Abstract
Background
At present, one in six men over age 50 carries the diagnosis of prostate cancer, but only one in 33 will die of the disease. In view of these facts, conservative strategies such as active surveillance (AS) are important in the management of prostate cancer.
Methods
To obtain information on active surveillance, the Medline database was searched from January 2002 to April 2008 for the terms "prostate cancer" OR "prostatic neoplasms," AND "active surveillance" OR "expectant management". In addition a manual search was performed in the reference lists of relevant publications on the treatment of prostate cancer and on active surveillance.
Results
88 relevant publications about active surveillance were found. The studies varied in methodological quality but consistently showed low rates of tumor progression and high rates of tumor-specific survival with active surveillance (99% to 100%). All 7 guidelines on the treatment of prostate cancer that have been published since 2006 list active surveillance in their recommendations as a therapeutic option for prostate cancer if there is a low risk of progression. In fact, the National Institute of Clinical Excellence (U.K.) recommends the active surveillance exclusively as the treatment strategy for such cases.
Conclusions
The guideline recommendations reflect a changed attitude toward the treatment of prostate cancer in the light of the early detection of these tumors and the data now available regarding active surveillance. A corresponding change in actual medical practice would be desirable. The treatment of prostate cancer should always be adapted to the individual needs of the patient, and risky treatments should only be used when absolutely necessary.
Keywords: prostate cancer, prostatectomy, surveillance, treatment, PSA test
For more than 25 years, radical prostatectomy (RP) has been the standard treatment for prostate cancer (PCa) and, according to 2006 DRG (diagnosis related groups) statistics, 68% of patients younger than 70 years undergo RP (4). This is based on two assumptions: that cure can be achieved only by organ removal and that the affected patient really is cured after the procedure. However, according to new insights over the past few years, some 30% of patients are not cured by surgery and develop PSA (prostate specific antigen) progression; a proportion of patients had tumors that did not require treatment, and the patients would not have died from their PCa, even without surgery. The reasons for the broad indication are mainly to be found in a lack of knowledge about tumor biology. Once a PCa has been diagnosed, doctors and patients often decide on RP owing to time pressure and worry, in order to alleviate the fear that the cancer might spread rapidly and threaten the patient’s life.
In the meantime, however, the situation has changed for patients with PCa: 1 in 6 men older than 50 currently receive a diagnosis of PCa, but only 1 in 33 actually die from the cancer in this age group (1). PSA screening enables the detection of PCa in its early stages. In contrast to earlier years, nowadays more than 90% of patients with a newly diagnosed PCa have non-metastatic tumors (1). PSA screening detects increasingly more tumors that would have remained undetected without early detection measures. Between 1979 and 2002, the incidence in men younger than 65 rose 4.28-fold (1).
Four in five of those affected will not experience clinically relevant progression due to age, comorbidities, or favorable tumor biology (1). These tumors, categorized as "low risk", have an excellent prognosis. The 10 year survival rate for grade 1 tumors exceeds 90%, regardless of whether the tumors are treated or not (2).
This background raises the question of which therapy is appropriate in PCa today, and whether curative measures—which may incur serious side effects—are justified in each and every case.
The diagnosis related shift in tumor stage and the favorable prognosis of well differentiated tumors with a low risk of progression have raised the general awareness of defensive strategies. Even as recently as 10 years ago, studies of "expectant management" were commented on with great skepticism (3). In the meantime, watch-and-wait strategies have become the topic of current studies. Active surveillance (AS) and watchful waiting (WW) are regarded as alternatives to the classic curative approaches such as RP and radiotherapy.
We searched Medline for studies and articles on the importance of active surveillance for the time period 01/2002 to 04/2008 by using the following search strategy: prostate and cancer OR prostatic and neoplasms AND active surveillance OR expectant management. We also hand-searched the reference lists of relevant publications.
