Abstract
A proposed mechanism for the persistence of low back pain due to clinical instability is a decrease in control of local spinal musculature, more specifically decreased recruitment of multifidus. Altered segmental mechanoreceptor input has been proposed as a contributing factor responsible for a decrease in local muscle recruitment. In this case report, immediate changes in the recruitment of the deep multifidus following manipulation were examined using needle EMG and isometric testing of trunk rotational force. Trunk rotational force appeared to improve while the multifidus demonstrated a decrease in activity as measured by needle EMG. No specific conclusions can be drawn from this report; however, the results do suggest that immediate multifidus function may be influenced with manipulation, resulting in improved muscular control of the trunk.
KEYWORDS: Instability, Manipulation, Multifidus, Needle EMG
Numerous definitions of clinical instability exist; as defined by Bogduk, instability occurs when at any time during movement, there is a change in the ratio of translation to rotation at a segment1. Panjabi described instability as the inability of the spine to maintain its pattern of displacement under physiologic loads2. Actual determination of the presence of clinical instability remains controversial3,4. Subjective reports from patients with clinical instability may include reports of long-term intermittent pain, increased pain with transitional movements, feelings of giving way or locking, pain with sustained positions, and a condition that is progressively worsening5–9. Signs of clinical instability include mild to moderate loss of trunk motion, Gower's sign, lateral shift, absence of neurological signs, and hypermobility with segmental stress testing6,7,9–11.
It has been proposed that this clinical problem may prove difficult to rehabilitate because of a decrease in the recruitment and cross-sectional area of the multifidus12 and an alteration in normal segmental articular mechanoreceptor input10,13–18. Several studies have suggested that activity of the trunk muscles is altered in the presence of lower back pain15,17,19–22.
Recruitment of the multifidus has been reported as an essential component during rehabilitation of patients with lower back pain20–22. However, it has also been proposed that restoration of normal articular motion precedes attempts at strengthening7,23, that segmental control is necessary for spinal stability7,8,21,22,24–26, and that multifidus activity is not directly related to direction of forces placed on the spinal column20,21,27–29. Other reports have suggested that reflexive muscular activity of the multifidus is diminished with laxity in the viscoelastic structures of the feline spine19, with prolonged positioning or loading19,30, and that multifidus recovery is not automatic following an episode of low back pain31.
Recent reports have suggested that multifidus function may be affected with spinal manipulation13 and that mechanical forces may alter EMG activity of the multifidus16. Additional studies have documented changes in strength32–34, muscular activity35–37, neuromuscular reflex38, and pain sensitivity39 following manipulation. It has not been reported that treatment focused at restoring normal articular motion or stimulating local mechanoreceptors will affect multifidus function when measured using needle EMG.
This case report attempts to identify the immediate changes in multifidus activity measured with needle EMG following manipulation of the lumbar spine that is targeted at a level identified as having decreased muscle bulk of the multifidus, abnormal findings in the biomechanical examination, and a decrease in trunk rotational control and strength. Findings may provide clinicians/researchers with ideas for further investigation into treatment of clinical instability.
Patient Characteristics
The patient was a 49-year-old male working as a golf course supervisor who attended therapy with an acute onset of low back pain 3 days prior. The patient reported having a 20-year history of intermittent low back pain of a similar nature, in which the pain usually lasted for 1–2 weeks and then resolved spontaneously. This pain was usually brought on with trivial activities or motions. He recalled no specific injury to his low back.
During these episodes, and as reported at the time of examination, he experienced a loss of trunk motion and moderate to severe pain in the left lower lumbar region. He reported having morning pain and stiffness, which usually resolved after a couple of hours of being up and about. He did not tolerate standing or sitting for periods greater than 2–3 hours without pain beginning. He denied any changes in bladder function, referred or radiating symptoms, sensory changes, or loss of strength or control of the lower extremities. He denied any relevant medical history. He rated his pain as a 6 on a 0–10 numeric rating scale and described it as moderately severe at the time of examination.
Examination
Observation
The patient stood with a flexed and left deviated trunk position. No other obvious deformities or malpositioning were noted.
