Abstract
In elderly individuals high levels of interleukin-1β (IL-1β) in the brain have been implicated in infection-related behavioral pathologies but this has not been directly tested. Therefore, the current study investigated if sickness behavior in aged animals elicited by peripheral injection of lipopolysaccharide (LPS) is mediated through central IL-1β. Adult and aged mice were injected intracerebroventricularly with either saline or IL-1ra (4 μg) immediately prior to intraperitoneal administration of saline or LPS (10 μg) and locomotor and social behaviors were assessed. As anticipated, LPS depressed locomotor activity and social behavior in both adult and aged mice but the behavioral deficits were markedly greater in the aged at 24 h. Pretreatment with IL-1ra did not affect LPS-induced sickness behavior in adults; however, in aged mice IL-1ra attenuated LPS-induced sickness behavior, restoring it to the level exhibited by young adults. Twenty-four h post injection hippocampal and hypothalamic tissues were collected to determine IL-1β mRNA expression. Neither LPS nor IL-1ra affected IL-1β mRNA levels in adults, presumably because any effect of LPS had dissipated by 24 h. In contrast, IL-1β mRNA was markedly higher in aged mice 24 h after LPS, and prior treatment with IL-1ra either blocked or attenuated this effect in the hippocampus and hypothalamus, respectively. Taken together these data provide the first direct evidence that central IL-1β is responsible for the severe sickness behavior observed in aged animals after LPS treatment. Thus, inhibiting the central actions of IL-1β may be useful for minimizing behavioral complications in older individuals with an infection.
Keywords: aging, brain, behavior, infection, IL-1β and IL-1ra
1. Introduction
Acute cognitive disorders such as delirium have been recognized as a frequent manifestation of peripheral infections in the elderly (Jackson et al., 2004; Wofford et al., 1996). Indeed demented elderly patients are routinely screened for bladder infections because of the close association between infection and behavioral disorders, and a striking characteristic of pneumonia in the elderly is that it commonly presents clinically as delirium (Janssens and Krause, 2004). The correlation between peripheral infection and behavioral disorders in the elderly is important because behavioral impairment leads to poor self care behavior (e.g., anorexia, weight loss, and noncompliance) and may ultimately increase rates of hospitalization and mortality (Armstrong et al., 1999; Jackson et al., 2004; Johnston et al., 1987; Wofford et al., 1996). Currently, the mechanisms underlying infection-related behavioral disorders in the elderly are unknown.
During a peripheral infection, the immune system conveys a message to the brain and microglial cells respond and produce proinflammatory cytokines that help coordinate a behavioral response that is normally adaptive (Hart, 1988). However, excessive production of proinflammatory cytokines in the brain can produce severe behavioral deficits that are maladaptive (Dantzer et al., 2008). Recent studies of possible causes of infection-related behavioral disorders in the elderly indicated that in aged rodents peripheral infection induced an exaggerated proinflammatory cytokine response in the brain and signs of behavioral pathology not seen in younger cohorts, including prolonged anorexia (Godbout et al., 2005), depressive-like behavior (Godbout et al., 2008), and cognitive dysfunction (Barrientos et al., 2002; Chen et al., 2008). Although aging appears to prime microglial cells to produce excessive levels of proinflammatory cytokines in response to signals from the peripheral immune system (Dilger and Johnson, 2008; Godbout et al., 2005; Godbout and Johnson, 2006; Henry et al., 2008; Perry, 2004), it is not known if the discordant central cytokine response underlies the aberrant behavioral response.