Therapeutic approaches
According to the latest data, RP is performed in Germany in up to 70% of patients (4, 5). For many, the procedure entails complications and late sequelae, depending on the operating technique:
Urinary incontinence in 3% to 74%
Stenosing anastomosis in 1% to 10%
Erectile dysfunction in at least 30%
Neurapraxia of the lower extremities in up to 25%
Fecal incontinence in 18%
Rectal lesions in 11% of patients (6).
Internal or external radiotherapy, which 15% to 30% of patients undergo (4, 5), can achieve long term curative rates of up to 80% in low risk PCa and is therefore comparable to RP. The commonest adverse effects are:
Impotence in up to 50%
Grade II (or higher) rectal hemorrhage in 2% to 25%
Severe late sequelae affecting the bladder and rectum are diagnosed in less than 3% of cases after a total radiation dose of 75 Gy (7).
On the other hand, conservative treatment strategies exist, which have been precisely defined (8). Active surveillance is an option for patients who are suitable for curative treatment but do not require an intervention at the time of their diagnosis. The strategy has two aims: to perform a curative measure only if the PCa is progressive and to avoid therapeutic complications if the tumor is non-progressive. A low risk of progression can be predicted on the basis of different models. The risk of active surveillance is that treatment may not be initiated sufficiently early in case of tumor progression.
WW means long term observation of patients who receive palliative treatment only once symptoms set in. Today, most tumors are diagnosed at the T1c stage, and the median time that these non-palpable cancers take from diagnosis to death is some 10 to 14 years—depending on the patient’s age and the aggressiveness of the tumor—and an elderly patient with multiple comorbidities would not expect an improvement in his quality of life as a result of active therapy (6). This is confirmed by studies pre-dating the PSA era and controlled studies from the PSA era (9).
Androgen deprivation may be considered in patients older than 70 with localized prostate cancer. In this age group, non-prostate cancer related causes may lead to death because of associated diseases. Hormones should be administered only if the PSA value has doubled within less than 12 months (10).
Studies of active surveillance
Our Medline search identified 88 articles on active surveillance. We excluded studies that investigated a therapeutic approach that was exclusively palliative (watchful waiting). Case reports were also excluded.
The publications were mainly methodologically heterogeneous phase 2 studies that investigated the strategy of active surveillance. Most studies were retrospective and had small case numbers, different inclusion criteria, and short observation periods. They all found high tumor specific survival rates. Except for a study reported by Klotz (17), all studies showed a survival rate of 100%. Five of the studies analyzed were of high methodological quality and investigated comparable parameters (11– 15):
Exclusively localized prostate cancer of category T1, 2 with a low aggressive potential, and PSA values below 15 ng/mL, which were suitable for curative treatment.
All patients thus met the criteria for active surveillance as a therapeutic option, as stipulated by current guidelines.
The studies had clearly defined, objective, and comparable parameters for tumor progression.
All patients were able to stop having active surveillance at any time and opt for an intervention.
These 5 studies therefore provide the best evidence of the importance of active surveillance (16) (table 1).
Table 1. Studies of active surveillance in clinically localized prostate cancer T1-T2 (16).
| Name | n | Age | Tumor category | Follow-up in months | Progression (%) | Tumor specific survival (%) | Abandonment of active surveillance |
| Mohler et al. (11) | 27 | 69 | T1c (100%) | 23 (6–62) | 33 | 100 | 4 patients because of progression |
| Choo et al. (12) | 206 | 70 | T1b (6%), T1c (57%), T2a (24%), T2b (13%) | 29 (2–66) | 17 | 100 | 69 patients: 15 because of clinical progression, 16 PSA, 5 histology, 23 patient’s wish, 6% protocol violation |
| Chen et al. (13) | 52 | 71 | T1a (100%) | 87 (6–180) | 8 | 100 | 4 patients because of progression (1 patient with bone metastases) |
| Khan et al. (14) | 78 | 65 | T1c (100%) | 23 | 29 | 100 | |
| Patel et al. (15) | 88 | 65 | T1a/b (20%), | 44 (7–172) | 25 | 100 | 31 patients: 17 because of progression, 7 because of anxiety, 7 because of anxiety and other reasons |
| T1c (58%), | |||||||
| T2a–c (22%) |
By searching Medline, the authors identified 88 publications of heterogeneous methodological quality. The 5 studies listed in this table are methodologically of the highest quality and form the evidence base for the recommendations of the NICE (National Institute for Health and Clinical Excellence) guideline for active surveillance (16).
