C. Cervical arterial dysfunction and manual therapy: A critical literature review to inform professional practice. Man Ther. 2008;13:278–288. doi: 10.1016/j.math.2007.10.006. DOI:10.1016/j.math.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Cote P, Kreitz BG, Cassidy JD, Thiel H. The validity of the extension-rotation test as a clinical screening procedure before neck manipulation. J Manipulative Physiol Ther. 1996;19:159–164. [PubMed] [Google Scholar]
- 70.Rivett D, Sharples K, Milburn P. Vertebral artery blood flow during pre-manipulative testing of the cervical spine. Proc IFOMT. 2000:387–390. Past-Present-Future. [Google Scholar]
- 71.Bakhtadze MA, Galaguza VN, Sidorskaya NV. Comparison of peak systolic blood flow velocity in extracranial segments of the vertebral arteries in patients with vertebrobasilar insuffciency and healthy volunteers. Duplex scan investigation. J Man Ther. 2006;4:7–12. [Google Scholar]
- 72.Friedenberg ZB, Edeiken J, Spencer HN, Tolentino SC. Degenerative changes in the cervical spine. J Bone Joint Surg. 1959;41:61–70. [PubMed] [Google Scholar]
- 73.Sheehan S, Bauer B, Meyer JS. Vertebral artery compression in cervical spondylosis: Arteriographic demonstration during life of vertebral artery insuffciency due to rotation and extension of the neck. Neurology. 1960;10:968–986. [Google Scholar]
- 74.McEwan AJ. The role of cervical spondylosis in the aetiology of cerebral embolism. Brit J Clin Pract. 1967;21:465–468. [PubMed] [Google Scholar]
- 75.Smith DR, Vander Ark GD, Kempe LG. Cervical spondylosis causing vertebrobasilar insuffciency: A surgical treatment. J Neurol Neurosurg Psychiatr. 1971;34:388–392. doi: 10.1136/jnnp.34.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lestini WF, Wiesel SW. The pathogenesis of cervical spondylosis. Clin Orthop Rel Res. 1989;239:69–93. [PubMed] [Google Scholar]
- 77.Gordon JV. Arthritis of the cervical spine. Mt Sinai J Med. 1994;61:204–211. [PubMed] [Google Scholar]
- 78.Ebraheim NA, Lu J, Brown JA, Biyani A, Yeasting RA. Vulnerability of the vertebral artery in anterolateral decompression for cervical spondylosis. Clin Orthop Rel Res. 1996;322:146–151. [PubMed] [Google Scholar]
- 79.Cavdar S, Dalcik H, Ercan F, Arbak S, Arifoglu Y. A morphological study on the V2 segment of the vertebral artery. Okajimas Folia Anat. 1996;73:133–137. doi: 10.2535/ofaj1936.73.2-3_133. [DOI] [PubMed] [Google Scholar]
- 80.Mitchell J. Differences between left and right suboccipital and intracranial vertebral artery dimensions: An influence on blood flow to the hindbrain? Physiother Res Internat. 2004;9:85–95. doi: 10.1002/pri.305. [DOI] [PubMed] [Google Scholar]