Lumbar Scan
A lumbar scan was performed as proposed in the curriculum taught by the North American Institute of Orthopaedic Manual Therapy (NAIOMT)7,9,10, consisting of range of motion of the lumbar spine and lower extremities with overpressure and resistance, and provocative testing (compression, torsion, traction, and postero-anterior shear testing) of the lumbar spine and of the sacroiliac region. The neurological component of the examination consisted of testing sensation to light touch, long tract involvement (clonus and Babinski's), tendon reflexes for L3-S1, slump and straight leg raise testing, and strength testing of root levels L2-S1.
Visual range of motion examination revealed a loss of right side-bending and extension. Gower's sign (walking the hands back up the thighs) was present with return from flexion. No deficits were noted with the neurological examination. Left torsion and postero-anterior shear testing revealed a painful hypermobility at the L4 segment. Palpation along the lumbar multifidus revealed an apparent area of hypotonicity at the right L4 region.
Biomechanical Examination
Following the scanning examination, a biomechanical examination consisting of passive intervertebral motion testing and segmental stress testing was performed. Passive accessory intervertebral motion testing revealed a loss of extension and right side-bending at L4–5 with a pathomechanical end feel9,10,40. Specific stress testing at L4–5 revealed increased laxity to anterior and left rotation stressors7,9. No abnormal findings were noted above L4 or at L5-S1 with intervertebral motion and stress testing. Following Meadows' proposal of assessment of clinical instability10, trunk rotation motor control was then assessed in sitting, with the patient asked to resist an isometric force into both right and left trunk rotation in neutral. A decrease in initial trunk resistance to a manually applied isometric force was found with right rotation in neutral. Testing in the flexion or extension quadrants was not performed because the deficit was discovered in the neutral position.
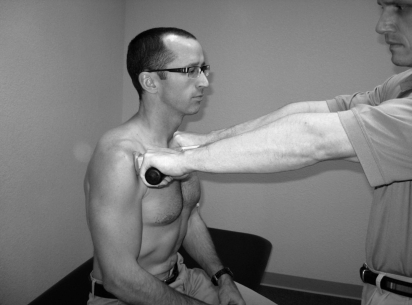
A handheld force transducer (Tech Medical Onsite Commander Portable Force Gauge, JTech Medical, Salt Lake City, UT) was used at this point to quantify trunk rotation strength (Figure 1). The patient was asked to resist an isometric force into right and left rotation, which was repeated three times in each direction. These data were gathered to serve as a comparison to post-EMG/manipulation trunk rotation forces (Table 1).
FIGURE 1.
Testing of trunk rotation strength with handheld dynamometer.
TABLE 1.
Pre-manipulation and post-manipulation neutral position trunk rotation force measured by handheld force transducer.
| Pre-manipulation maximum (pounds) | Pre-manipulation average (pounds) | Post-manipulation maximum (pounds) | Post-manipulation average (pounds) | |
|---|---|---|---|---|
| Right rotation | ||||
| Trial 1 | 8 | 4 | 28 | 16 |
| Trial 2 | 16 | 8 | 28 | 22 |
| Trial 3 | 16 | 10 | 26 | 20 |
| Left rotation | ||||
| Trial 1 | 18 | 12 | 26 | 16 |
| Trial 2 | 16 | 8 | 22 | 16 |
| Trial 3 | 20 | 14 | 26 | 20 |
Clinical Impression
The history given by the patient, combined with the findings of the examination, appeared to indicate the presence of clinical instability. There were no subjective reports, medical history, or examination findings to indicate systemic involvement, neurological involvement, or impending neurological compromise. The pain was well localized and did not refer. Hip motion was full bilaterally in all directions and quadrants. Palpation revealed an obvious hypotonicity of the multifidus to the right of L4.
Articular assessment revealed hypermobility at L4–5 with anterior and left rotation stress testing, with a hypomobility in right side-bending and extension. The end-feel of this hypomobility was pathomechanical. Trunk rotational control and strength were also diminished in right rotation.
If the assumption can be made that clinical instability is caused by a breakdown of the structure of the disc or surrounding structures, it appears possible that there is increased translation present at a particular segment during movement, allowing it to become the proverbial locked back. The decision to manipulate in this case study was based on the history, clinical presentation, and end-feel in an attempt to return the segment to a neutral position.