A total of 451 patients were selected on the basis of these criteria in these 5 studies. After a mean follow-up of 40 months, 8% to 33% of cases experienced cancer progression, which occurred within 33 months in half of them (16). Tumor specific survival in the studies was 100%. Bone metastases were found in 1 patient (13). Klotz, in an earlier study, reported on 2 of 229 patients who died 5 years after their tumor diagnosis after defensive treatment (17). These patients would have been excluded from active surveillance in the 5 studies because of their unfavourable prognosis. The results were so convincing that they led to phase 3 studies comparing defensive strategies with curative strategies: PRIAS (e1), START (e2), ProtecT (e3), PIVOT (e4), and HAROW (e5). Whether the randomized controlled studies currently under way will be able to confirm the clear superiority of one of the strategies in 15 years’ time cannot be predicted because of the small difference in tumor specific survival.
Guidelines
Our search identified 58 guidelines for PCa. We excluded those that focused on individual aspects of PCa therapy or special stages, as well as all guidelines that were completed before 2006. We evaluated 7 guidelines (table 2). All current guidelines mention active surveillance as an equally valuable therapeutic option for tumors with a low risk of progression. The American Urological Association (18) actually mentions this option even for patients at medium or higher risk, because there are no phase 3 trials to prove the superiority of other therapeutic procedures.
Table 2. Current guidelines for the treatment of prostate cancer.
| Organization / country / date | Active surveillance as a therapeutic option? |
| NICE / U.K. / 02/2008 | As the exclusive therapeutic recommendation for low risk PCa |
| Nederlandse Vereniging voor Urologie [Dutch urological association] / 07/2007 | As an equally valid therapeutic option for localized PCa |
| American Urological Association /USA / 2007 | As a licensed therapeutic option for localized PCa |
| Duodecim, Finnish Medical Society /Finland / 2007 | As an equally valid therapeutic option for low risk PCa (T1–2, Gleason = 6, PSA = 10) |
| European Urological Association 2007 | As a licensed therapeutic option for low risk PCa |
| Association française de l’Urologie (French urological association) / France / 2006 | As a licensed therapeutic option for low risk PCa |
| Sociedade Brasileira de Urologia (Brazilian urological association) / Brazil / 06/2006 | As an equally valid therapeutic option for low risk PCa |
The February 2008 guideline from the UK’s National Institute for Health and Clinical Excellence (NICE) goes one step further in that it recommends active surveillance for the treatment of localized, low risk prostate cancers, while naming RP, brachytherapy, and external radiation as options. The recommendation to offer each low risk patient active surveillance initially is based on expert consensus. Cancer registry data thus indicate a tendency to overtreat.
Risk prediction and surveillance
All current guidelines stratify prostate cancer into 3 risk groups for their therapeutic recommendations. The basis for this is the model of D’Amico, which enables predicting progression on the basis of 3 criteria (20). Low risk for which active surveillance is primarily recommended according to the guidelines is defined as follows:
PSA ≤ 10 ng/mL
Tumor stage T1, 2a
Gleason score ≤ 6. The Gleason score describes the histological assessment of the degree of differentiation of the two most common types of tumor cells. It enables conclusions about the aggressiveness of a tumor.
The decision in favor of active surveillance is based on the ability to predict an indolent prostate cancer. D’Amico’s risk stratification is suitable for this to a limited degree only; it was originally developed to predict biochemical progression after active treatment (e6). The models for identifying a tumor that does not require treatment are based mainly on the studies of Epstein and can predict a low risk of progression with a greater degree of certainty (e6, 21).
