Abstract
Background
Deep brain stimulation, known to be effective in the treatment of movement disorders, is now attracting increasing interest in the treatment of other neurological and psychiatric diseases, particularly pain syndromes and epilepsy. It may be a new treatment option for intractable epilepsy.
Methods
Selective literature review of human applications of deep brain stimulation in epilepsy presented together with the author’s own experimental and clinical experience.
Results
Conceptually, deep brain stimulation might be used to prevent the spread of epileptic discharges or to suppress their generation. Various target structures in the brain, including the thalamus, the subthalamic nucleus, and foci in the hippocampus and neocortex, are currently of interest and are being analyzed in multicenter clinical studies. In parallel, experimental models of epilepsy are being used to help determine the suitable stimulation parameters, e.g., frequency or type of stimulation. Recent clinical studies on stimulation of epileptic foci indicate a favorable ratio of efficacy to adverse effects in the treatment of temporal lobe epilepsy, although only a small number of patients have been so treated to date.
Conclusions
Large-scale studies involving stimulation of the thalamus and of cortical foci are now underway in the United States. On the basis of the favorable results of focus stimulation, a multicenter study in Europe is currently comparing the safety and efficacy of hippocampal stimulation to that of surgical treatments. These studies are expected to yield benchmark findings in the next few years that will determine the role deep brain stimulation will play in the treatment of epilepsy.
Keywords: epilepsy, treatment, efficacy, electrostimulation, surgery
In recent years deep brain stimulation has become established as an effective treatment for movement disorders that is usually also safe over the long term (1). Currently, there is great interest in broadening the spectrum of diseases that can be treated with this approach. In addition to the treatment of pain syndromes and psychiatric disorders, one of the possibilities is that deep brain stimulation could become an innovative treatment for epilepsy.
At present, about a third of patients with epilepsy continue to be subject to seizures even after attempted treatment with a wide variety of anticonvulsive drugs. In Germany there are at present more than 200 000 patients with medically intractable epilepsy (e1). Even the second generation of anticonvulsives have failed to alter this situation significantly.
For some forms of focal epilepsy, surgery can achieve freedom from seizures in about two-thirds of patients (e2– e4). However, not all patients are candidates for this method of treatment, in some cases because no circumscribed focus can be identified, in others because, given the site of the focus, its removal would entail risking the impairment of cognitive or motor function.
This is why it is particularly important to develop alternative therapeutic techniques. For 20 years, vagus nerve stimulation has provided a stimulation technique that can significantly reduce the frequency or severity of seizures by stimulating the tenth cranial nerve (2, 3). In addition to its short-term anticonvulsive effect, the efficacy of electrical stimulation increases if continued over the long term, indicating an additional neuromodulatory effect. So far as is known at present, diffuse projection systems of the brain stem (especially the locus ceruleus [e5]) play a central role in this disease-modifying effect.
Vagus nerve stimulation is safe in respect of cognition and affect; no interactions are seen. Typical side effects can arise from infection of the implant (2) or from irritation of the recurrent laryngeal nerve, and are usually transient (e6). The efficacy of vagus nerve stimulation is comparable to that of an additional drug treatment (e7).
Techniques for deep brain stimulation could open up the possibility of targeted modulation of active epileptic networks via other mechanisms of effect, and are therefore worth investigation as novel approaches to treatment. This article provides a selective review of stimulation techniques for the treatment of epilepsy that are currently undergoing clinical trials.
Anticonvulsive mechanisms of effect of therapeutic stimulation techniques
An epileptic brain is hyperexcitable. It might therefore appear surprising that additional electrical stimulation could have a beneficial effect on the occurrence of epileptic seizures. And indeed, depending on the type of such stimulations, they can in fact potentially trigger epileptic activity or induce "kindling," i.e. the modulation of neural networks in a way that favors epileptic activity (e8, e9).