To begin understanding this issue, the present study examined the contribution of interleukin-1β (IL-1β) in the brain to infection-related behavioral deficits in aged mice. Specifically, we evaluated sickness behavior in young adult and aged mice treated intracerebroventricularly (ICV) with IL-1 receptor antagonist (IL-1ra) followed by intraperitoneal injection of LPS. Additionally, IL-1β mRNA was measured in the hippocampus and hypothalamus. We focused on IL-1β because central administration of recombinant IL-1β is well known to induce a constellation of behavioral changes reminiscent of an infection (Dantzer, 2001; Kent et al., 1992). Moreover, the expression of IL-1β has been shown to be consistently higher in the brains of old mice compared to younger cohorts after peripheral injection of LPS (Chen et al., 2008; Godbout et al., 2005; Henry et al., 2008). The results showed that centrally administered IL-1ra abrogated sickness behavior in aged mice but not adults. Therefore, inhibiting the central actions of IL-1β may be useful for minimizing behavioral complications in older individuals with an infection.
2. Materials and Methods
2.1. Animals
Adult (3- to 6-mo-old) and aged (22- to 24-mo-old) male BALB/c mice from our in-house specific pathogen free colony were used. Mice were housed individually in polypropylene cages and maintained at 21°C under a reverse phase 12 h light-dark cycle with ad libitum access to water and rodent chow. Male juvenile conspecifics (4- to 5-wk-old) used in the social exploratory behavior paradigm were maintained under identical conditions. At the end of each study, mice were examined postmortem for signs of disease (e.g. splenomeglia and tumors). Data from mice determined to be unhealthy were excluded from analysis. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Intracerebroventricular Cannulation
Mice were prepared with an intracerebroventricular (ICV) cannula as described previously (Abraham et al., 2008). In brief, mice were anesthetized with an intraperitoneal (i.p.) injection of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). They were positioned in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA) so that the plane formed by the frontal and parietal bones was parallel to horizontal zero. A 26-gauge stainless steel cannula (Plastics One, Roanoke, VA) was placed in the left lateral cerebral ventricle according to predetermined coordinates (lateral 1.6 mm and anteroposterior 1 mm to the bregma, and horizontal 2 mm from the dura mater). The cannula was secured using stainless steel screws and cranioplastic cement (Plastics One). Mice were injected subcutaneously with buprenorphine (0.05 mg/kg) following surgery and then again 8–12 h later. Mice were provided a minimum of 5 d to recover before any treatment or behavioral test.
2.3. Behavioral Tests
Locomotor activity and social exploratory behavior were measured as described previously (Abraham et al., 2008). In brief, mice were handled 2 min each day for 5 d before experimentation to acclimate them to routine handling. Tests were conducted during the dark phase (between 0800 and 1700) of the photoperiod under infrared lighting to aid video recording.
Locomotor activity
Mice were maintained in their home cage and locomotor activity was video recorded during 3 min tests. On the video records, cages were divided into six identical rectangles and a trained observer who was blind to experimental treatments determined the incidence of line crossing.
Social exploratory behavior
Immediately after the locomotor test, a novel juvenile conspecific was introduced into the test subject’s home cage for a 10 min period to assess the motivation to engage in social exploratory behavior. Mice were videotaped, and the duration engaged in social investigation was determined from the video records by a trained observer who was blind to experimental treatments. Social behavior was determined as the amount of time that the experimental subject spent investigating (e.g., anogenital sniffing, trailing) the juvenile and the results are expressed as percent depression in time engaged in social behavior compared with age-matched baseline controls.