The CAPRA (Cancer of the Prostate Risk Assessment) score is used in competition with this and other risk prediction models (e8). It assigns point scores to the patient’s age, PSA value, Gleason score, local tumor spread cT, and the proportion of tumor mass in the biopsy specimen (table 3). Active surveillance is possible for 0–2 points; 3–4 points mean local therapy with curative intent; and for 5–6 points, the curative measure is supported by hormone therapy. Systemic androgen deprivation is required in a scenario of 7–10 points.
Table 3. Risk assessment of PCa according to clinical and histological criteria, so called CAPRA (Cancer of the Prostate Risk Assessment) score (e8).
| Variable | Range | Points |
| PSA ng/mL | 2.0–6.0 | 0 |
| 6.1–10.0 | 1 | |
| 10.1–20.0 | 2 | |
| 20.1–30.0 | 3 | |
| > 30 | 4 | |
| Gleason score | 1–3 / 1–3 | 0 |
| 1–3 / 4–5 | 1 | |
| 4–5 / 1–5 | 3 | |
| Category of primary tumor | cT1 / cT2 | 0 |
| cT3a | 1 | |
| Positive biopsies | <34 % | 0 |
| ≥ 34 % | 1 | |
| Age | <50 years | 0 |
| ≥ 50 years | 1 |
The CAPRA score assigns points to the patient’s age, PSA value, Gleason score, local tumor spread (cT), and proportion of tumor mass in the biopsy specimen. In patients with a score of 0–2 points, active surveillance is possible; in those whose score is 3–4 points, local treatment with curative intent is indicated; and in those with a score of 5–6 points this curative measure is supported by adjuvant hormone therapy. 7–10 points require systemic androgen deprivation.
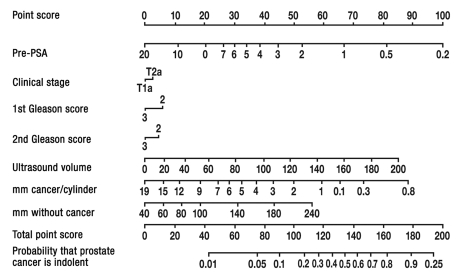
The most popular model to predict an indolent cancer of <0.5 mL is the Kattan nomogram (Figure). The receiver operating characteristic (ROC) curves to evaluate these analytic models reach values of up to 0.79 and therefore confirm sufficiently high reliability. The main function of surveillance is to spare patients with lacking tumor activity radical interventions, but provide curative treatment to those whose tumors progress. Signs of progression include rectal palpability, a rise in PSA values or changes in tumor kinetics (PSA velocity or doubling time), and an increased Gleason score, as well as an increase in tumor mass in the control biopsy specimen (16). To detect a deterioration in tumor biology, control examinations are recommended in the literature, which differ from each other merely in detail: in the first year, clinical examinations with PSA measurements to be repeated at 3-monthly intervals and at 6-monthly intervals after 2 years (12). Ultrasound guided biopsies were performed if clinical parameters deteriorated or six months after diagnosis (15), or annually (14) or every 3 years if a rebiopsy had been taken after the first year.
Figure 1.
Kattan nomogram to predict indolent prostate cancer. The values in the top line are assigned to 7 tumor criteria, and the totals are entered on the penultimate line. The ultrasound volume is recorded in mL. The probability of an indolent cancer can then be read along the bottom line (23).
In spite of different monitoring strategies, no study has reported a tumor death due to late detection of progression; bone metastases were observed in 1 of 451 patients.