On the other hand, Galen (e10) already described how, under certain circumstances, sensitive or sensory stimuli can contribute to acute interruption of an epileptic seizure. Similar clinical observations were reported by famous neurologists of the nineteenth century, such as Brown-Séquard (e11), Jackson (e12), and Gowers (e13). The stimulation therapies being investigated today use primarily electrical stimuli of varying frequencies to interfere with epileptic activity. These can, for example, inactivate neurons by blocking depolarization, or reduce the recruitability of neurons on the basis of the rhythmic activity they induce. Furthermore, the activation of inhibiting neurons and their projections, and changes in the properties of networks (desynchronization, anti-kindling effects), can have an antiepileptic effect.
Although there is some experimental evidence for such effects from animal studies (e14, e15), the most important individual mechanisms of the various stimulation treatments are to date still insufficiently understood. In particular, the significance of each of these mechanisms for the efficacy of therapeutic stimulation depends critically on the targeted site of effect and on the exact nature (parameters) of the stimulus.
Target regions for efficacious antiepileptic deep brain stimulation
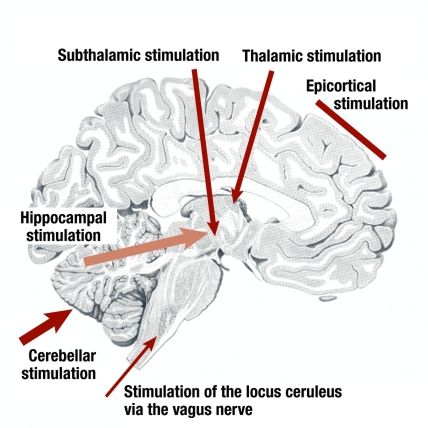
In the treatment of movement disorders, the deep brain stimulation sites are in the immediate area of the basal ganglia. To treat epilepsy, by contrast, a variety of sites are approached. The most important of these are shown in Figure 1. A basic distinction is made between stimulation at a distance from the epileptic focus, to modulate extensive networks, and stimulation to control the epileptogenic region itself.
Figure 1.
Target structures for deep brain stimulation as a treatment for epilepsy
Cerebellum
The first stimulation treatments were carried out as early as the 1970s and 1980s, and involved stimulation of the cerebellum. The idea was to cause inhibition of thalamic nuclei by modulating the activity of efferent cerebellar nuclei. Although the first open series of treatments led to improved seizure control for most patients (e16– e18), controlled trials did not confirm these effects (4, 5), and neither did a series of experimental studies on animals (e19). However, now that a recent double-blind randomized study, albeit with only a small number of patients, has found a marked reduction in seizures after an average follow-up of 24 months (6), the role of cerebellar stimulation in the treatment of epilepsy must remain an open subject.
Subthalamic nucleus
Stimuli to the subthalamic nucleus work via modulation of the dorsal midbrain anticonvulsant zone. In animal experiments, generalized and focal seizures can be suppressed by stimulation of the subthalamic nucleus (e20, e21). Although pilot studies in Grenoble and Cleveland showed a beneficial effect on seizure frequency (7, 8), the reduction achieved with the stimulation parameters used did not seem great enough to justify further investigation in larger clinical trials.
Caudate nucleus
In agreement with experimental results, one group described seizure-reducing effects of stimulation of the caudate nucleus (e22– e24). The quality of the data in the description of the antiepileptic effect has been criticized, however, and the role of this therapeutic option is as yet undecided.
Thalamus
Stimulation of the thalamus as a treatment for epilepsy has been carried out in the region of the centromedian nucleus (9, 10) and the anterior nucleus. The results of stimulation of the centromedian nucleus were somewhat variable, and this method is now being pursued by only one group (11). Stimulation of the anterior nucleus, on the other hand, is now at the center of scientific interest. This thalamic nucleus, the central relay station of the limbic system, is closely connected both to the hippocampi and also to extensive areas of the neocortex (figure 2). For this reason, it is a worthwhile target area for network modulation, which could have an effect both on the epileptogenic focus and on the further spread of epileptic activity.
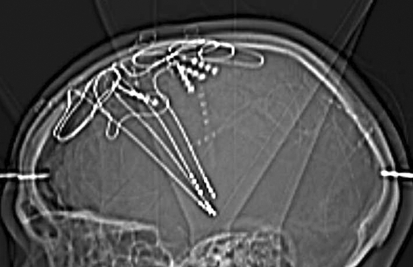
Figure 2.