2.4. Quantitative Real-Time PCR
Total hippocampal RNA was isolated from brain using the Tri Reagent protocol (Sigma, St. Louis, MO). RNA samples were subjected to a DNAse I digestion procedure and then reverse transcribed to cDNA using a RT Retroscript kit (Ambion, Austin, TX). Total hypothalamic RNA was isolated using the Arcturus PicoPure™ RNA isolation kit as described by the manufacturer. DNase treatment was performed on a PicoPure column with a Qiagen RNase-free DNase set (Qiagen, Valenica, CA). Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Abraham et al., 2008). In brief, cDNA was amplified by PCR where a target cDNA (IL-1β, Mm00434228_m1) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed at the following conditions: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
2.5. Experimental Protocol
Recombinant IL-1ra (Cat. No. 480-RM/CF, R&D Systems, Minneapolis, MN) and Escherichia coli LPS (Serotype 0127:B8, Sigma, St. Louis, MO) were dissolved in sterile saline immediately prior to an experiment. At the onset of the dark phase, spontaneous locomotor activity and social exploratory behavior of adult (n=40) and aged (n=40–44) mice were evaluated. Immediately after the behavioral tests, mice were infused ICV with 2 μl of sterile saline or IL-1ra (4 μg) over a 30 sec period using a 28-gauge injection cannula, Hamilton syringe and syringe pump. After each injection, the injection cannula was left in place for an additional 1 min to allow diffusion of the solution away from the cannula tips. Immediately following ICV injection, mice received a 100 μl i.p. injection of sterile saline or LPS (10 μg). Thus the eight treatments comprised the 2 × 2 × 2 factorial arrangement of age (adult or aged), IL-1ra (0 or 4μg) and LPS (0 or 10 μg). After injections, mice were returned to their home cage and locomotor and social behaviors were determined again 2, 4, 8 and 24 h later. After completion of behavioral testing, mice were killed by CO2 asphyxiation and hippocampal and hypothalamic brain regions were collected to determine steady-state levels of IL-1β cytokine mRNA. In brief, the brain was placed ventral side up and using curved forceps whole hypothalamus was removed. The boundaries of the hypothalamus were: rostral, just posterior to the optic chiasm; lateral, the choroidal fissures; and caudal, just anterior to the mammillary bodies. The brain was then placed dorsal side up and the cerebral cortex was carefully removed to expose the hippocampus. The whole hippocampus was removed from surrounding brain tissue for subsequent analysis.
2.6. Statistical Analysis
