SUMMARY
The Disrupted In Schizophrenia 1 (DISC1) gene is disrupted by a balanced chromosomal translocation (1; 11) (q42; q14.3) in a Scottish family with a high incidence of major depression, schizophrenia and bipolar disorder. Subsequent studies provided indications that DISC1 plays a role in brain development. Here we demonstrate that suppression of DISC1 expression reduces neural progenitor proliferation, leading to premature cell cycle exit and differentiation. Several lines of evidence suggest that DISC1 mediates this function by regulating GSK3β. First, DISC1 inhibits GSK3β activity through direct physical interaction, which reduces β-catenin phosphorylation and stabilizes β-catenin. Importantly, expression of stabilized β-catenin overrides the impairment of progenitor proliferation caused by DISC1 loss-of-function. Furthermore, GSK3 inhibitors normalize progenitor proliferation and behavioral defects caused by DISC1 loss-of-function. Together, these results implicate DISC1 in GSK3β/β-catenin signaling pathways and provide a framework for understanding how alterations in this pathway may contribute to the etiology of psychiatric disorders.
Keywords: DISC1, neural progenitor, GSK3β, behavior, adult neurogenesis
INTRODUCTION
Schizophrenia is a severe brain illness that affects 0.5–1% of the world population. While the etiology is poorly understood, accumulating evidence suggest that neurodevelopmental defects are involved (Ross et al., 2006). Recent studies have identified many risk genes associated with schizophrenia (International-Schizophrenia-Consortium, 2008; Stefansson et al., 2008; Walsh et al., 2008). Among these genetic factors, the (1; 11) (q42; q14.3) translocation allele of the DISC1 gene closely segregates with the manifestation of psychiatric disorders in a large Scottish pedigree (Blackwood et al., 2001). Further genetic evidence from different populations have identified several SNPs in the DISC1 gene associated with schizophrenia, and multiple haplotypes and SNPs along this gene are also associated with bipolar disorder and autism spectrum disorders (Chubb et al., 2008). These data suggest that alterations in DISC1 function play a role in the pathophysiology of these mental illnesses.
Several proteins interact with DISC1, including NudE-like 1 (Ndel1), Lis1, phosphodiesterase 4B (PDE4B), and the transcription factors ATF4 and ATF5 (Chubb et al., 2008). Functional studies revealed that DISC1 is involved in neurite outgrowth, neuronal migration, integration of newborn neurons, and cAMP signaling (Chubb et al., 2008). However, the mechanisms by which DISC1 contributes to the wide spectrum of psychiatric disorders remain elusive.
Several DISC1 transgenic mouse lines have been established to assess the effect of the human DISC1 (hDISC1) truncation on behavior. Over-expression of the DISC1 Scottish mutant (Hikida et al., 2007; Pletnikov et al., 2007) or the C-terminal portion of DISC1 (Li et al., 2007) in mouse brains results in behavioral phenotypes reminiscent of schizophrenia. Likewise, point mutations in exon 2 of mouse DISC1 (mDISC1) lead to the manifestation of schizophrenia- or depression-like behaviors (Clapcote et al., 2007). Furthermore, mouse mutant containing two stop codons (in exon 7 and 8), which results in the expression of a truncated transcript (Kvajo et al., 2008) was described that show abnormal morphology of newborn neurons and reduced synapse number accompanied by a working memory deficit.
In this study, we show that in addition to its known role in regulating neuronal functions, DISC1 is highly expressed in neural progenitor cells and is required for their proliferation. This function of DISC1 involves regulation of the β-catenin/GSK3β pathway, whereby DISC1 stabilizes β-catenin by inhibiting GSK3β activity through a direct interaction. We further narrowed down the DISC1-GSK3β interaction domain and generated a DISC1 peptide that strongly inhibits GSK3β In the context of the adult brain, DISC1 loss-of-function in the dentate gyrus resulted in reduced neural progenitor proliferation and elicited hyperactive and depressive behaviors in mice. Importantly, these behavioral abnormalities were normalized by treatment with a chemical inhibitor of GSK3β. These findings provide compelling evidence that DISC1 is a central player in the GSK3β/β-catenin signaling pathway that impinges on neural progenitor proliferation.
RESULTS
DISC1 regulates cell proliferation in vitro
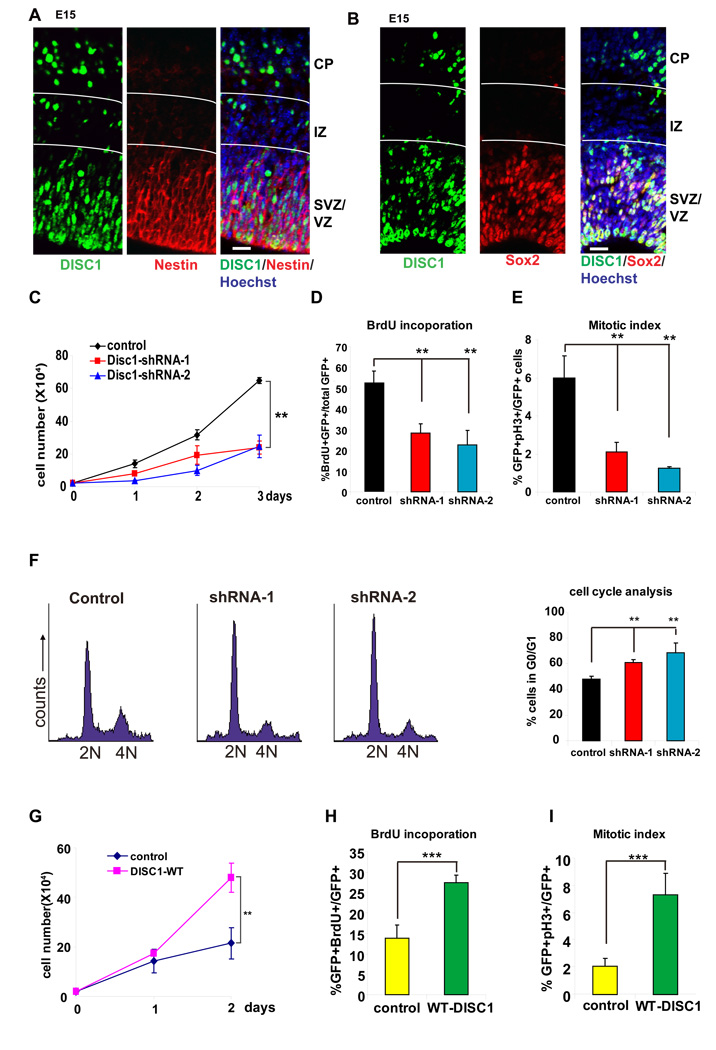
Expression of DISC1 peaks at E14–E15 in mouse embryonic brains, a period of active neurogenesis in the cortex, and gradually declines as development proceeds (data not shown). In adult mice, DISC1 is robustly expressed in the dentate gyrus (DG) and olfactory bulb, two regions displaying active neurogenesis (Ma et al., 2002). In the embryonic cerebral cortex, we found that DISC1 was robustly expressed in nestin and Sox2 positive neural progenitors residing in the ventricular zone (VZ)/subventricular zone (SVZ), but reduced in doublecortin (DCX) positive neurons (Figure 1A, B, S2A). DISC1 expression was reduced in the intermediate zone (IZ) but reappeared in the cortical plate (CP), consistent with its known function in post-mitotic neurons (Figure 1A, B). The specificity of DISC1 staining was verified using embryonic mouse brains electroporated with a DISC1 short hairpin RNA sequence (shRNA) (Figure S1B).
Figure 1. DISC1 regulates progenitor cell proliferation in vitro.
(A) E15 embryonic brain sections were co-stained with anti-DISC1 and anti-Nestin antibody. Scale bar=20 µm.
(B) E15 embryonic brain sections were co-stained with anti-DISC1 and anti-Sox2 antibody. Scale bar=20 µm.
(C) Cell proliferation is reduced in AHP cells infected with lentivirus expressing DISC1 shRNAs. GFP expression is used as a marker for viral infection. Both DISC1 shRNAs significantly reduced cell proliferation (n=3, p<0.01).