Combined bilateral deep stimulation of the thalamus and subthalamic nucleus to treat a patient with myoclonic epilepsy (implant; image from the Department of Stereotactic Surgery, by kind permission of Prof. G. Nikkhah)
A Canadian study achieved seizure reduction by more than 50% in four patients subjected to high-frequency stimulation of the anterior thalamic nucleus. The results suggested, however, that the effects merely of the bilateral insertion of the depth electrodes made an important contribution to the outcome (12– 14). Other pilot series (15, 16) consisting of respectively five and four patients with inoperable focal epilepsy reported excellent results, with seizure reduction in four of five and four of four patients respectively; in the latter study, the reduction was by 84% to 92%. The efficacy and safety of this treatment is currently being investigated in the USA in a large controlled prospective multicenter study (SANTE) (e25). Results are expected within the next year.
Epileptic focus
Finally, direct stimulation of the epileptic focus is of particular interest in relation to the antiepileptic mechanisms of action mentioned above: from acute blockade of epileptic discharges to raising thresholds for epileptic activity by means of synaptic or neural network mechanisms.
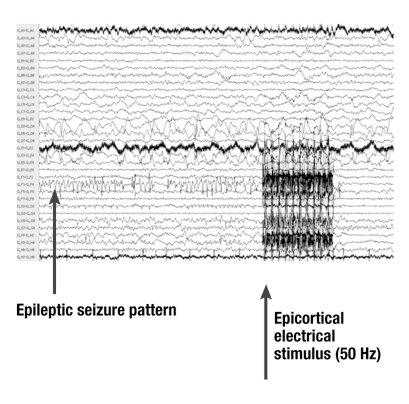
Neocortical foci present particular challenges: they are often extensive, and it is difficult to modulate the entire area by electrical fields. From the first descriptions by Penfield and Jasper (1954), and from systematic investigations in which presurgically eloquent areas (e.g., the region of speech) were blocked off by implanted epicortical electrodes, we know that an early epicortical stimulus can interrupt seizure patterns (figure 3) (e26, e27). If only a part of the zone of origin is stimulated, seizure activity can spread from the non-inactivated or non-modulated areas to the rest of the brain (17). This could limit the effectiveness of the type of stimulation at present being carried out in a multicenter study in the USA. However, in individual cases marked seizure reduction, lasting even for years, has been reported as a result of focal stimulation (18).
Figure 3.
Electrocorticographic evidence of the suppression of a local seizure pattern (channel GF3-F4), triggered by an epicortical stimulus (stimulus artifact; intracranial EEG using a subdural electrode grid)
The anatomical situation in circumscribed nuclear regions and in the hippocampus is more favorable, because deep stimulation here can modulate the activity of the entire focus. Eight years ago a Mexican group reported excellent clinical and electrophysiological effects for high-frequency stimulation via implanted depth electrodes, which had been temporarily left in place after the end of the diagnostic phase (19, 20). Interestingly, hippocampal stimulation led to an increase in the temporal reduced perfusion on the stimulated side on single-photon emission computed tomography (SPECT), which may be an expression of inhibition of the focal region.
This was the starting point for further controlled studies using a variety of stimulation parameters and durations of stimulation. One of the four studies found no significant effects for monthly alternation of stimulation and no stimulation (21). In contrast, two studies published in 2007 in which stimulation was continuous reported lasting success even after periods of 18 to 36 months. Some patients became completely seizure-free, and more than half of patients with drug-resistant epilepsy experienced marked seizure reduction (22, 23). This treatment approach is therefore now being intensively pursued in further clinical studies, including in Europe.