Intervention
A signed release was obtained from the patient after the EMG procedure was explained by the physician, and the proposed treatment was explained by the therapist. A free-run EMG of 10 milliseconds/division with a filter setting of 20Hz–10,000Hz, and sensitivity set at 500 µv/division (Nicolet Viking Quest, Nicolet Biomedical, Madison, WI), to test for recruitment of the deep lumbar multifidus was then performed by the physician. While the reliability and validity of EMG to determine muscle activity remains controversial41,42, the use of needle EMG for attempted assessment of multifidus function has been reported superior to surface EMG43. Prior to the EMG, an explanation of the procedure was given again by the physician to the patient, and the patient again gave verbal consent to participate.
The patient was placed in a prone position; the previously noted area of apparent hypotonicity at L4 was then identified by the therapist and marked by the physician. The EMG needle was inserted by the physician at the marked area to the right side of the L4 spinous process to the deep layer of the multifidus at a 45° angle until the base of the L4 transverse process was encountered, then retreating until the needle tip was no longer in bony contact. The ground electrode was then attached adjacent to the right side of the spinous processes of L1–2 over the paraspinal muscles without marking.
After a period of less than 1 minute following needle insertion, a baseline EMG reading was obtained by the physician to determine resting tone of the multifidus, which was verbally reported as zero by the physician. The patient then performed a modified Biering-Sorensen test and held the position for approximately 10 seconds while a reading of multifidus EMG activity was obtained by the physician. The Biering-Sorensen test has been previously reported as one method of assessing erector spinae and multifidus strength7.
After the pre-intervention reading was obtained, the ground electrode was removed along with the wire to the EMG needle. The needle was left in place in an attempt to keep the needle in the same location so that EMG data could be collected again from the same motor neuron pool. Removal of the ground electrode and needle wire was to ensure the patient was not connected to the EMG machine during manipulation to avoid inadvertent electrical shock.
The patient was then placed in left side-lying, an explanation of the proposed manipulation was given to the patient, and his verbal consent was obtained before proceeding. A neutral position high-velocity/low-amplitude manipulation in left side-lying was chosen based on the history23 and clinical presentation44–46.
As an increased laxity to left rotation and anterior stressors were found during the segmental stress tests, the patient was placed in a left side-lying position. This position theoretically provides a right rotational stress that allows a specific lumbar segment to be targeted for manipulation; thereby avoiding additional stress in the direction of perceived hypermobility.
A high-velocity/low-amplitude manipulation was chosen because of the pathomechanical23,40 end-feel that was encountered during the biomechanical examination. With the patient in left side-lying, the neutral position of L4–5 was found by using the therapist's left arm to position the patient's legs to move the lumbar spine, with the right hand palpating for the neutral position. The therapist's palpating hand changed from the right to the left, so that a neutral extension lock from above, down to but not into L4, could be performed with the right hand, by drawing the patient's left arm vertically.
The therapist's palpation hand then changed to the right, and the patient's right hip was flexed until the right knee was ahead of the left thigh, which allowed the patient's pelvis to begin to rotate to the left, up and into the L4–5 segment. Once the manipulation position was obtained, an overpressure was applied and held for 10 seconds. No pain, discomfort, or other symptoms were reported by the patient during the overpressure. A neutral position, high-velocity/low-amplitude manipulation targeting the L4–5 segment was performed (Figure 2). A single audible click was noted, which was also felt under the therapist's superior thumb at L4. The process from patient set-up to manipulation took approximately 30 seconds.
FIGURE 2.