Data analysis was completed using the Mixed Procedure of the Statistical Analysis System (SAS Inst., Cary, NC). All data were subjected to a univariate analysis to ensure normality. Behavioral results were subjected to a three-way ANOVA using repeated measures in which test hour (0, 2, 4, 8 and 24 h) was a within subjects measure, and age (adult or aged), IL-1ra (saline or 4 μg/mouse), and LPS (saline or 10 μg/mouse) were between subjects measures. Cytokine mRNA levels were analyzed using a three-way ANOVA in which age (adult or aged), IL-1ra (saline or 4 μg/mouse) and LPS (saline or 10 μg/mouse) were between subjects measures. Post hoc Student’s t test of least square means with a Tukey adjustment was employed to determine if treatment means were significantly different from one another (p<0.05). All data are presented as means ± standard error of mean.
3. Results
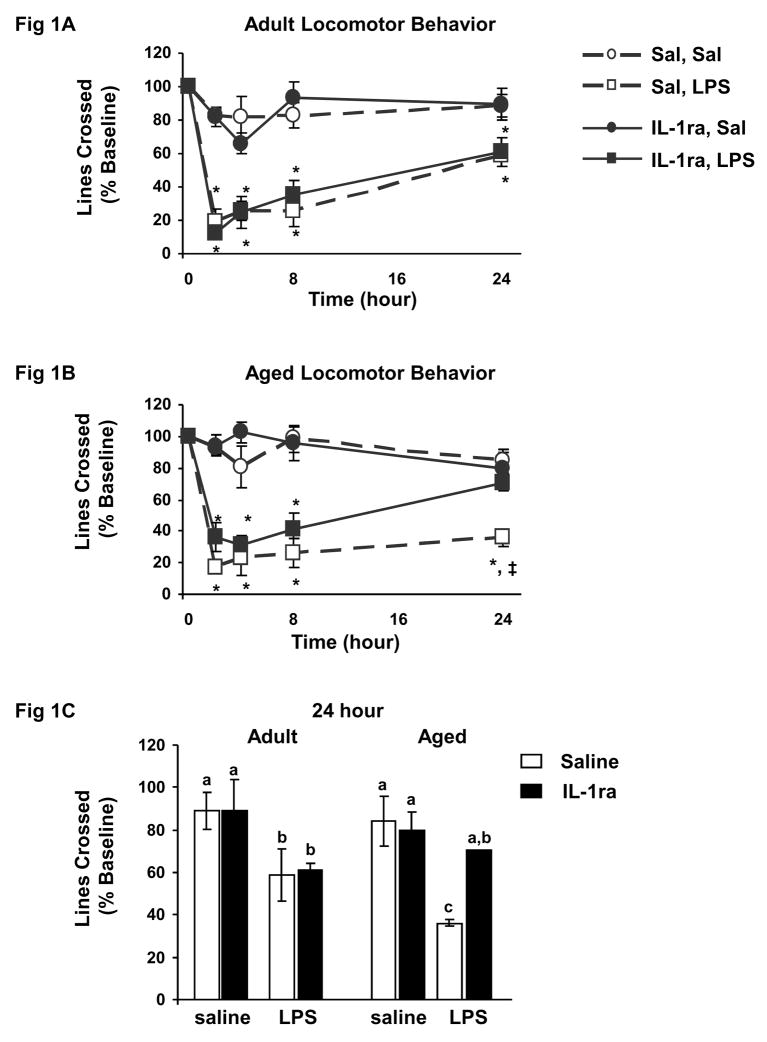
To assess the specific role of IL-1β in mediating behavioral deficits associated with peripheral immune activation in the aged, young adult and aged mice were pretreated centrally with either saline or IL-1ra and immediately injected peripherally with saline or LPS. Three-way ANOVA of spontaneous locomotor activity (Fig. 1A–C) revealed significant main effects of IL-1ra (F (1, 234) = 4.68, p=0.03) and LPS (F (1, 234) = 95.04, p<0.0001), as well as a trend for an age × IL-1ra interaction (F (1, 234) = 2.74, p=0.06). Post-hoc comparisons showed LPS reduced spontaneous locomotor activity similarly in both adult and aged mice 2–8 h post injection. However, while adult mice showed improvement (i.e., locomotor activity approached baseline level) 24 h after injection of LPS, depressed locomotor activity persisted in aged mice (t (41) = −3.07, p=0.06). Pretreatment with IL-1ra did not affect LPS-induced sickness behavior in adults at anytime post injection. However, the longer lasting depression of locomotor activity seen in aged mice treated with LPS was completely blocked by IL-1ra. To better illustrate this point, Figure 1C shows spontaneous locomotor activity of the eight treatment groups 24 h post injection.
Figure 1. IL-1ra protected aged mice but not adult mice from LPS-induced deficits in locomotor behavior.
Adult and aged mice were injected ICV with saline or IL-1ra and then injected i.p. with saline or LPS. Locomotor activity in adult mice (A) and locomotor activity in aged mice (B) were measured before injections and 2, 4, 8 and 24 h after injections. Data points represent the mean ± S.E.M. (n=10–11). Means with * are significantly different (p<0.05) from age-matched controls and means with ‡ are significantly different from mice given IL-1ra and LPS. Locomotor behavior for both adult and aged mice at 24 h is also shown (C). Bars represent the mean ± S.E.M. (n=10–11). Means with different letters (a, b or c) are significantly different (p<0.05) from each other.