Choice of stimulation parameters
Electrical deep brain stimulation is usually carried out using bipolar orthogonal impulses. Often the stimulation frequency used in the treatment of movement disorders, 130 Hz, is employed; more rarely, other frequencies such as 50 Hz or low frequencies in the region of 0.1 to 1 Hz are used. One critical problem for clinical studies is that, so far—on the basis of current knowledge—there are no "optimal" stimulation parameters to choose. Firstly, the choice of suitable parameters depends on the intended effect. Secondly, because of the episodic nature of the disease, there are difficulties that are particular to epileptology: whereas when treating a movement disorder it is often possible to test the effect of deep brain stimulation while still in the operating room, and thus quickly identify the stimulation parameters that are best for that patient, the effects of epilepsy treatment only become apparent over the long term. Systematic modulation of all relevant parameters of the stimulation (especially the frequency, pulse type, and the pattern of stimuli) is possible only to a very limited extent in clinical studies. For this reason, in a few centers (Ghent, Freiburg), a preselection of suitable stimulus types is being investigated in parallel animal experimental models (e28, e29) (www.bccn.unifreiburg.de/research/projects/c3).
In addition to the type of the stimulus, an increasing subject of discussion in relation to epilepsy is the possibility of "closed-loop" stimulation. For this, a closed regulating loop of epileptic activity or prodromal activity is used to suppress seizures. Whereas treatment of movement disorders requires more or less continuous stimulation to suppress symptoms, epileptic seizures occur only intermittently, occupying less than 1% of the lifetime of the patients. Targeted intervention based on early recognition or prediction of seizures (e30) could help to increase the life of the stimulator batteries (which currently last a few years) many times over, and to minimize any side effects. However, this would require both technical improvements and improved algorithms. Only one group (16, 24) used a seizure detection algorithm in thalamic stimulation treatment. The current multicenter study in the USA on focal stimulation, described as "responsive neurostimulation" (e31, e32), aims at time-targeted intervention of this kind based on the recognition of seizure patterns. Because of the low specificity of seizure recognition, however, it is questionable whether this can be called seizure-triggered closed-loop stimulation.
Methodological aspects and risks
In deep brain stimulation, stimulation electrodes are implanted stereotactically in target structures with a precision of about 1 to 2 mm. This often takes place under local anesthesia. The exact position is ascertained using magnetic resonance imaging and by means of recordings of the electrical activity and stimulation. The stimulator unit with impulse generator, control unit, and battery are placed in the torso and programmed transcutaneously.
Side effects can occur in association with the electrode implantation or as a consequence of the stimulation. In Freiburg, the risk of side effects of stereotactic implantation is around 0.5% to 1% for symptomatic bleeding and a similarly low risk of local infection along the connecting cable or at the site of the implanted stimulators (e33). One review reports infection rates of 6.1%, misplacement of electrodes in 4.4%, electrode breakage in 1.8%, and skin ulcerations in 1.3% of cases. In German centers, asymptomatic bleeding was reported in 1.6% of cases and symptomatic bleeding in 1.3%, with resulting permanent morbidity of 0.8% and mortality 0.4% (e34). In the context of epilepsy treatments, so far one case of infection has been reported in a patient treated with cerebellar stimulation (6). One case of asymptomatic bleeding and one of infection have been reported in patients who underwent hippocampal electrode implantation (25).
The stimulation parameters are chosen so as to avoid tissue damage (e35). In the above series side effects were mild and rare or were absent. Of course, the case numbers in all series of patients treated with deep brain stimulation are still low. With subthalamic stimulation, there were a few cases of slight muscle contractions in the facial region or of numbness in the extremities, which regressed when the stimulus intensity was reduced (7). Among epilepsy patients given anterior thalamic stimulation, one patient experienced episodes of disturbed consciousness and behavioral arrest in an intensity-dependent manner; these episodes occurred only above a well-tolerated intensity threshold (16). One case of paranoid psychosis 5 months after the start of stimulation to the anterior thalamus was interpreted as a possible consequence of sudden, complete suppression of epileptic discharges amounting to forced normalization (16). Interestingly, in patients undergoing chronic stimulation of the hippocampus, no deterioration of memory function was reported (22, 25).
The future
At present, deep brain stimulation as a new method of treating epilepsy is at the center of clinical research interest throughout the world. Many questions remain, such as how to choose the best target area, the best stimulation parameters, and how best to match the individual patients with the stimulation type best suited to them. If the good results shown in some of the pilot studies are confirmed in the multicenter clinical trials now under way, we will be a step closer to wider implementation of the technique, as is now the case for vagus nerve stimulation (Figures 4 and 5).