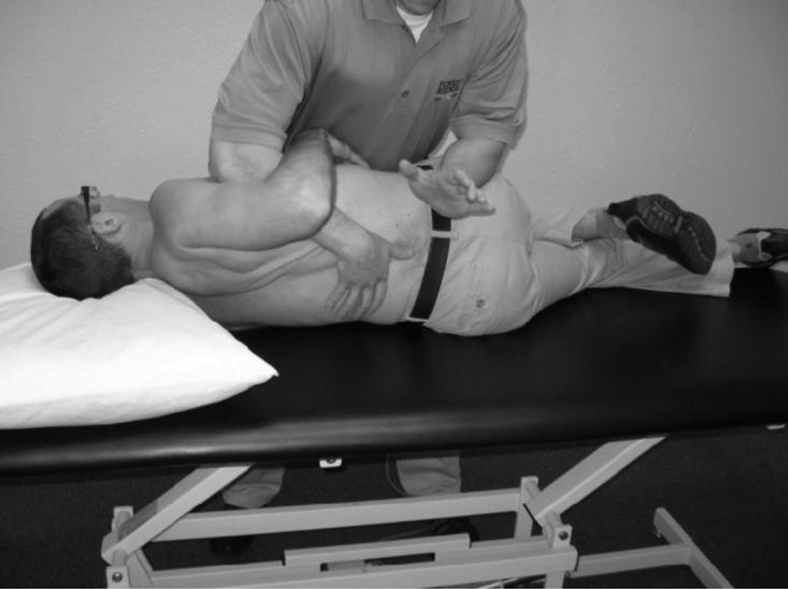
Neutral position gapping manipulation technique as described by Pettman44.
Immediately following the manipulation, the patient returned to the prone position. The needle wire was re-attached, and the ground electrode was re-attached over the right L1–2 paraspinals by the physician. After a period of less than 1 minute, multifidus EMG resting tone was again measured and verbally reported as zero by the physician. As before, the patient was then asked to perform the modified Biering-Sorensen test holding for a period of 10 seconds, while the EMG reading of multifidus recruitment was obtained by the physician. The needle and ground electrode were removed after the final reading was obtained.
Outcome
Immediately following the removal of the needle and ground electrode, the biomechanical examination was conducted again, and it revealed normal passive accessory intervertebral motion at the L4–5 segment9,40. Visual assessment of trunk range of motion was full, without deviation, and reported as painless by the patient. The patient reported an immediate improvement in his symptoms and movement ability, rating his pain as 0 on a 0–10 numeric pain rating scale. Trunk control was assessed as described previously; the response to a manually applied isometric force by the therapist appeared to improve, and the handheld force transducer was used again. Trunk rotational strength appeared to improve, in particular right rotation (Table 1). As shown in Table 2, the results of the EMG demonstrated less activity of the multifidus post-manipulation compared to pre-manipulation readings.
TABLE 2.
Needle EMG readings pre-manipulation and post-manipulation.
| Pre-manipulation EMG reading | Post-manipulation EMG reading | |
|---|---|---|
| Recruitment frequency | 8Hz | 8Hz |
| Motor unit action potentials | 3–4 | 2 |
| Amplitude | 1500–3500 microvolts | 500–1000 microvolts |
The patient reported a long history of similar occurrences as described in this report. Short-term prognosis would appear fair, with unguarded movements or trauma being the most likely to result in another episode. While long-term outcome following treatment designed to enhance multifidus recruitment in combination with addressing articular dysfunction or arthrogenic influence is unknown, long-term prognosis of this particular patient remains guarded.
Discussion
The results of trunk rotation strength appear to support the initial hypothesis that manipulation can affect trunk muscular control in addition to improvements in the subjective, objective, and biomechanical examinations. Although numerous variables exist that could compromise the readings, from use of a handheld force transducer for assessment of trunk strength, it did appear that the amount of rotation force generated by the patient improved. Handheld dynamometers have been shown to be a reliable method of assessing strength47–49, although the use as described in this case report has not been widely reported.
While the extent of the response to initial manual loading of the trunk into either left or right rotation could not be accurately assessed due to the limitations of the equipment available, it was noted by both the patient and therapist that a more immediate and controlled response to loading was present with initiation of resistance into right rotation. Use of more sophisticated and sensitive equipment might demonstrate more accurate measurement of trunk forces generated at the initial onset of rotational loading.
The EMG data, however, did not support the hypothesis that multifidus EMG activity would increase following manipulation. Theoretically, it is a possibility that manipulation of the segment either allowed for improvement in the efficiency of multifidus recruitment resulting in a lower output noted with EMG testing, or quite simply that reduction in pain resulted in less local muscle activation. It has previously been reported that weakened subjects produce higher EMG readings to generate a given absolute force50, and that removal of neurogenic inhibition may affect strength after mobilization51.