(D) BrdU incorporation is decreased in DISC1 knockdown cells. AHPs infected with control, DISC1 shRNA-1, or shRNA-2 lentivirus were pulse labeled with 10 µM BrdU and stained with BrdU antibody. The percentage of GFP positive cells that are also BrdU positive cells is shown (n=4, p<0.01).
(E) Mitotic index is reduced in DISC1-silenced AHPs. The percentage of GFP positive cells that are also pH3 positive is shown (n=3, p<0.01).
(F) Histograms for FACS analysis of N2a cells transfected with DISC1 shRNAs. Bar graph depicts the percentage of GFP positive cells in G0/G1 (n=3, p<0.01).
(G) Cell proliferation is increased in DISC1 overexpressing cells. AHPs were infected with either control or DISC1-WT lentivirus. Cell number was counted for 2 days (n=3, p<0.01).
(H) BrdU incorporation is increased in DISC1-overexpressing AHPs. The percentage of GFP positive cells that are also BrdU positive cells is shown (n=4, p<0.001).
(I) Increased mitotic index in DISC1 overexpressing AHPs. The percentage of GFP positive cells that are also pH3 positive is shown (n=4, p<0.001).
Based on its expression in neural progenitors, we looked into a potential role for DISC1 in progenitor proliferation. To approach this, we generated two specific shRNAs that silenced endogenous DISC1 expression in adult hippocampal progenitors (AHPs) (shRNA1 and shRNA2; Figure S1A). Using lentivirus expressing control or DISC1 shRNAs, we observed that DISC1 knockdown markedly decreased cell proliferation compared to control shRNA in AHPs (Figure 1C). Moreover, DISC1 knockdown decreased BrdU labeling (2hr pulse) by 2-fold (Figure 1D, S3A) and mitotic index by 3-fold (Figure 1E, S3B). Fluorescence-activated cell sorting (FACS) analysis revealed that DISC1 knockdown significantly increased the proportion of cells in G0/G1 (Figure 1F). The reduction of proliferation by DISC1 knockdown prompted us to pursue the reciprocal DISC1 gain-of-function experiment. Notably, overexpression of full length hDISC1 resulted in a 2-fold increase in cell proliferation, 2-fold increase in BrdU labeling, and 3-fold increase in mitotic index (Figure 1G-I, Figure S3C, Figure S3D). Thus, DISC1 is required for the normal proliferation of AHPs, and its overexpression promotes proliferation.
DISC1 regulates cortical progenitor proliferation in utero
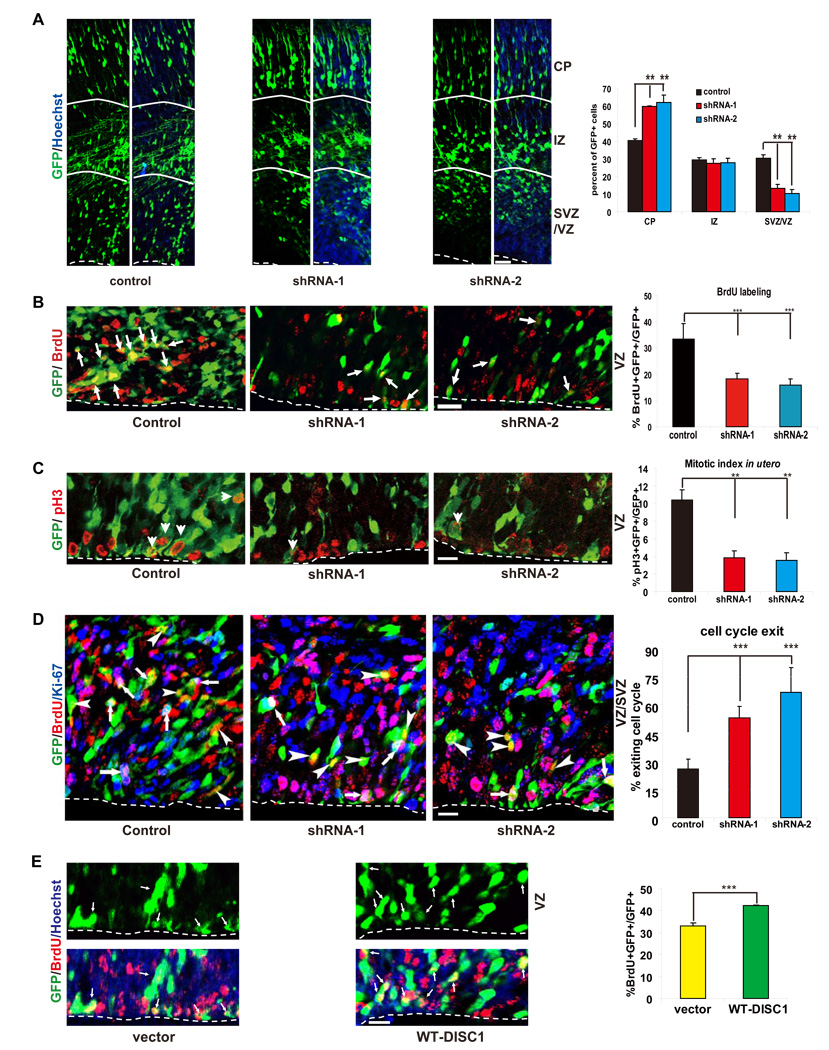
We next investigated whether DISC1 regulates neural progenitor proliferation in vivo. Control or DISC1 shRNA constructs were electroporated together with a GFP-expressing vector into E13 mouse brains, and the brains were analyzed 2 days later. The positioning of GFP positive cells revealed that DISC1 knockdown resulted in a substantial reduction of cells in the VZ/SVZ (Figure 2A), with a corresponding increase in GFP positive cells in the CP. Therefore, DISC1 loss-of-function causes a depletion of cells from the proliferative VZ/SVZ.
Figure 2. DISC1 regulates progenitor cell proliferation in utero.
(A) DISC1 knockdown cells exhibit cell positioning defects in utero. Control or DISC1 shRNA constructs were electroporated into E13 embryonic mouse brains and the mice were sacrificed at E15. The percentage of GFP cells in each region is shown (n=4, p<0.01). Scale bar=20 µm. CP, cortical plate; IZ, intermediate zone; VZ/SVZ, ventricular zone/subventricular zone.
(B) BrdU incorporation is reduced in DISC1 knockdown brains. Brains of mice electroporated at E13 were pulse labeled with BrdU (100 mg/kg) for 2 hours. Arrows indicate GFP and BrdU double positive cells. The bar graph shows the percentage of BrdU and GFP double positive cells to total GFP positive cells in SVZ (n=4, p<0.0005). Scale bar=20 µm.
(C) Mitotic index of DISC1 silenced cells is reduced in utero. The percentage of GFP positive cells that are also pH3 positive in the VZ is shown (n=4, p<0.01). Scale bar=20 µm. Arrowheads indicate GFP and pH3 double positive cells.
(D) DISC1 knockdown in progenitor cells causes premature cell cycle exit in utero. Control or DISC1 shRNA constructs were electroporated into E13 embryonic brains and BrdU was injected at E15. Mice were sacrificed at E16. The cell cycle exit index is measured as the percentage of the GFP-positive cells that exited the cell cycle (GFP+ BrdU+ Ki67−) divided by total GFP and BrdU double positive (GFP+ BrdU+) cells (n=5, p<0.001). Scale bar=20 µm. White arrows indicate GFP+BrdU+Ki67+ cells. Arrowheads indicate GFP+BrdU+Ki67− cells.
(E) BrdU labeling is increased in DISC1 overexpressing cells in utero. Control or WT-DISC1 plasmids were electroporated into embryonic brains at E14 and BrdU was injected 2 hours before sacrificing at E15. The percentage of GFP positive cells in the SVZ that are also BrdU positive cells is shown (n=3, * p<0.05, ***p<0.005). Scale bar=10 µm.