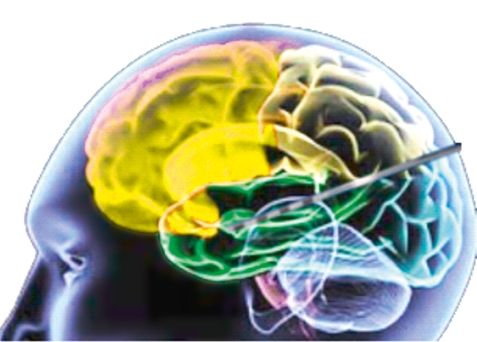
Figure 4.
Hippocampal deep stimulation for epilepsy treatment (schema). Approaching from the occipital direction, the stimulation electrode is placed longitudinally in the head of the hippocampus; the stimulus is delivered via a stimulation system implanted in the torso
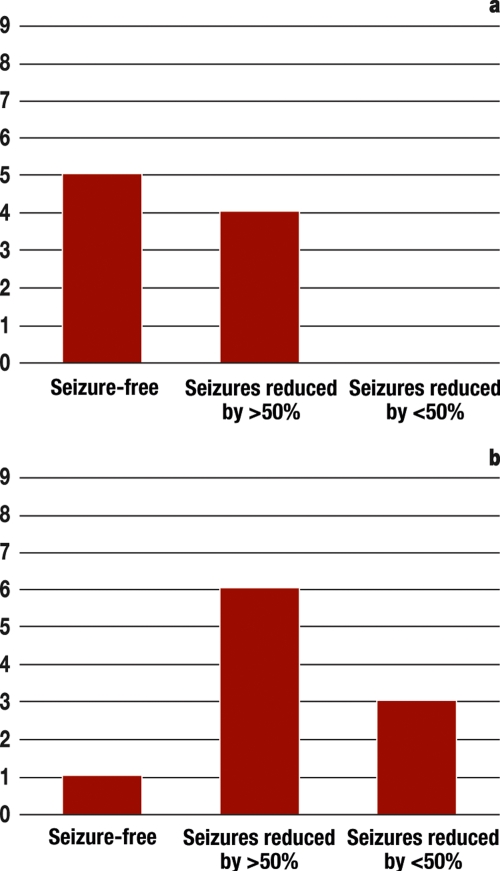
Figure 5.
Results of recent prospective studies of hippocampus stimulation
a) Randomized double-blind studies of the effectivity of bilateral hippocampus stimulation in 9 patients with temporal lobe epilepsy due to uni- or bilateral hippocampal sclerosis. Follow-up time: 18 months. Observed side effects were: skin erosions requiring treatment in 3 patients 24 months after implantation (data from Velasco et al. 2007 [22])
b) Open prospective studies of the efficacy of bilateral hippocampus stimulation in 10 patients with temporal lobe epilepsy, who underwent invasive monitoring. Follow-up time: 31 months. No subjective or objective side effects of treatment were observed (data from Boon et al. 2007 [25])
For this reason, several multicenter prospective double-blind studies on stimulation of the thalamus and the epileptic focus are currently under way in the USA; these have already completed enrollment. Results are expected in 2009. For the first time, a study of deep brain stimulation (CoRaStiR) is also at present under way in Germany. In this study, patients with drug-resistant epilepsy and a seizure origin in the hippocampus have the opportunity to receive treatment by electrical stimulation of the hippocampus in the framework of a European multicenter study. Patients are randomized to undergo epilepsy surgery or deep brain stimulation, in order to allow a comparison of efficacy (seizure reduction) and safety (including cognitive performance) in a large patient group.
Even if deep brain stimulation may not be expected to prove more effective than surgical resection of the hippocampus, it may still become an established form of treatment on the basis that it is less invasive and safer. Existing studies of hippocampus stimulation report unanimously that this stimulation does not impair memory function (22). Moreover, if side effects do arise, the type and intensity of stimulation can be adjusted by the physician or the patient at any time. In addition to the minimally invasive implantation of the stimulators, the lower risk of unwanted effects give this method a decided advantage over conventional operative procedures such as resection of the temporal lobe or selective removal of the amygdala and hippocampus. If deep brain stimulation proves not to be effective enough, epilepsy surgery can be carried out after a year. If seizure control is partial, individual optimizations of the stimulation are possible.