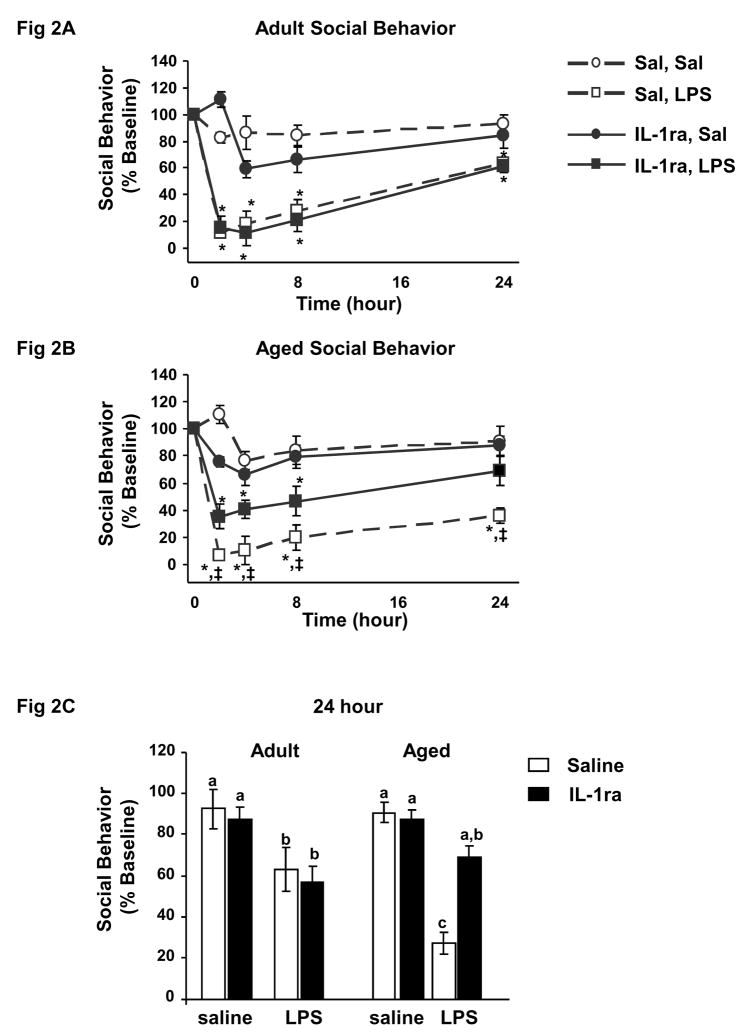
A similar but more pronounced effect was observed in the social exploration task (Fig. 2A–C). Three-way ANOVA of social behavior revealed significant main effects of IL-1ra (F (1, 222) = 4.74, p=0.03), LPS (F (1, 222) = 111.52, p<0.0001) as well as a significant age × IL-1ra interaction (F (1, 222) = 5.67, p=0.01). As expected, post-hoc analyses revealed that LPS depressed social behavior similarly in both adult and aged mice 2–8 h post injection. As seen in locomotor activity, social behavior of adult mice showed improvement 24 h after injection of LPS but that of aged mice remained substantially depressed (t (38) = −6.69, p<0.0001). Pretreatment with IL-1ra did not affect LPS-induced depression of social behavior in adult mice at anytime post injection. However, pretreatment with IL-1ra ameliorated LPS-induced depression in social behavior in aged mice as early as 4 h post injection (t (38) = 3.50, p=.02; Fig. 2A–B). At 24 h, social behavior of aged mice given IL-1ra prior to LPS returned to baseline, while social behavior of aged mice given saline prior to LPS was still depressed by 73%. To better illustrate this point, Figure 2C shows the social behavior of the eight treatment groups 24 h post injection. Taken together, these data show that centrally administered IL-1ra inhibits sickness behavior caused by a peripheral injection of LPS in aged mice but not in young adult mice.
Figure 2. IL-1ra protected aged mice but not adult mice from LPS-induced deficits in social behavior.
Adult and aged mice were injected ICV with saline or IL-1ra and and then injected i.p. with saline or LPS. Social behavior in adult mice (A) and social behavior in aged mice (B) were measured before injections and 2, 4, 8 and 24 h after injections. Data points represent the mean ± S.E.M. (n=10–11). Means with * are significantly different (p<0.05) from baseline controls and means with ‡ are significantly different from mice given IL-1ra and saline. Social behavior for both adult and aged mice at 24 h is also shown (C). Bars represent the mean ± S.E.M. (n=10–11). Means with different letters (a, b or c) are significantly different (p<0.05) from each other.