To gain insight into the mechanism accounting for the observed difference in cell positioning caused by DISC1 knockdown, we injected BrdU into the pregnant dams 2 hours prior to collection of the electroporated brains. DISC1 knockdown resulted in a marked reduction in BrdU labeling (Figure 2B) and mitotic index (Figure 2C) that could be rescued by human DISC1 cDNA (not targeted by shRNA-1) (Figure 4C, D), demonstrating that DISC1 is required to maintain cortical progenitor proliferation in vivo. Conversely, DISC1 overexpression increased both the percentage of cells remaining in the VZ/SVZ and the BrdU labeling index (Figure 2E).
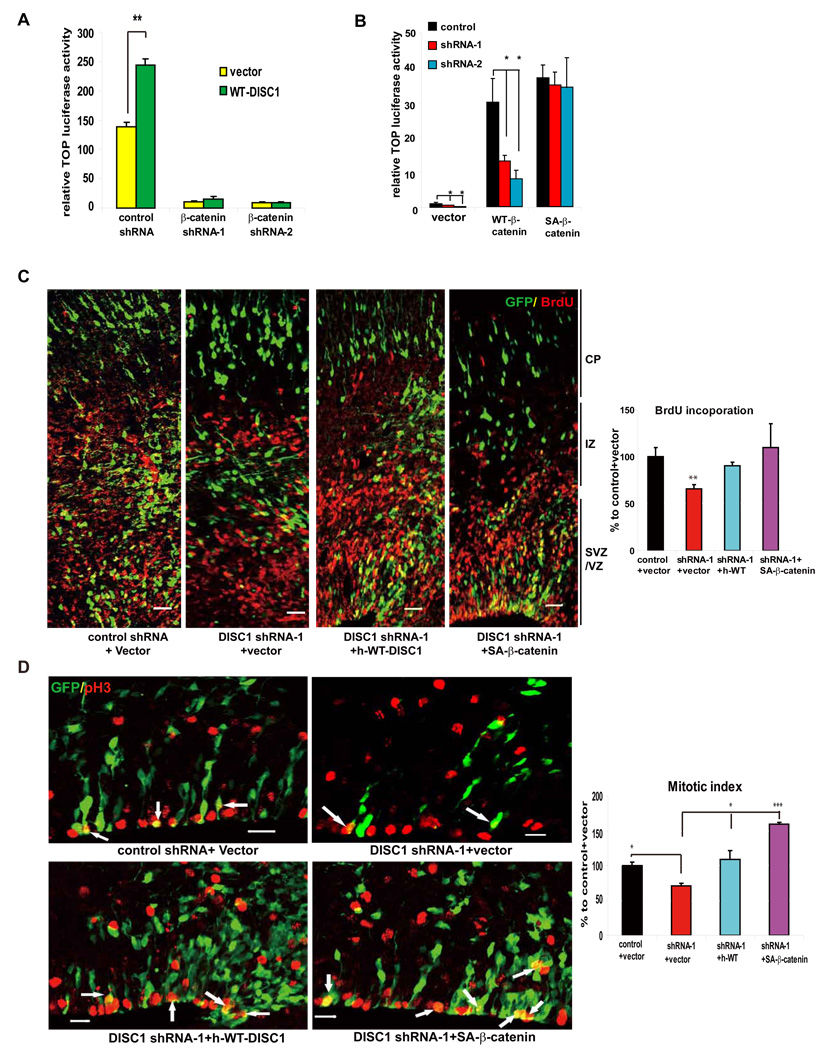
Figure 4. Stable β-catenin rescues DISC1-induced defects.
(A) Enhancement of TOP activity by DISC1 overexpression is abolished by β-catenin shRNAs in embryonic primary progenitors (n=6, p<0.01).
(B) Vector, WT-β-catenin, or SA-β-catenin was cotransfected with the TOP reporter into embryonic progenitors transduced with lentiviruses expressing DISC1 shRNAs. Only SA-β-catenin rescued the TOP activation defect in DISC1 knockdown cells (n=4, *p<0.05, **p<0.01).
(C) Cell positioning and BrdU incorporation defects caused by DISC1 loss-of-function are rescued by human WT-DISC1 or SA-β-catenin. The GFP positive cells that are also BrdU positive cells is shown as the percentage to control plus vector (n=5, **, p<0.01; ***, p<0.005). Scale bar=20 µm.
(D) The reduction in mitotic index caused by DISC1 knockdown in embryonic brains is rescued by human WT-DISC1 or SA-β-catenin. Arrows indicate GFP and pH3 double positive cells. The GFP positive cells that are also pH3 positive cells is shown as the percentage to control plus vector (n=4, *, p<.0.05; ***, p<0.005). Scale bar=20 µm.
Our observations that DISC1 knockdown reduced neural progenitor proliferation and decreased positioning in the VZ/SVZ suggest that cells may be prematurely differentiating into neurons. To test this possibility, we measured the cell cycle exit index (Sanada and Tsai, 2005). E13 mouse brains were electroporated with DISC1 shRNA constructs and BrdU was injected 2 days later at E15 into pregnant dams. At E16, brains were collected and analyzed by immunohistochemistry using anti-GFP, -BrdU, and -Ki67 antibodies (Figure 2D). We observed a 2–3 fold increase in cell cycle index in DISC1 shRNA transfected embryonic brains, suggesting that the reduction of proliferating progenitors in DISC1 shRNA treated brains likely results from increased cell cycle exit. Consistent with this, a significant increase in DCX positive cells and decrease in Sox2 positive cells was observed in DISC1 shRNA transfected brains compared to control shRNA transfected brains (Figure S4A, B). Supporting the premature neuronal differentiation, we found that DISC1 knockdown in E15 brains resulted in a significantly higher proportion of Cux2 (layer 2 and 3 marker) positive cells at postnatal day 7 compared to the control. Furthermore, more control shRNA transfected cells were found to be migrating towards layer 3 (Figure S5A). Taken together, these results suggest that DISC1 knockdown causes premature neuronal differentiation at the expense of the progenitor pool.
Regulation of β-catenin signaling by DISC1
β-Catenin signaling is a conserved pathway implicated in maintenance of the stem cell pool (Lie et al., 2005; Zechner et al., 2003), neuronal differentiation (Hirabayashi et al., 2004), and development of the central nervous system (Schuller and Rowitch, 2007). Both DISC1 and β-catenin are highly enriched in neural progenitors in the VZ. Previous reports demonstrated that ablation of β-catenin expression in the developing brain results in the depletion of neural progenitors, whereas transgenic mice expressing stabilized β-catenin exhibit a drastically expanded progenitor pool in embryonic brains (Chenn and Walsh, 2002; Zechner et al., 2003). Thus, β-catenin and DISC1 share similar properties in regulating neural progenitors.