Both hippocampal and thalamic stimulation are particularly interesting as treatment options for patients in whom surgery is contraindicated by the absence of a clear, unilateral focus and the presence of a high risk of memory impairment with a high cognitive requirement.
If the successes seen in treatment series to date are confirmed in the ongoing multicenter studies of continuous stimulation, further tasks will remain. These are: optimization of the stimulus types—which will require experimental animal studies in addition to clinical trials —and the further development of time-targeted stimulation therapy. Progress in computer-assisted recognition (24) and prediction of seizures (e30) will play a central part in this.
Key messages.
Deep brain stimulation is currently under evaluation as a new therapeutic procedure for the treatment of epilepsy.
Stimulation of various target sites in the brain, amongst others the thalamus, subthalamic nucleus, and the epileptic focus, can prevent the occurrence and propagation of epileptic activity.
Pilot studies in epilepsy patients suggest that the treatment is safe and effective even in those whose epilepsy is drug-resistant.
The optimum forms of stimulation are still being developed in clinical and experimental studies.
A multicenter clinical study is currently under way in Germany comparing the efficacy and safety of hippocampus stimulation with epilepsy surgery.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
For further information about the European Study of Hippocampus Stimulation (Controlled, Randomized Stimulation versus Resection, CoRaStiR) visit www.uniklinik-freiburg.de/epilepsie/live/aktuelles.html
The study is being carried out at the Freiburg Epilepsy Center (Epilepsiezentrum Freiburg) in close collaboration with the Department of General Neurosurgery and the Department of Stereotactic Neurosurgery.
Conflict of interest statement
Professor Schulze-Bonhage is taking part in a European multicenter study of hippocampus stimulation for the treatment of epilepsy. The stimulators used in this study are provided free of charge by Medtronic.
References
- 1.Wojtecki L, Südmeyer M, Schnitzler A. Therapie des ideopathischen Parkinson-Syndroms. Dtsch Arztebl. 2007;104(37):A-2513–A-2522. [Google Scholar]
- 2.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 3.Salinsky M, Wernicke J, Rutecki P, et al. The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45:224–230. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 4.Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48:407–416. doi: 10.3171/jns.1978.48.3.0407. [DOI] [PubMed] [Google Scholar]
- 5.Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769–774. doi: 10.1136/jnnp.47.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasco F, Carrillo-Ruiz JD, Brito F, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1071–1081. doi: 10.1111/j.1528-1167.2005.70504.x. [DOI] [PubMed] [Google Scholar]
- 7.Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(Suppl 3):S83–S93. [PubMed] [Google Scholar]
- 8.Loddenkemper T, Pan A, Neme S, et al. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18:514–532. doi: 10.1097/00004691-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Velasco F, Velasco M, Jimenez F, Velasco AL, Marquez I. Stimulation of the central median thalamic nucleus for epilepsy. Stereotact Funct Neurosurg. 2001;77:228–232. doi: 10.1159/000064611. [DOI] [PubMed] [Google Scholar]
- 10.Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 11.Velasco AL, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 12.Andrade DM, Zumsteg D, Hamani C, et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66:1571–1573. doi: 10.1212/01.wnl.0000206364.19772.39. [DOI] [PubMed] [Google Scholar]
- 13.Wennberg R. Chronic anterior thalamic deep brain stimulation as a treatment for intractable epilepsy. In: Schelter B, Timmer J, Schulze-Bonhage A, editors. Seizure Prediction in Epilepsy. Weinheim: Wiley-VCH Verlag; 2008. pp. 307–316. [Google Scholar]
- 14.Dennig D, Trippel M, Carius A, Schulze-Bonhage A. Anfallskontrolle durch intrahippocampale Tiefenelektrodenimplantation. Aktuel Neurol. 2008;35(Suppl 1):S57–S58. [Google Scholar]