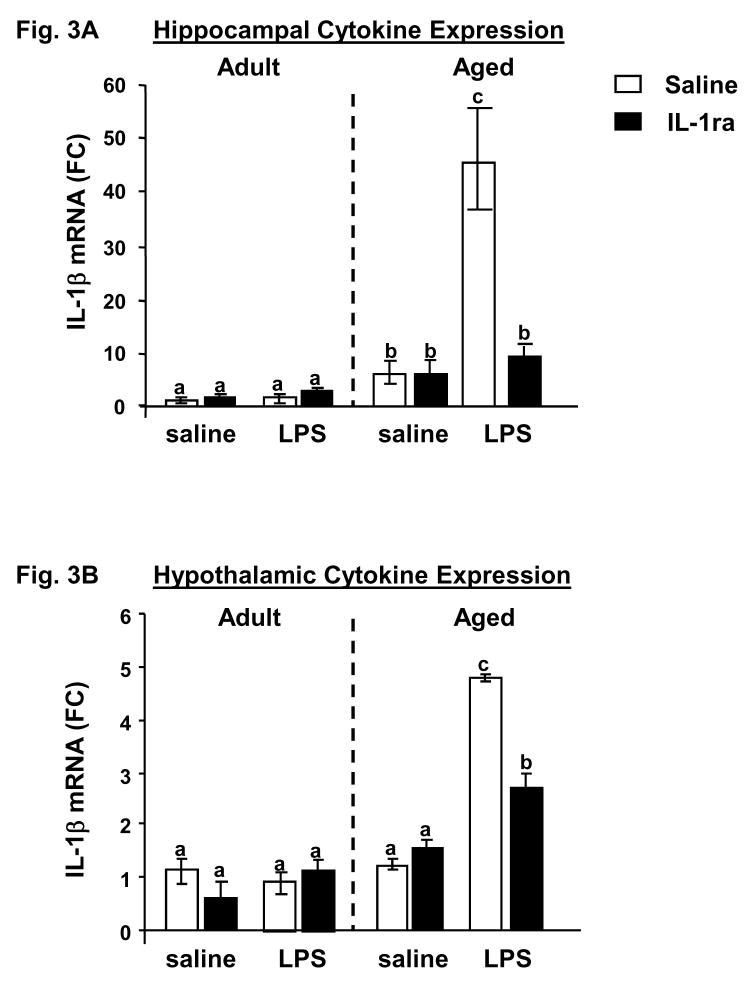
Immediately after assessing social behavior at the 24 h time point, hippocampal and hypothalamic tissues were collected to determine the IL-1β mRNA level. The hippocampus was of particular interest due to its sensitivity to the insults of inflammation and aging. And the hypothalamus was selected because of its role in behavioral, thermoregulatory, and endocrine responses to LPS. Figure 3A shows the effects of IL-1ra and LPS on IL-1β mRNA in the hippocampus of adult and aged mice. There were significant main effects of age (F (1, 24) = 30.16, p<0.0001), IL-1ra (F (1, 24) = 5.87, p=0.03), and LPS (F (1, 24) = 16.33, p=0.0006) as well as significant age × IL-1ra (F (1, 24) = 6.88, p=0.02), age × LPS (F (1, 24) = 13.64, p=0.002), IL-1ra × LPS (F (1, 24) = 15.46, p<0.0007) and age × IL-1ra × LPS (F (1, 24) = 16.15, p=0.0006) interactions. Regardless of LPS or IL-1ra treatments, levels of IL-1β mRNA in the hippocampus in young mice did not differ from saline controls, probably because any effect of LPS had dissipated by 24 h. However, hippocampal levels of IL-1β mRNA in aged animals were significantly elevated post LPS injection (t (24) = 7.84, p<0.0001). Notably, the LPS-induced increase in IL-1β mRNA in aged mice was reduced to basal levels by pretreatment with IL-1ra (t (24) = −6.50, p<0.0001). A similar effect was observed in the hypothalamus (Fig. 3B). There were significant main effects of age (F (1, 28) = 18.11, p=0.0002), IL-1ra (F (1, 28) = 3.85, p=0.05), and LPS (F (1, 28) = 34.16, p<0.0001) as well as significant age × LPS (F (1, 28) = 5.86, p=0.03) and age × IL-1ra × LPS (F (1, 28) = 6.39, p=0.02) interactions. Neither LPS nor IL-1ra affected IL-1β mRNA levels in adults at 24 h, while hypothalamic levels of IL-1β mRNA in aged animals were significantly elevated post LPS injection (t (28) = 6.38, p<0.0001). Notably, this LPS-induced increase in IL-1β mRNA in aged mice was inhibited by pretreatment with IL-1ra (t (28) = −3.83, p=0.01). It should also be noted that at 24 h levels of IL-1β mRNA did not differ between any treatment group or age in cortical, cerebellar and striatal tissue (data not shown). These data demonstrate that IL-1ra administered centrally to aged mice reduced IL-1β expression in discrete brain areas after peripheral injection of LPS. Collectively, these studies suggest a critical role for brain IL-1β in the exaggerated behavioral response to peripheral immune stimulation in aged mice.