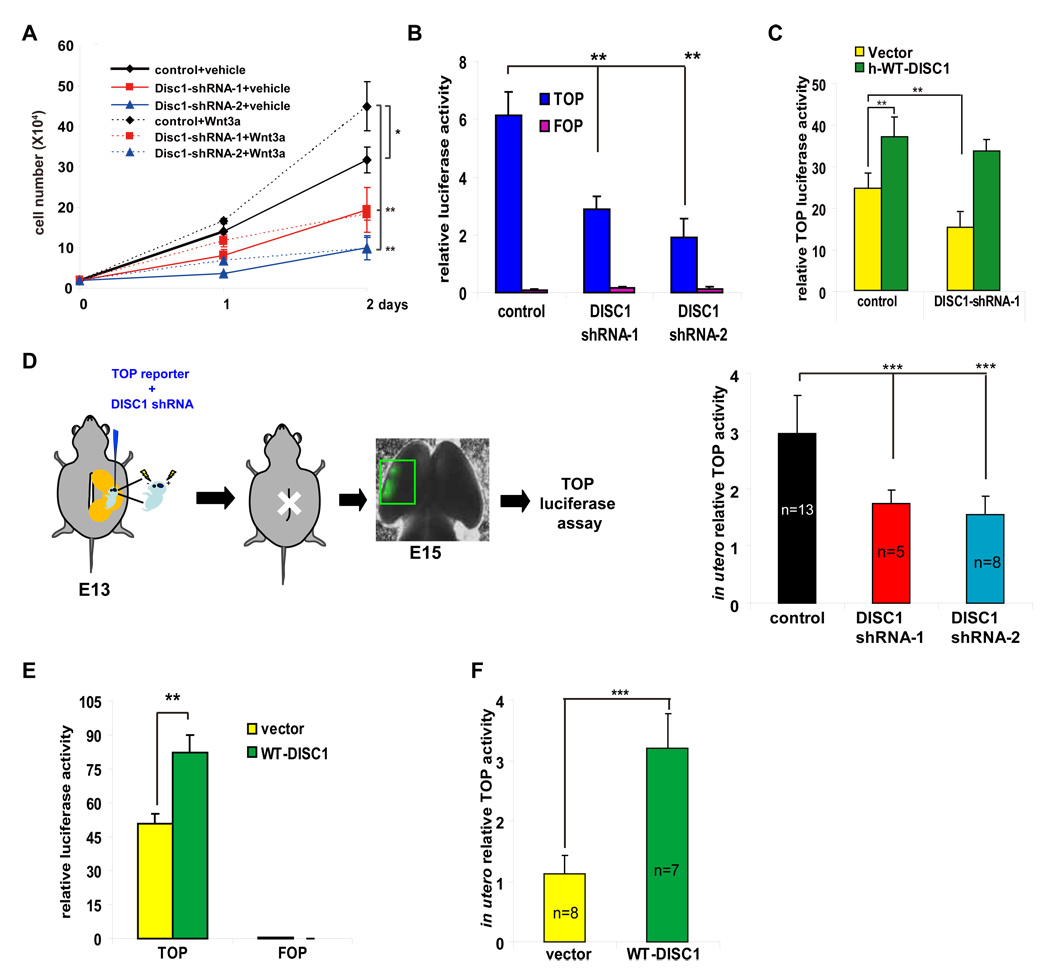
Since β-catenin is a central downstream effector of canonical Wnt signaling, we investigated the possibility that DISC1 may modulate Wnt-mediated proliferation. The addition of Wnt3a (200 ng/ml) to culture medium significantly stimulated the proliferation of AHPs, and this increase in proliferation was abolished by DISC1 knockdown (Figure 3A). This suggests that DISC1 function converges with downstream mediators of Wnt-dependent proliferation.
Figure 3. DISC1 regulates the β-catenin pathway.
(A) DISC1 knockdown cells exhibit proliferation defects in response to Wnt3a. Transduced AHPs were grown in medium with or without Wnt3a (200 ng/ml) and cell numbers were counted for 2 days (n=3, *p<0.05, **p<0.01).
(B) LEF/TCF activity (TOP) is decreased in DISC1-silenced primary neural progenitors. Luciferase activity with mutated LEF/TCF binding sites (FOP) is not affected by DISC1 shRNAs. The relative firefly luciferase activity normalized to Renilla luciferase activity is shown (n=6, p<0.01).
(C) TOP activity is rescued by human WT-DISC1 expression in DISC1-silenced primary neural progenitors (n=3, p<0.01).
(D) In utero TOP luciferase assay in DISC1 knockdown brains. Control or DISC1 shRNAs were coelectroporated with TOP and pRL-TK plasmids into E13 brains. Embryonic brains were harvested and subjected to luciferase assays 48 hours later. The relative TOP luciferase activity normalized to Renilla luciferase activity is shown (p<0.001).
(E) TOP activity is increased in primary neural progenitors overexpressing DISC1. Shown is the relative firefly luciferase activity normalized to Renilla luciferase activity (n=3, p<0.01).
(F) In utero TOP activity is increased with DISC1 overexpression. Luciferase activity is measured 24 hours after in utero electroporation (p<0.001).
β-Catenin exerts its function in part through nuclear translocation to stimulate the transcription of genes containing binding sites for the lymphoid enhancer factor-T cell factor (LEF/TCF) family (Gregorieff and Clevers, 2005). The transcription complex containing nuclear β-catenin activates the expression of target genes important for cell proliferation (Adachi et al., 2007). Since DISC1 knockdown abrogated Wnt-dependent proliferation, we determined whether DISC1 is required for LEF/TCF activation using a luciferase reporter construct containing 8 copies of the LEF/TCF binding site (8XSuperTOPFLASH (TOP)) or mutated LEF/TCF binding sites (8XSuperFOPFLASH (FOP)) (Veeman et al., 2003). DISC1 knockdown significantly reduced TOP, but not FOP, reporter activity in cultures treated with Wnt3a (Figure 3B). Importantly, TOP reporter activity could be rescued by co-expressing human DISC1 cDNA with DISC1 shRNA-1 (Figure 3C). The reduction in LEF/TCF activity by DISC1 knockdown could also be recapitulated in the developing brain (Figure 3D). Furthermore, we found that DISC1 knockdown had no effect on irrelevant CRE and C/EBP-ATF reporters, confirming the specific effect on LEF/TCF activity (Figure S6A, B). If DISC1 loss-of-function attenuates Wnt signaling, then DISC1 gain-of-function should potentiate this signaling pathway. DISC1 overexpression increased TOP, but not FOP, reporter activity in primary neural progenitor cultures (Figure 3E) and in embryonic brains by more than 2-fold (Figure 3F). Together, these results indicate that DISC1 participates in the Wnt signaling pathway and in Wnt-mediated cell proliferation.
Stable β-catenin expression overrides progenitor proliferation defects induced by DISC1 knockdown
To further decipher the mechanism by which DISC1 influences β-catenin and LEF/TCF activity, we created two β-catenin shRNAs that efficiently silenced endogenous β-catenin expression (Figure S7A). We found that the enhancement of LEF/TCF reporter activity by DISC1 overexpression was completely abolished when the expression of endogenous β-catenin was silenced (Figure 4A), indicating that the effects of DISC1 on LEF/TCF reporter activity require β-catenin. We further evaluated the effects of co-expressing WT-β-catenin, or a degradation-resistant mutant of β-catenin-S33A (SA-β-catenin) with control or DISC1 shRNAs on LEF/TCF reporter activity. We found that both WT and mutant β-catenin potentiated TOP reporter activity (Figure 4B). However, while DISC1 knockdown significantly down-regulated reporter activity in WT-β-catenin expressing cells, it had no effect on SA-β-catenin-mediated reporter activation (Figure 4B). This suggests that DISC1 acts upstream of β-catenin and impacts LEF/TCF transcription by regulating β-catenin abundance.
To further examine the relationship between DISC1 and β-catenin in brain development, we determined whether SA-β-catenin can rescue progenitor proliferation in vivo when DISC1 expression was silenced. As mentioned earlier, DISC1 knockdown reduced the percentage of GFP positive cells in the VZ/SVZ, increased GFP positive cells in the CP, and reduced BrdU labeling and the mitotic index (Figure 2A, B, C). Remarkably, co-expression of SA-β-catenin with DISC1 shRNA-1 completely rescued these phenotypes (Figure 4C, D). This observation underscores a major role for DISC1 in regulating progenitor proliferation by modulating β-catenin levels.
DISC1 regulates β-catenin abundance
Increased β-catenin levels rescued the defects caused by DISC1 knockdown, suggesting that DISC1 may regulate β-catenin abundance. Indeed, we found that DISC1 shRNAs significantly decreased β-catenin levels in AHPs (Figure 5A). GSK3β regulates β-catenin stability by phosphorylating serine and threonine residues (Ser33/37 and Thr41) important for targeting β-catenin for ubiquitin-dependent proteasomal degradation (Aberle et al., 1997). Notably, we observed that the reduction in β-catenin levels caused by DISC1 knockdown was accompanied by increases in Ser33/37 and Thr41 phosphorylation (Figure 5A) and β-catenin ubiquitination (Figure S7B). Thus, DISC1 loss-of-function reduces β-catenin abundance. We further evaluated the effect of DISC1 gain-of-function on β-catenin levels. Overexpression of WT-DISC1 reduced β-catenin S33/37/T41 phosphorylation, decreased ubiquitination, and increased total β-catenin levels in progenitors (Figure 5B & S7C). Collectively, these results suggest that DISC1 regulates β-catenin stability.
Figure 5. DISC1 regulates β-catenin stability.
(A) Phosphorylation of β-catenin is increased in DISC1 silenced AHPs. Bar graph shows the percentage of relative band intensity of pS33/37/T41-β-catenin compared to control (mean±S.E.M., n=3, p<0.05) .