- 15.Kerrigan JF, Litt B, Fisher RS, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45:346–354. doi: 10.1111/j.0013-9580.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 16.Osorio I, Overman J, Giftakis J, Wilkinson SB. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. 2007;48:1561–1571. doi: 10.1111/j.1528-1167.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 17.Lüders HO, editor. Deep brain stimulation and epilepsy. London: Dunitz Verlag; 2004. [Google Scholar]
- 18.Elisevich K, Jenrow K, Schuh L, Smith B. Long-term electrical stimulation-induced inhibition of partial epilepsy. Case report. J Neurosurg. 2006;105:894–897. doi: 10.3171/jns.2006.105.6.894. [DOI] [PubMed] [Google Scholar]
- 19.Velasco F, Velasco M, Velasco AL, Menez D, Rocha L. Electrical stimulation for epilepsy: stimulation of hippocampal foci. Stereotact Funct Neurosurg. 2001;77:223–227. doi: 10.1159/000064610. [DOI] [PubMed] [Google Scholar]
- 20.Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. 2000;41:158–169. doi: 10.1111/j.1528-1157.2000.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 21.Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66:1490–1494. doi: 10.1212/01.wnl.0000209300.49308.8f. [DOI] [PubMed] [Google Scholar]
- 22.Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48:1895–1903. doi: 10.1111/j.1528-1167.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 23.Boon P, Vonck K, De H V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 24.Osorio I, Frei MG, Sunderam S, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258–268. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- 25.Boon P, Vonck K, De H V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- e1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- e2.Fauser S, Bast T, Altenmuller DM, et al. Factors influencing surgical outcome in patients with focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2008;79:103–105. doi: 10.1136/jnnp.2007.116038. [DOI] [PubMed] [Google Scholar]
- e3.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- e4.Schulze-Bonhage A. Langzeit-Outcome nach epilepsiechirurgischen Eingriffen. Z Epileptol. 2008;21:17–25. [Google Scholar]
- e5.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- e6.Ben-Menachem E, French JA. VNS Therapy versus the latest antiepileptic drug. Epileptic Disord. 2005;7:22–26. [PubMed] [Google Scholar]
- e7.Schulze-Bonhage A. In need of stimulation: the role of the vagus nerve in treating epilepsy and depression. Epilepsy Professional. 2007;7:15–17. [Google Scholar]
- e8.Velasco F, Velasco M, Marquez I, Velasco G. Role of the centromedian thalamic nucleus in the genesis, propagation and arrest of epileptic activity. An electrophysiological study in man. Acta Neurochir Suppl (Wien) 1993;58:201–204. doi: 10.1007/978-3-7091-9297-9_48. [DOI] [PubMed] [Google Scholar]
- e9.McIntyre DC, Racine RJ. Kindling mechanisms: current progress on an experimental epilepsy model. Prog Neurobiol. 1986;27:1–12. doi: 10.1016/0301-0082(86)90010-9. [DOI] [PubMed] [Google Scholar]
- e10.Galenus: On the affected parts. Vol. 96. Basel: Krager Verlag; 1976. [Google Scholar]
- e11.Brown-Séquard CE. Researches on Epilepsy: its artificial production in animals, and its etiology and treatment in man. Boston Med J. 1857:55–57. [Google Scholar]
- e12.Jackson JH. Case of convulsive attacks arrested by stopping the aura. Lancet. 1868;196:618–619. [Google Scholar]
- e13.Gowers WR. Epilepsy and other chronic convulsive diseases: their causes, symptoms and treatment. London: William Wood; 1885. pp. 235–236. [Google Scholar]
- e14.Bikson M, Lian J, Hahn PJ, Stacey WC, Sciortino C, Durand DM. Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J Physiol. 2001;531:181–191. doi: 10.1111/j.1469-7793.2001.0181j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Tass PA. A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol Cybern. 2003;89:81–88. doi: 10.1007/s00422-003-0425-7. [DOI] [PubMed] [Google Scholar]