Conclusions
Although randomized controlled studies are lacking, current data of active surveillance permit the conclusion that doctors have an obligation to alert patients to this option. Current guidelines on the treatment of PCa also confirm that the treatment of localized prostate cancer has undergone a rethink. However, this is barely reflected in clinical practice, at least not in Germany. In spite of the shift in tumor stage to earlier diagnosis as described earlier, the number of radical prostatectomies is rising steadily, particularly in the group of patients for whom active surveillance as laid out in the latest guidelines would be a suitable option. According to 2006 DRG statistics, almost 69% of men younger than 70 undergo RP (4). The numbers of operations have risen in recent years (4). The Brandenburg cancer registry shows that the following therapies were applied in men younger than 70 with tumor categories pT1 to pT3, for 2003–2005:
Radical prostatectomy in 70% of patients
Exclusively radiotherapy in 15% of patients
Defensive strategies such as hormone therapy, WW, or active surveillance were used in only 15% of patients.
Almost two thirds of PCa found were at stage T1 or T2 and would have been suitable for active surveillance (5).
There are no more precise data from Germany that show how many patients have a low risk PCa and would therefore be suitable to undergo active surveillance. Currently the best data source on PCa is the longitudinal data collection of CaPSURE (24). Of more than 10 000 patients included between 1989 and 2003, 29.7% had low risk tumors, for which NICE recommends active surveillance as the first therapeutic option. In 1989/90, 31% were in this risk group, but the proportion rose to 47% in 2001/2002. Over the same time period, the proportion of high risk tumors fell from 41% to 15% (24). These data give rise to the assumption that in Germany, the 69% of prostatectomies in men younger than 70 include many cases with an excellent prognosis.
PCa treatment should aim to provide each patient with the treatment that is appropriate for his personal needs, individual medical history, and tumor biology. Radical, risk prone interventions should be considered if they are unavoidable and the patient’s survival gain justifies the risks associated with the intervention. In the range of options, active surveillance is the strategy that enables risk assessment and making considered, unhurried therapeutic decisions: a retrospective controlled study with 188 participants (level of evidence 2b) showed that radical prostatectomy delayed by 26 months does not impair the chances of curative treatment in small and well differentiated tumors if they are category T1c, have a PSA density <0.15 ng/mL/cm3, have a Gleason score <7, and are detectable in no more than two positive biopsy cores with less than 50% of tumor mass in each core (25).
Knowledge of the tumor biology and giving appropriate consideration to all available treatment strategies can enable primary care physicians to become important decision-makers jointly with their patients. Some food for thought: an editorial, suitably entitled "Prostate Cancer: are we over-diagnosing—or under-thinking?", concludes with the following advice: "Think more!" (e7).
Key messages.
More than 90% of patients diagnosed with PCa today have a non-metastatic tumor.
By measuring PSA values, increasingly more cancers are diagnosed that would have remained undetected without screening due to age, comorbidities, or favorable tumor biology.
In Germany, up to 70% of patients <70 undergo radical prostatectomy, associated with the risk of late sequelae including fecal or urinary incontinence and erectile dysfunction.
Studies suggest that active surveillance is a therapeutic option in tumors with a low risk of progression. This is also reflected in guidelines.
In localized tumors of category T1, 2 and PSA values <15 mg/mL progression occured in 8% to 33% of patients and tumor specific survival was 100% with a mean follow-up of 40 months.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Professor Weißbach is a member of the steering group for the development of the S3 guideline for prostate cancer and of the urological advisory boards of Novartis and AstraZeneca. Professor Altwein declares that he has no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.National Cancer Institute. SEER-Studie. www.seer.cancer.gov/statfacts/html/prost.html?statfacts_page=prost.html&x=18&y=16.
- 2.Lu-Yao G, Lao S. Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet. 1997;349:906–910. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]
- 3.Catalona WJ. Expectant management and the natural history of localized prostate cancer. J Urol. 1994;152:1751–1752. doi: 10.1016/s0022-5347(17)32377-7. [DOI] [PubMed] [Google Scholar]
- 4.DRG-Statistik 2006: InEK. Datenveröffentlchung gemäß §21 KHEntgG. www.gdrg.de/cms/index.php/inek_site_de/content/view/full/1629.