(B) Phosphorylated β-catenin is reduced by DISC1 overexpression in AHPs. Bar graph shows the percentage of relative band intensity of pS33/37/T41-β-catenin to vector (mean±S.E.M., n=3, p<0.005) .
(C) Cyclin D1 and axin2 expression are reduced in DISC1 silenced AHPs. Bar graph shows the percentage of relative band intensity of cyclin D1 to control (mean±S.E.M., n=3, *p<0.05, **p<0.01).
(D) Cyclin D1 and axin2 expression is enhanced in DISC1 overexpressing AHPs. Bar graph shows the percentage of relative band intensity of cyclin D1 to vector (mean±S.E.M., n=3, p<0.05).
(E) GSK3β activity increases in DISC1 knockdown cells. Lysates from transduced AHPs were immunoblotted with the anti-pY216-GSK3β, pS231/234 Ngn2, or pT220/226 C/EBPα antibodies. Bar graph shows the percentage of relative band intensity of pY216-GSK3β or pS231/234 Ngn2 to control (mean±S.E.M., n=3, p<0.01).
(F) Overexpression of DISC1 suppresses GSK3β activity. Bar graph shows the percentage of relative band intensity of pY216-GSK3β or pS231/234 Ngn2to corresponding vector (mean±S.E.M., n=3, p<0.01).
If DISC1 is crucial for stabilizing β-catenin, one might predict that β-catenin transcriptional targets may be elevated by DISC1 gain-of-function. Cyclin D1 (Tetsu and McCormick, 1999) and axin2 (Leung et al., 2002) are well-established targets of β-catenin, and cyclin D1 promotes G1 progression during the cell cycle. In AHPs, cyclin D1 levels were reduced by 50% in DISC1 shRNA-1 expressing cells and by 70% in shRNA-2 expressing cells (Figure 5C). A similar decrease was observed with Axin2. Conversely, cyclin D1 and axin2 expression was markedly upregulated in DISC1 overexpression cells (Figure 5D). These data support the notion that DISC1 controls cell proliferation by regulating β-catenin abundance, thereby fine-tuning cell cycle progression.
DISC1 regulates GSK3β activity
The increase in β-catenin phosphorylation caused by DISC1 knockdown raised the possibility that GSK3β activity may be directly or indirectly influenced by DISC1. Growth factors such as insulin activate AKT, which in turn phosphorylates GSK3β at Ser9, an inhibitory phosphorylation site (Cross et al., 1995). Furthermore, GSK3β autophosphorylates itself at Tyr216 (Lochhead et al., 2006), which is required for its activity. Upon transduction of AHPs with DISC1 shRNA lentiviruses, we observed a significant increase in Y216 phosphorylation, suggesting that DISC1 negatively impacts GSK3β activity (Figure 5E). In contrast, Ser9 phosphorylation was not affected by DISC1 knockdown (Figure 5E). Furthermore, DISC1 overexpression reduced Y216 phosphorylation (Figure 5F), further supporting the role of DISC1 in inhibition of GSK3β activity. Notably, phosphorylation of other known GSK3β substrates, Ngn2 (Ma et al., 2008) and C/EBPα (Ross et al., 1999), was not affected by DISC1 shRNAs or overexpression. Thus, DISC1 selectively regulates the phosphorylation of certain GSK3β substrates.
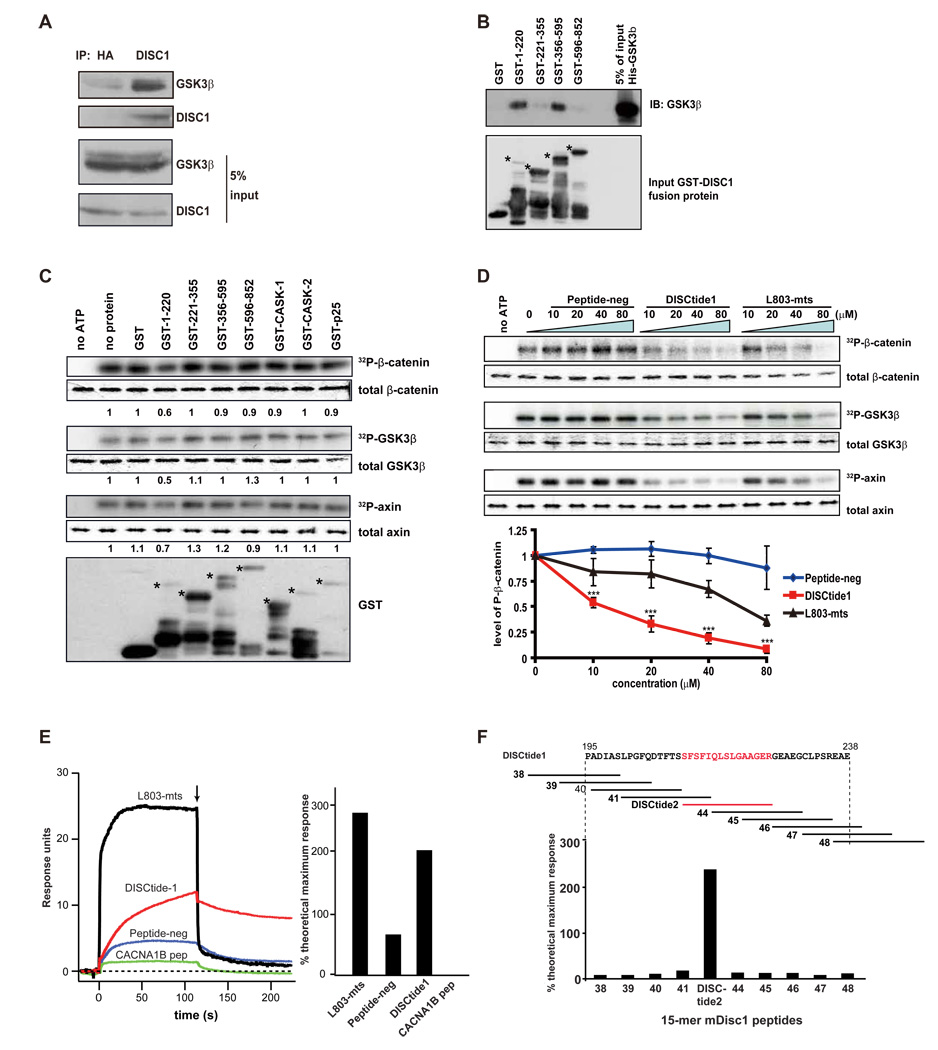
Consistent with its ability to regulate GSK3β activation, we found that DISC1 associates with GSK3β in E15 mouse embryonic brains (Figure 6A). To determine whether DISC1 and GSK3β directly bind each other and to map the region(s) of DISC1 required for the interaction, we generated GST-tagged DISC1 protein fragments and performed an in vitro association assay using purified His-tagged GSK3β (Figure 6B). Only DISC1 fragments spanning residues 1–220 and 356–595 exhibited strong interactions with GSK3β. Collectively, these results suggest that DISC1 directly interacts with GSK3β and inhibits its activity.
Figure 6. DISC1 regulates the GSK3β signaling pathway.
(A) DISC1 interacts with endogenous GSK3β. E14 brain lysate was subjected to immunoprecipitation with anti-HA (negative control) or anti-DISC1 antibody and immunoblotted with anti-GSK3β or DISC1 antibody.
(B) GSK3β directly binds to DISC1 in vitro. Purified His-GSK3β interacts directly with DISC1 fragments 1–220aa and 356–595aa. Stars indicate the intact GST fusion proteins.
(C) GSK3β activity is reduced by DISC1 GST-fragments in vitro. Numbers in the panel indicate the relative intensity of the band compared to the no protein lane (n=3). Stars indicate the different intact GST fusion proteins.
(D) Inhibition of GSK3β activity by DISC1 peptide 1(195–238aa) is dose-dependent. The dose-response curve is shown (n=3, ***, p<0.005).