Several factors could have affected the post-manipulation EMG results such as less volitional effort by the patient, inadvertent repositioning of the EMG needle during the set-up for the manipulation, lack of specific marking and control of the ground electrode position, or repositioning of the needle during the manipulation. The initial introduction of the EMG needle may have also caused an increase in activity of the multifidus, which would not reflect true multifidus activity pre-manipulation compared to post-manipulation readings. Further study is needed to elucidate this possibility.
It is also unknown if manipulative techniques that do not attempt to direct and control forces to a particular level, such as one described by Flynn et al52, would provide the same or different results, or if this particular patient would have tolerated such forces23,44. Further study examining manipulative techniques and outcomes in this particular patient population would appear necessary. It is also unknown what differences might be noted with comparison of rehabilitative ultrasound imaging to needle EMG testing in this specific patient population13. Additionally, examination of responses of the superficial multifidus to manipulation would be warranted. No definitive conclusions can be drawn from this case report; however, the results here suggest that immediate multifidus function may be influenced with manipulation, resulting in improved muscular control of the trunk.
Conclusion
This case report attempted to identify immediate changes in multifidus motor activity measured by needle EMG and trunk rotational strength measured with a handheld force gauge following manipulation of the lumbar spine. This was based on the clinical observation of improved trunk control and strength following either mobilization or manipulation of a segment perceived to have hypotonicity of the adjacent multifidus and abnormal accessory motion. While trunk strength appeared to improve, EMG measurement revealed less multifidus recruitment post-intervention compared to pre-intervention readings. Further study examining the effect of mobilization or oscillation of a segment may provide additional information on possible arthrogenic influence on the multifidus.
Acknowledgements
This case report was originally completed as part of the fellowship requirements for the North American Institute of Orthopaedic Manual Therapy (NAIOMT). The author would also like to thank Timothy Pettingell, MD for performing the EMG study and providing the EMG data for this case report.
REFERENCES
- 1.Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum, 3rd ed. Syndey, Australia: Churchill Livingstone; 1997. [Google Scholar]
- 2.Panjabi M, White A. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: JB Lippincott; 1990. [Google Scholar]
- 3.Hicks GE, Fritz JM, Delitto A, Mishock J. Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84:1858–1864. doi: 10.1016/s0003-9993(03)00365-4. [DOI] [PubMed] [Google Scholar]
- 4.Abbott JH, McCane B, Herbison P, Moginie G, Chapple C, Hogarty T. Lumbar segmental instability: A criterion-related validity study of manual therapy assessment. BMC Musculoskelet Disord. 2005;6:56. doi: 10.1186/1471-2474-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biely S, Smith S, Silfies S. Clinical instability of the lumbar spine: Diagnosis and intervention. Ortho Phys Ther Prac. 2006;3:11–18. [Google Scholar]
- 6.Cook C, Brismee J, Sizer P. Subjective and objective descriptors of clinical lumbar spine instability: A Delphi study. Man Ther. 2006;11:11–21. doi: 10.1016/j.math.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Dutton M. Orthopaedic Examination, Evaluation, & Intervention. St. Louis, MO: McGraw-Hill; 2004. [Google Scholar]
- 8.O'Sullivan P. Lumbar segmental “instability”: Clinical presentation and specific stabilizing exercise management. Man Ther. 2000;5:2–12. doi: 10.1054/math.1999.0213. [DOI] [PubMed] [Google Scholar]
- 9.Pettman E. NAIOMT Level 2 Intermediate Lower Quadrant. Berrien Springs, MI: NAIOMT; 2003. [Google Scholar]
- 10.Meadows J. NAIOMT Level 3 Advanced Lower Quadrant. Dallas, TX: NAIOMT; 2007. [Google Scholar]