- 5.Tumorzentrum Brandenburg. www.tumorzentrum-brandenburg.de/pwp/(S(temi1ujch4e0rmza1bo4wfyl))/uploads/Sachbericht_2007.pdf.
- 6.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–2705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MU, Chan H, Hunt M. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004;5:101–106. doi: 10.1016/S1470-2045(04)01384-1. [DOI] [PubMed] [Google Scholar]
- 9.Allaf ME, Carter HB. The results of watchful waiting for prostate cancer. AUA update Series. 2005;24:2–7. [Google Scholar]
- 10.Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0-4 N0-2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891) Eur Urol. 2008;53:941–949. doi: 10.1016/j.eururo.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Mohler JL, Williams BT, Freeman JA. Expectant management as an option for men with stage T1c prostate cancer: a preliminary study. World J Urol. 1997;15:364–368. doi: 10.1007/BF01300184. [DOI] [PubMed] [Google Scholar]
- 12.Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–1669. [PubMed] [Google Scholar]
- 13.Chen WM, Yang CR, Ou YC, et al. Clinical outcome of patients with stage T1a prostate cancer. J Chin Med Assoc. 2003;66:236–240. [PubMed] [Google Scholar]
- 14.Khan MA, Carter HB, Epstein JI, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant managemant criteria for prostate cancer? J Urol. 2003;170:2274–2278. doi: 10.1097/01.ju.0000097124.21878.6b. [DOI] [PubMed] [Google Scholar]
- 15.Patel MI, DeConcini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–1524. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 16.Martin RM, Gunnell D, Hamdy F, Neal D, Lane A, Donovan J. Continuing contro-versy over monitoring men with localized prostate cancer: a systematic review on programs in the prostate specific antigen era. J Urol. 2006;176:439–449. doi: 10.1016/j.juro.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz L. Active Surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23:8165–8169. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 18.American Urological Association. Guideline prostate cancer. www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm.
- 19.National Institute for Health and Clinical Excellence. Prostate cancer. www.nice.org.uk/guidance/index.jsp?action=byID&o=11924.
- 20.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 21.Epstein JI, Chan DW, Sokoll LJ, et al. Nonpalpable stage T1c prostate cancer: prediction of insignificant disease using free/total prostate specific antigen levels and needle biopsy findings. J Urol. 1998;160:2407–2411. [PubMed] [Google Scholar]
- 22.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated,confined tumors. J Urol. 2003;170:1792–1797. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 24.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: Lessons from the Cancer of the Prostate Urologic Strategic Research Endeavour (CaPSURE), a National Disease Registry. J Urol. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 25.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98:355–357. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.van den Bergh RC, Roemeling S, Roobol MJ, Roobol W, Schröder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: The PRIAS Study. Eur Urol. 2007;52:1560–1563. doi: 10.1016/j.eururo.2007.05.011. [DOI] [PubMed] [Google Scholar]
- e2.Klotz L, Nam RK. Active surveillance with selective delayed intervention for favorable risk prostate cancer: clinical experience and a ‘number needed to treat’ analysis. Can J Urol. 2006;(13 Suppl):48–55. [PubMed] [Google Scholar]
- e3.Bott SRJ, Birtle AJ, Taylor CJ, Kirby RS. Prostate cancer management: (1) an update on localised disease. Postgrad Med J. 2003;79:575–580. doi: 10.1136/pmj.79.936.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Wilt TJ, Brawer MK, Barry MJ. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;1:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- e5.Stiftung Männergesundheit Berlin. Versorgungsstudie zum PCa. www.harow.de.
- e6.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- e7.Jones JS. Prostate cancer: are we over-diagnosing—or under-thinking? Eur Urol. 2008;53:10–12. doi: 10.1016/j.eururo.2007.08.045. [DOI] [PubMed] [Google Scholar]
- e8.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco cancer of the prostate risk assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]