(E) Disctide-1(195–238aa) directly binds to GSK3β Surface plasmon resonance sensorgrams for GSK3β binding assays with peptide-neg, Disctide-1, L803-mts, and a peptide from CACNA1B are shown in the left panel. The arrow indicates the injection of buffer without compound. The percent theoretical maximal response is summarized in the right hand panel for these SPR experiments.
(F) Disctide2 (211–225aa) directly binds to GSK3β The schematic shows Disctide1 (mDisc aa 195–238). Each numbered line represents a 15-mer peptide (i.e. peptide2–38 – - 48) that corresponds to the DISC1 amino acids above it. The percent theoretical maximal response for SPR binding assays at 25 µM between (Disctide1 15-mers and GSK3β is summarized at the bottom of the panel. Disctide2, indicated in red, was found to bind to GSK3β.
To examine the effect of DISC1 fragments on GSK3β activity, GST-DISC1 fragments, GST-CASK fragments, or GST-p25 were incubated with purified active GSK3β in the presence of the GSK3β substrates β-catenin and axin (Thomas et al., 1999) in vitro. We then analyzed the extent of GST-β-catenin phosphorylation, GST-axin phosphorylation, and GSK3β Y216 autophosphorylation. Consistent with its ability to bind GSK3β, the DISC1 fragment spanning residues 1–220 potently inhibited β-catenin, axin, and GSK3β phosphorylation at 0.5 µM, whereas none of the other DISC1 fragments, GST-p25, or GST-CASK proteins inhibited GSK3β activity at this concentration (Figure 6C). However, at higher concentrations (2 µM), DISC1 fragments spanning 221–355 and 356–595 started to inhibit GSK3β autophosphorylation (Figure S7D). This suggests that the inhibitory activity of DISC1 on GSK3β may reside on multiple domains, but that fragment 1 (1–220) possesses the most potent inhibitory activity. Furthermore, a dose response curve was established for DISC1 1–220 whereby no inhibition of GSK3β Y216 phosphorylation was observed at 0.1 µM and the inhibition of phosphorylation plateaued at 1 µM (Figure S7E). None of the GST-DISC1 fragments inhibited AKT autophosphorylation (data not shown) indicating that DISC1 is not a general kinase inhibitor. To narrow down the domain in DISC1 that inhibits GSK3β, we synthesized two peptides from mDISC1 which are highly conserved between human and mouse (Figure S8). The first peptide spanned amino acids 40–77 (Peptide-neg) and the second peptide spanned amino acids 195 to 238 (DISCtide-1). In vitro kinase assays demonstrated that Peptide-neg did not inhibit GSK3β at 80 µM, whereas DISCtide-1 inhibited GSK3β at 10 µM. We further found that DISCtide-1 inhibited GSK3β more potently than L803-mts, a previously described GSK3β peptide inhibitor (Plotkin et al., 2003) (Figure 6D).
Surface plasmon resonance (SPR) was used to determine whether DISCtide-1 directly binds to GSK3β. GSK3β SPR binding assays were carried out with mDISC1 Peptide-neg, DISCtide1, the GSK3β inhibitor L803-mts, and as a negative control, a peptide from the N-type calcium channel, CACNA1B (Figure 6E). We found that both DISCtide1 and L803-mts bound to GSK3β (percent theoretical maximal response 197% and 259%, respectively) at a concentration of 25 µM (Figure 6E), while both Peptide-neg and the calcium channel peptide showed much lower binding (percent theoretical maximal response 62% and 29%, respectively). Interestingly, the super-stoichiometric binding exhibited by both L803-MTS and DISCtide1 (259% and 197%, respectively) suggests that both bound to GSK3β in these assays at a peptide:protein ratio of 2:1. To identify the DISCtide1 sequences that mediate this interaction with GSK3β, we designed overlapping 15-mer peptides that covered DISCtide1 and tested their binding to GSK3β by SPR (Figure 6F). One 15-mer peptide that encompasses mDISC1 amino acids 211 to 225, number 43 (DISCtide2), bound to GSK3β, while all other DISCtide1 15-mers showed background levels of binding (Figure 6F). Similar to DISCtide1, DISCtide2 also showed super stoichiometric binding to GSK3β in these assays with a peptide:protein ratio of 2:1 (percent maximal binding 238%). Further characterization of DISCtide2 is shown in Figure S9. Importantly, a reversed DISCtide2 sequence displayed negligible binding to GSK3β demonstrating that the binding of DISCtide2 to GSK3β is specific. Despite the specific binding to GSK3β, DISCtide2 failed to inhibit GSK3β kinase activity (data not shown), suggesting that other regions of DISCtide1 are also required for the inhibition.
DISC1 regulates progenitor proliferation by inhibiting GSK3β
If DISC1 inhibits GSK3β activity, we speculated that the negative effects of DISC1 knockdown on proliferation should be alleviated by GSK3β loss-of-function. To test this possibility, we examined the consequence of treating DISC1 knockdown AHP cells with SB-216763, a specific chemical inhibitor of GSK3. SB-216763 restored cell proliferation as evaluated by BrdU incorporation (Figure S10A). SB-216763 also rescued neural progenitor proliferation defects in vivo. SB216763 increased BrdU labeled cells in control shRNA electroporated mouse embryonic brains (Figure S10C) and rescued the BrdU labeling index in DISC1 shRNA electroporated embryonic brains. Consistent with these results, we further found that the GSK3β inhibitors SB-216763 and CHIR-99021 rescued TCF activity in DISC1 knockdown cells to control levels (Figure S10B). Finally, we tested the consequence of overexpressing GSK3β in the embryonic brain. While GSK3β overexpression reduced the number of BrdU labeled cells (Figure S10D), this was suppressed by DISC1 coexpression. Taken together, these results provide further evidence that DISC1 regulates neural progenitor proliferation by inhibiting GSK3β.
DISC1 regulates adult neural progenitor proliferation by inhibiting GSK3β
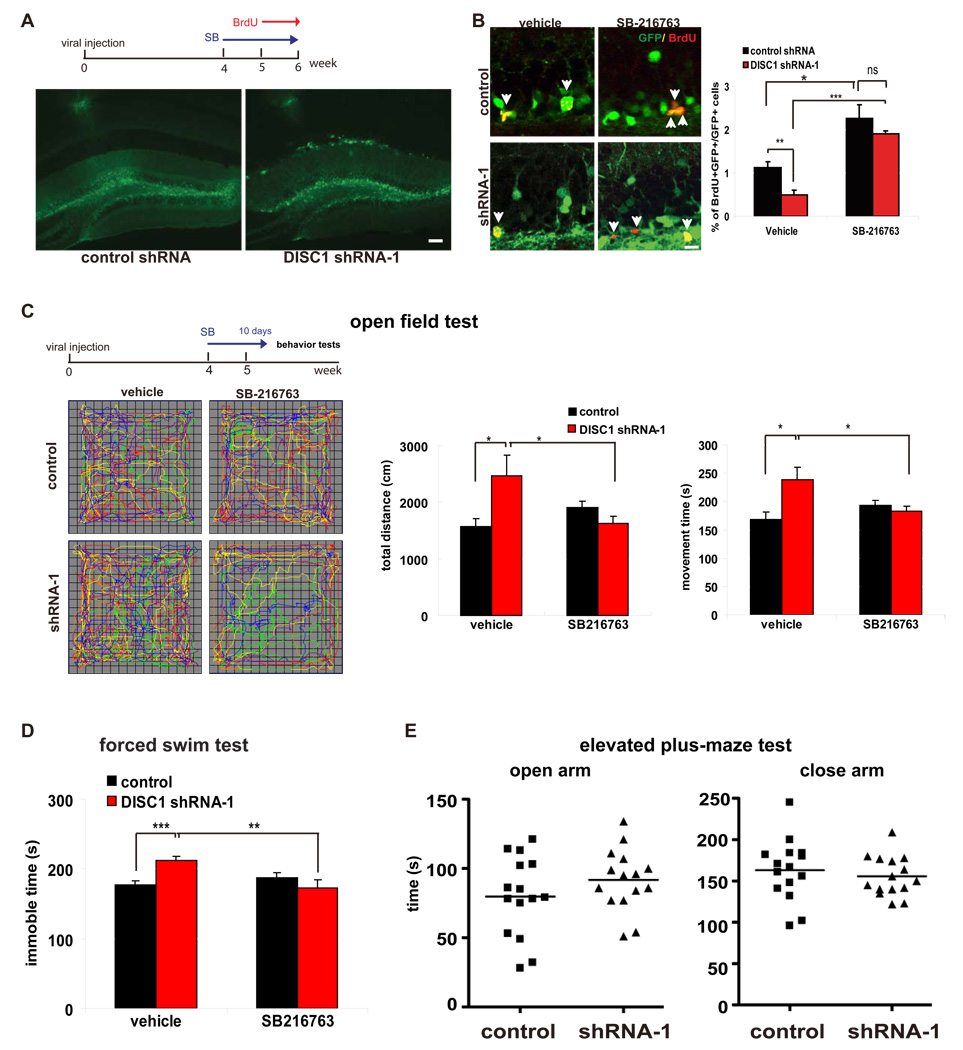
In the adult dentate gyrus, DISC1 is expressed in neural progenitors and neurons, but is absent in astrocytes (Figure S11A–C). To determine whether DISC1 regulates hippocampal progenitor proliferation, we stereotactically injected control or DISC1 shRNA-1 lentivirus into the dentate gyrus of adult mouse brains. Five weeks later, we administered daily injections of BrdU over the course of 7 days (Figure 7A). Lentivirus expressing control or DISC1 shRNA showed comparable infection rates in the dentate gyrus as revealed by the GFP signal (Figure 7A). Consistent with our observations in embryonic brains, we observed a significant reduction in BrdU incorporation in the DISC1 shRNA-1 group compared to the control shRNA group (Figure 7B). This reduction was not due to increased cell death, as there was no increase in active caspase 3 labeling of GFP positive cells in the dentate gyrus (Figure S12). To determine whether this effect was mediated through GSK3β activation, vehicle or SB-216763 (2 mg/kg) was administered to mice every other day for 2 weeks (4 weeks after the administration of virus) (Figure 7A). Intriguingly, SB-216763 treatment significantly increased the number of BrdU positive cells in mice infected with the control virus (Figure 7B) and rescued the reduction in BrdU positive cells in DISC1 shRNA-1 injected mice. These results suggest that DISC1 also regulates the proliferation of adult hippocampal progenitors by inhibiting GSK3β activity. Consistent with a previous report (Duan et al., 2007), we also observed aberrant positioning and increased complexity of dendritic morphology in DISC1 knockdown granule neurons (data not shown).