- 11.Paris S. Physical signs of instability. Spine. 1985;10:277–279. doi: 10.1097/00007632-198504000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Richardson C, Hodges P, Hides J. Therapeutic Exercise for Lumbopelvic Stabilization 2nded. Sydney, Australia: Churchill Livingstone; 2004. [Google Scholar]
- 13.Brenner A, Gill N, Buscema C, Kiesel K. Improved activation of lumbar multifidus following spinal manipulation. J Ortho Sports Phys Ther. 2007;37:613–619. doi: 10.2519/jospt.2007.2470. [DOI] [PubMed] [Google Scholar]
- 14.Cassisi J, Robinson M, O'Connor P, MacMillan M. Trunk strength and lumbar paraspinal muscle activity during isometric exercise in chronic low back pain. Spine. 1993;18:245–251. doi: 10.1097/00007632-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Danneels L, van der Straeten G, Cambier D, Witvrouw E, Cuyper H. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Euro Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine. J Electro Kines. 2002;12:219–234. doi: 10.1016/s1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 17.Elfving B, Dedering A, Nemeth G. Lumbar muscle fatigue and recovery in patients with long-term low back trouble: Electromyography and health related factors. Clin Biomech. 2003;18:619–630. doi: 10.1016/s0268-0033(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 18.Hodges P, Mosely G. Pain and motor control of the lumbopelvic region: Effect and possible mechanisms. J Electro Kines. 2003;13:361–370. doi: 10.1016/s1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 19.Gedalia U, Solomonow M, Zhou B, Baratta R, Lu Y, Harris M. Biomechanics of increased exposure to lumbar injury caused by cyclic loading. Part 2. Recovery of reflexive muscular stability with rest. Spine. 1999;24:2461–2467. doi: 10.1097/00007632-199912010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hodges P, Richardson C. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–1012. doi: 10.1016/s0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 21.Hodges P, Richardson C. Inefficient muscular stabilization of the lumbar spine associated with low back pain: A motor control evaluation of transversus abdominus. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 22.Vleeming A, Mooney V, Dorman T, Snijders C, Stoeckart R. Movement, Stability & Low Back Pain: The Essential Role of the Pelvis. Sydney, Australia: Churchill Livingstone; 1999. [Google Scholar]
- 23.Meadows J. Orthopedic Differential Diagnosis in Physical Therapy. St. Louis, MO: McGraw-Hill; 1999. [Google Scholar]
- 24.Hides J, Jull G, Richardson C. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26:243–248. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Jull G, Richardson C. Motor control problems in patients with spinal pain: A new direction for therapeutic exercise. J Manipulative Physiol Ther. 2000;23:115–117. [PubMed] [Google Scholar]
- 26.Wohlfart D, Jull G, Richardson C. The relationship between dynamic and static function of abdominal muscles. Aust Physiother. 1993;39:9–13. doi: 10.1016/S0004-9514(14)60464-5. [DOI] [PubMed] [Google Scholar]
- 27.Hodges P, Cresswell A, Daggfeldt K, Thorstensson A. Three-dimensional preparatory trunk motion precedes assymetrical upper limb movement. Gait Posture. 2000;11:92–101. doi: 10.1016/s0966-6362(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 28.Mosely G, Hodges P, Gandevia S. Deep and superficial fibers of the lumbar multifidus are differentially active during voluntary arm movements. Spine. 2002;27:29–36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Ng J, Richardson C. Reliability of electromyographic power spectral analysis of back muscle endurance in healthy subjects. Arch Phys Med Rehabil. 1996;77:259–264. doi: 10.1016/s0003-9993(96)90108-2. [DOI] [PubMed] [Google Scholar]
- 30.Jackson M, Solomonow M, Zhou B, Baratta R, Harris M. Multifidus EMG and tension-relaxation recovery after prolonged static lumbar flexion. Spine. 2001;7:715–723. doi: 10.1097/00007632-200104010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hides J, Richardson C, Jull G. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 32.Cleland J, Stowell T, Selleck B. Short-term effects of thoracic manipulation on lower trapezius muscle strength. J Man Manip Ther. 2004;12:82–90. [Google Scholar]