- e16.Cooper IS, Amin I, Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192–196. [PubMed] [Google Scholar]
- e17.Davis R, Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg. 1992;58:200–208. doi: 10.1159/000098996. [DOI] [PubMed] [Google Scholar]
- e18.Cooper IS, Amin I, Upton A, Riklan M, Watkins S, McLellan L. Safety and efficacy of chronic stimulation. Neurosurgery. 1977;1:203–205. doi: 10.1097/00006123-197709000-00019. [DOI] [PubMed] [Google Scholar]
- e19.Krauss GL, Koubeissi MZ. Cerebellar and thalamic stimulation treatment for epilepsy. Acta Neurochir Suppl. 2007;97:347–356. doi: 10.1007/978-3-211-33081-4_40. [DOI] [PubMed] [Google Scholar]
- e20.Vercueil L, Benazzouz A, Deransart C, et al. High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res. 1998;31:39–46. doi: 10.1016/s0920-1211(98)00011-4. [DOI] [PubMed] [Google Scholar]
- e21.Bressand D, Dermateis M, Kahane P, Banazzouz A, Benabid AL. Involvement of the subthalamic nucleus in the conrtol of temporal lobe epilepsy. Soc Neurosci. 2008;25 [Google Scholar]
- e22.Chkhenkeli SA, Sramka M, Lortkipanidze GS, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–329. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- e23.Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereotact Funct Neurosurg. 1997;69:221–224. doi: 10.1159/000099878. [DOI] [PubMed] [Google Scholar]
- e24.Sramka M, Fritz G, Galanda M, Nadvornik P. Some observations in treatment stimulation of epilepsy. Acta Neurochir (Wien) 1976:257–262. doi: 10.1007/978-3-7091-8444-8_41. [DOI] [PubMed] [Google Scholar]
- e25.Fisher RS Sante study group. Interim Report. Abstract #4.122, presented at the American Epilepsy Society Annual Meeting. 2006 [Google Scholar]
- e26.Lesser RP, Kim SH, Beyderman L, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53:2073–2081. doi: 10.1212/wnl.53.9.2073. [DOI] [PubMed] [Google Scholar]
- e27.Motamedi GK, Lesser RP, Miglioretti DL, et al. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia. 2002;43:836–846. doi: 10.1046/j.1528-1157.2002.24901.x. [DOI] [PubMed] [Google Scholar]
- e28.Wyckhuys T, De Smedt T, Claeys P, et al. High frequency deep brain stimulation in the hippocampus modifies seizure characteristics in kindled rats. Epilepsia. 2007;48:1543–1550. doi: 10.1111/j.1528-1167.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- e29.Feddersen B, Vercueil L, Noachtar S, David O, Depaulis A, Deransart C. Controlling seizures is not controlling epilepsy: a parametric study of deep brain stimulation for epilepsy. Neurobiol Dis. 2007;27:292–300. doi: 10.1016/j.nbd.2007.05.005. [DOI] [PubMed] [Google Scholar]
- e30.Schelter B, Timmer J, Schulze-Bonhage A, editors. From Basic Mechanisms to Clinical Applications. Weinheim: Wyley; 2008. Seizure Prediction in Epilepsy. [Google Scholar]
- e31.Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, Jenkins PD. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83:1530–1538. doi: 10.1159/000088656. [DOI] [PubMed] [Google Scholar]
- e32.Sun FT, Morrell MJ, Wharen RE., Jr Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5:68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Vesper J, Haak S, Ostertag C, Nikkhah G. Subthalamic nucleus deep brain stimulation in elderly patients - analysis of outcome and complications. BMC Neurol. 2007;7 doi: 10.1186/1471-2377-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e34.Voges J, Hilker R, Bötzel K, et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord. 2007;22:1486–1489. doi: 10.1002/mds.21481. [DOI] [PubMed] [Google Scholar]
- e35.Agnew WF, McCreery DB. Considerations for safety with chronically implanted nerve electrodes. Epilepsia. 1990;31(Suppl 2):S27–S32. doi: 10.1111/j.1528-1157.1990.tb05845.x. [DOI] [PubMed] [Google Scholar]
- e36.Boon P, Vonck K, De Heerd V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- e37.Meier R, Dittrich H, Schulze-Bonhage A, Aertsen A. Detecting epileptic seizures in long-term human EEG: a new approach to automatic online and real-time detection and classification of polymorphic seizure patterns. J Clin Neurophysiol. 2008;25:119–131. doi: 10.1097/WNP.0b013e3181775993. [DOI] [PubMed] [Google Scholar]