Figure 7. GSK3β inhibitor rescues the proliferation and behavior defect caused by DISC1 knockdown in adult mice.
(A) Control or DISC1 shRNA-1 lentivirus was stereotactically injected into adult dentate gyrus. After 4 weeks of recovery, mice received SB216763 (2 mg/kg) every other day for 2 weeks and BrdU (100 mg/kg) daily for 7 days. The GFP signal represents lentiviral infected cells in dentate gyrus. Scale bar=50 µm.
(B) DISC1 knockdown reduced adult progenitor proliferation in dentate gyrus, but this defect can be rescued by SB216763. The percentage of GFP and BrdU double positive cells is shown (n=5, *, p<.0.05; **, p<0.01; ***, p<0.005; ns, not significant). Scale bar=10 µm.
(C) DISC1 knockdown in adult mice leads to hyperlocomotion in a novel environment. DISC1 knockdown mice showed ieaterg distance traveled, movement time in a novel open field, which can be reversed by SB216763 treatment (n≥8 for each group, p<0.05).
(D) DISC1 knockdown in adult mice increased immobility in the forced swimming test, which was rescued by SB216763 (n≥8 for each group, **, p<0.01; ***, p<0.005).
(E) No behavior changes were detected in the levated plus maze test (n=15, for each group).
Behavioral consequences of DISC1 loss-of-function and GSK3β inhibition
Alterations in neurogenesis are implicated in the development of depression and other abnormal behaviors. To examine the behavioral consequences of disrupting the DISC1/GSK3β pathway, we silenced DISC1 expression in the adult dentate gyrus and evaluated behavioral consequences. Four weeks after the injection of control or DISC1 shRNA-1 lentivirus, mice were treated with vehicle or SB-216763 (2 mg/kg i.p.) every other day for 10 days. Compared to control mice, DISC1 shRNA injected mice exhibited hyperlocomotion in response to novelty, which is considered a model of positive symptoms of schizophrenia. DISC1 shRNA injected mice traveled a greater distance and spent more time moving in a novel open field than control mice (Figure 7C). These behaviors were normalized by SB-216763 treatment. We further tested whether DISC1 loss-of-function had consequences on depression-like behavior in the forced swim test. DISC1 shRNA infected mice displayed greater immobility (Figure 7D), an indicator of depressive behavior, which was also suppressed by SB-216763. Importantly, swimming velocity was unchanged (data not shown). DISC1 shRNA infected mice did not display increased anxiety, as there was no difference in time spent in the closed arms versus the aversive open arms in the elevated plus-maze (Figure 7E). Thus, increased GSK3β activity caused by DISC1 loss-of-function is associated with schizophrenia- and depression-like behaviors.
Discussion
The DISC1 gene shows close association with psychiatric illness in humans. We demonstrate here that DISC1 is required for neural progenitor proliferation during embryonic brain development and in the adult dentate gyrus. This is a surprising result given that previous studies have focused on roles for DISC1 in postmitotic neurons. We provide multiple lines of evidence supporting a role for DISC1 in regulating progenitor proliferation by modulating GSK3β activity and β-catenin abundance. Our results provide important insight into the pathophysiology of psychiatric disorders and offer potential avenues for therapeutic intervention.
DISC1 inhibits GSK3β by direct physical interaction
We found that two different domains of DISC1, spanning amino acids 1–220 and 356–595, directly associate with purified GSK3β in an in vitro binding assay. The finding that GSK3β can bind to multiple distinct DISC1 domains is similar to that observed with the DISC1-PDE4B interaction (Murdoch et al., 2007). The interaction of DISC1 with GSK3β directly impacts GSK3β catalytic activity, as evidenced by the inhibition of recombinant GSK3β catalytic activity by the DISC1 195–238 (DISCtide-1) fragment in vitro (Figure 6D). Inhibition of GSK3β by this peptide was dose dependent and more potent than L803-mts, a commercial peptide inhibitor. L803-mts is structurally similar to a GSK3β substrate and acts as a selective, substrate-specific, competitive inhibitor (Plotkin et al., 2003). The amino acid sequence of DISCtide1 shows no similarity to L803-mts and was not phosphorylated by GSK3β (data not shown), suggesting that DISCtide1 is not a direct substrate of GSK3β However, we cannot exclude the possibility that the structure of this peptide may mimic a GSK3β substrate and act as a substrate-specific inhibitor.
Implication of the DISC1/GSK3β/β-catenin interaction in behavioral outputs
GSK3β is involved in several signaling pathways implicated in mental illness. It is a downstream mediator of dopamine signaling via the dopamine D2 receptor/β-arrestin 2/phosphatase 2A complex (Beaulieu et al., 2005). The dopamine D2 receptor is a target of many anti-psychotic drugs. Likewise, neuregulin-1 signaling via Akt, both implicated in schizophrenia risk, regulates GSK3 activity. Furthermore, our observation that constitutively stable SA-β-catenin and specific GSK3β inhibitors can override the negative effects of DISC1 loss-of-function on cell proliferation in vitro and in vivo also suggest that GSK3 may be a reasonable target for therapeutic intervention.
Additional support for the involvement of DISC1/GSK3β/β-catenin in psychiatric illness comes from rodent behavioral models. It was shown previously that genetic or pharmacological inhibition of GSK3β reduced amphetamine-induced hyperactivity in mice (Beaulieu et al., 2004), a model of mania-like behavior. Furthermore, the overexpression of β-catenin in the mouse brain phenocopies the effects of lithium chloride in the amphetamine model (Gould et al., 2007). Interestingly, the forebrain-specific β-catenin conditional knockout (includes the hippocampus) exhibits depression-like behavior in the forced swim test, but not in other mood- or anxiety-related tests (Gould et al., 2008). This is consistent with our finding that compromising DISC1 function in the adult dentate gyrus resulted in hyperactivity and depression-like behaviors, and that these behaviors were rescued by treatment with SB216763 (Figure 7C&D). These results not only link DISC1 regulated adult neurogenesis with behavioral outputs, but also underscores a critical role for DISC1 in fine-tuning GSK3β-mediated signaling events. In this light, DISC1 function somewhat resembles lithium chloride, a well established medication for bipolar disorder that directly and indirectly inhibits GSK3 activity (Beaulieu et al., 2008; Harwood, 2005). Future studies will be needed to address whether lithium and DISC1 inhibit GSK3 through the same mechanism.