- 33.Keller T, Colloca C. Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: A comparative clinical trial. J Manipulative Physiol Ther. 2000;23:585–595. doi: 10.1067/mmt.2000.110947. [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe S, Reese H, Sydenham R. Effect of high-velocity low-amplitude manipulation on cervical spine muscle strength. J Man Manip Ther. 2006;14:152–158. [Google Scholar]
- 35.Gill N, Teyhen D, Lee I. Improved contraction of the transversus abdominus immediately following lumbopelvic manipulation: A case study using real-time ultrasound imaging. Man Ther. 2007;3:280–285. doi: 10.1016/j.math.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Herzog W, Scheele D, Conway P. Electromyographic responses of back and limb muscles associated with spinal manipulative therapy. Spine. 1999;24:146–153. doi: 10.1097/00007632-199901150-00012. [DOI] [PubMed] [Google Scholar]
- 37.Suter E, McMorland G, Herzog W, Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22:149–153. doi: 10.1016/S0161-4754(99)70128-4. [DOI] [PubMed] [Google Scholar]
- 38.Colloca C, Keller T. Stiffness and neuromuscular reflex response of the human spine to postero-anterior manipulative thrusts in patients with low back pain. J Manipulative Physiol Ther. 2001;24:489–500. doi: 10.1067/mmt.2001.118209. [DOI] [PubMed] [Google Scholar]
- 39.George S, Bishop M, Bialosky J, Zeppieri G, Robinson M. Immediate effects of spinal manipulation on thermal pain sensitivity. BMC Musculoskeletal Disorders. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaltenborn F, Evjenth O. Manual Mobilization of the Joints. Volume 2: The Spine, 4th ed. Minneapolis, MN: OPTP; 2003. [Google Scholar]
- 41.Kim BJ, Date ES, Derby R, et al. Electromyographic technique for lumbar multifidus examination: A comparison of previous techniques used to localize the multifidus. Arch Phys Med Rehabil. 2005;86:1325–1329. doi: 10.1016/j.apmr.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 42.Kendall R, Werner RA. Interrater reliability of the needle examination in lumbosacral radiculopathy. Muscle Nerve. 2006;34:238–241. doi: 10.1002/mus.20554. [DOI] [PubMed] [Google Scholar]
- 43.Stokes IA, Henry SM, Single RM. Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin Biomech. 2003;18:9–13. doi: 10.1016/s0268-0033(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 44.Pettman E. Manipulative Thrust Techniques: An Evidence-Based Approach. Abbotsford, Canada: Aphema; 2006. [Google Scholar]
- 45.Pettman E. NAIOMT Level 3 Advanced Lower Quadrant. Berrien Springs, MI: NAIOMT; 2003. [Google Scholar]
- 46.Bronfort G, Haas M, Evans RL, Bouter LM. Effcacy of spinal manipulation and mobilization for low back pain and neck pain: A systematic review and best evidence synthesis. Spine. 2004;4:335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Roy MA, Doherty TJ. Reliability of hand-held dynamometry in assessment of knee extensor strength after hip fracture. Am J Phys Med Rehabil. 2004;83:813–818. doi: 10.1097/01.phm.0000143405.17932.78. [DOI] [PubMed] [Google Scholar]
- 48.Wang CY, Olson SL, Protas EJ. Test-retest strength reliability: Hand-held dynamometry in community-dwelling elderly fallers. Arch Phys Med Rehabil. 2002;83:811–815. doi: 10.1053/apmr.2002.32743. [DOI] [PubMed] [Google Scholar]
- 49.Kilmer DD, McCrory MA, Wright NC, Rosko RA, Kim HR, Aitkens SG. Hand-held dynamometry reliability in persons with neuropathic weakness. Arch Phys Med Rehabil. 1997;78:1364–1368. doi: 10.1016/s0003-9993(97)90311-7. [DOI] [PubMed] [Google Scholar]
- 50.Gaudreault N, Arsenault A, Larivière C, DeSerres S, Rivard C. Assessment of the paraspinal muscles of subjects presenting an idiopathic scoliosis: An EMG pilot study. BMC Musculoskelet Dis. 2005;6:14. doi: 10.1186/1471-2474-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makofshy H, Panicker S, Abbruzzese J, et al. Immediate effect of grade IV inferior hip joint mobilization on hip abductor torque: A pilot study. J Man Manip Ther. 2007;15:103–111. doi: 10.1179/106698107790819927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine. 2002;27:2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]