Ablation of adult neural progenitors is associated with impaired learning behaviors and synaptic plasticity. Furthermore, anti-depressants increase neurogenesis in the dentate gyrus and ablation of adult neurogenesis renders animals resistant to anti-depressants (Santarelli et al., 2003). Conversely, increased neuronal excitability in the hippocampus is associated with hyperactivity and impaired learning (Peters et al., 2005). Adult-generated immature neurons integrate into preformed spatial memory circuitry (Kee et al., 2007) and a failure of newly born neurons to integrate into previously established circuits results in impaired learning (Farioli-Vecchioli et al., 2008). Taken together, we suggest that behavioral abnormalities resulting from DISC1 loss-of-function in the dentate gyrus likely involve a combination of reduced numbers of newly born neurons and their aberrant integration into the existing circuitry, as well as effects on mature neurons.
Gestational disruption of brain development in rats leads to a reduction of prefrontal cortex and hippocampus size, resulting in schizophrenic behavior changes (Flagstad et al., 2004). Decreases in neural stem cell proliferation have been reported in schizophrenia patients (Reif et al., 2006), and anti-psychotics increase neurogenesis in the hippocampus (Newton and Duman, 2007). In this study, we found that DISC1 is essential for neural progenitor proliferation in embryonic brains and in the dentate gyrus of adult brains through its ability to fine-tune GSK3β activity. Thus, DISC1 loss-of-function may tip the balance between progenitor proliferation, neuronal differentiation, and integration of newly born neurons, ultimately impacting the neural circuitry and predisposing to an increased risk for compromised cognition and behavioral abnormalities (Figure S13).
Experimental procedures
Cell proliferation analysis
N2a and AHP cells were transduced with lentivirus expressing DISC1 shRNA-1, DISC1 shRNA-2 or WT-hDISC1 for 2 days. The transduced cells were sorted by FACS based on GFP expression. 2×104 cells were seeded into 12 well plates with medium and cell number was counted each day for 2 days (AHP) or 3 days (N2a cells). In the AHP proliferation assay, recombinant Wnt3a protein (200ng/ml) or vehicle was added to the medium to determine the proliferation in response to Wnt3a stimulation.
In utero electroporation
E13 mice were used for in utero electroporation as described previously (Sanada and Tsai, 2005). For cell cycle exit and rescue experiments, mice were intraperitoneally (i.p.) injected with BrdU (100 mg/kg) 2 days after electroporation and sacrificed 24 hours later. Detailed procedures are provided in the supplemental methods.
Immunohistochemistry and immunocytochemistry
Immunohistochemistry and immunocytochemistry were performed as described previously (Sanada and Tsai, 2005). Transduced AHPs were seeded onto polyornithine and fibronectin coated coverslips. BrdU (10 µM) was added to the culture medium for 2 hours. Cells were fixed in 4% paraformaldehyde and stained with anti-BrdU or pH3 antibodies. Five random fields from each experiment were obtained and over 500 cells were counted for each experiment.
Luciferase assays
5×105 transduced AHPs, embryonic progenitors, or 293T cells were seeded into 24-well plates and transfected with 0.8 µg of Super8XTOPFLASH or Super8XFOPFLASH and 0.1 µg of pRL-TK using Lipofectamine 2000 (Invitrogen). 24 hours after transfection, transfected cells were stimulated with Wnt3a-conditioned medium (Wnt3a CM) for 14 hours and TCF reporter activity was measured using the Dual-Luciferase Assay System (Promega). Detailed procedures are provided in the supplemental methods.
Stereotaxic injection
1 µl of high titer lentivirus (~2×109 transducing units/ml) was injected into the dentate gyrus of 8 week old mice bilaterally (AP -2, ML ±1.5, DV −1.8 from Bregma, n=15 per group). Viral spread was determined by determining the presence of GFP positive cells along the anterior–posterior axis of the dentate gyrus (about 1.2–1.6 mm) (Fig. S14). Following 4 weeks of recovery, mice i.p. injected with SB216763 (2 mg/kg) every other day for another two weeks. SB-216763 has been previously reported to cross the blood-brain barrier after intraperitoneal injection (Selenica et al., 2007). BrdU (100mg/kg) was injected daily for the last week. The mice were then perfused with 4% paraformaldehyde and the brains were processed for staining with an anti-BrdU antibody.
Mouse behavior
C57/B6J male mice were obtained from Jackson Lab. 4 weeks after stereotaxic injection, mice were treated with either vehicle or SB216763 (2 mg/kg i.p.) every other day for another 10 days before behavior test. Activity in a novel open field (40cm × 40cm × 30cm) over a 10 minute period was measured using an Accuscan Instruments VersaMax Activity Monitoring system. The forced swim test was carried out in a 20cm diameter × 25cm high plexiglass cylinder filled to 13cm depth with water. Behavior over a 6 minute period was recorded and analyzed with a Noldus EthoVision XT video behavior recognition system. The elevated plus-maze was conducted as described previously (Clapcote et al., 2007). Statistical analysis was performed using both the student t-test and ANOVA (n≥8 for each group, mean ± SEM).
Surface plasmon resonance
For complete details see supplemental methods.
Supplementary Material
Acknowledgements
We thank Dr. A. Sawa for providing the human DISC1 cDNA construct, Dr. C. Lois for the FUGW construct, Dr. X. He for FLAG-Dvl2, HA-GSK3β, FLAG-WT-β-catenin, and SA-β-catenin constructs, Dr. Y. Ma and Dr. M. Greenberg for Ngn2 antibodies, and Dr. F. Gage for the AHP cells. We acknowledge Drs. E. Scolnick, B.A. Samuels, Z. Xie, D. Meletis, M. Carlen, C. Bragg, J. Buchman, P. Thanawala, and M. Dobbin for technical support, helpful discussion, and critical reading of the manuscript. L.-H. T. is an investigator of the Howard Hughes Medical Institute and the Director of the Neurobiology program at the Stanley Center for Psychiatric Research. Y.W.M. is a recipient of the NARSAD Young Investigator Award.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- International-Schizophrenia-Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lonnqvist J, Huttunen MO, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu Y, Ky B, Shughrue PJ, Austin CP, Morris JA. Cloning and characterization of Disc1, the mouse ortholog of DISC1 (Disrupted-in-Schizophrenia 1) Genomics. 2002;80:662–672. doi: 10.1006/geno.2002.7012. [DOI] [PubMed] [Google Scholar]
- Ma YC, Song MR, Park JP, Henry Ho HY, Hu L, Kurtev MV, Zieg J, Ma Q, Pfaff SL, Greenberg ME. Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron. 2008;58:65–77. doi: 10.1016/j.neuron.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Duman RS. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21:715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- Plotkin B, Kaidanovich O, Talior I, Eldar-Finkelman H. Insulin mimetic action of synthetic phosphorylated peptide inhibitors of glycogen synthase kinase-3. J Pharmacol Exp Ther. 2003;305:974–980. doi: 10.1124/jpet.102.047381. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBPalpha kinase. Mol Cell Biol. 1999;19:8433–8441. doi: 10.1128/mcb.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schuller U, Rowitch DH. Beta-catenin function is required for cerebellar morphogenesis. Brain Res. 2007;1140:161–169. doi: 10.1016/j.brainres.2006.05.105. [DOI] [PubMed] [Google Scholar]
- Selenica ML, Jensen HS, Larsen AK, Pedersen ML, Helboe L, Leist M, Lotharius J. Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol. 2007;152:959–979. doi: 10.1038/sj.bjp.0707471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.