Figure 3. Pretreatment with IL-1ra inhibited the LPS-induced increase in IL-1β mRNA in aged mice.
Adult and aged mice were injected ICV with saline or IL-1ra and then injected i.p. with saline or LPS. After the final behavioral test (24 h after injection) hippocampal tissue (A) and hypothalamic tissue (B) were collected and IL-1β mRNA was measured by quantitative real-time PCR. Bars represent means ± SEM (n=10–11). Means with different letters (a, b or c) are significantly different (p<0.05) from each other.
4. Discussion
Microglial cells are considered to be the principal source of proinflammatory cytokines in the brain. They are normally quiescent and express low or undetectable levels of major histocompatibility complex (MHC) class II. During aging, however, microglial cell activity increases, as evidenced by increased expression of MHC class II (Henry et al., 2008; Perry, 1998; Rogers et al., 1988; Sheffield and Berman, 1998; Streit et al., 2004), and they become more responsive to signals from the peripheral immune system (Dilger and Johnson, 2008). Consequently, when LPS is administered i.p. to mimic a peripheral infection, an exaggerated proinflammatory cytokine response in the brain ensues such that the magnitude and duration of central IL-1β expression is consistently greater in old mice injected with LPS compared to younger cohorts (Chen et al., 2008; Henry et al., 2008).
The excessive production of proinflammatory cytokines in the brain has been suggested to underlie the more severe behavioral response seen in older mice given LPS to mimic a peripheral infection. For example, anorexia, depression-like behavior, and deficits in social behavior are prolonged in old mice administered LPS or HIV gp120 (Abraham et al., 2008; Godbout et al., 2005; Godbout et al., 2008). Recently, old mice were found to have more microglial staining in the hippocampus and, following peripheral LPS administration, more IL-1β-positive cells (Chen et al., 2008). In that study, LPS-treated old mice showed a deficit in hippocampal-dependent working memory that was not evident in young adult cohorts. More recently we reported that a peripheral injection of LPS that induced an exaggerated proinflammatory cytokine response in the hippocampus of old mice also induced atrophy of dendrites of pyramidal neurons in the CA1 region (Richwine et al., 2008). Collectively, these studies indicate peripheral infection in the aged can lead to excessive production of proinflammatory cytokines in the brain and more severe behavioral deficits, but none of them established a causal relationship between the central proinflammatory cytokine response and behavioral pathology.
In the present study, ICV injection of IL-1ra in adult mice neither inhibited LPS-induced behavioral deficits nor enhanced recovery. However, ICV injected IL-1ra effectively inhibited LPS-induced sickness behavior in the aged. In addition, 24 h after LPS injection, hippocampal and hypothalamic levels of IL-1β mRNA in aged animals were significantly elevated compared to all other treatment groups. More importantly, this LPS-induced increase in IL-1β mRNA in aged animals was inhibited by pretreatment with IL-1ra, probably by disrupting the self perpetuating induction of central IL-1β. Thus, the present findings provide the first direct evidence suggesting IL-1β is involved in the behavioral pathology evident in old mice given LPS peripherally. That IL-1ra was effective in old mice but not adults seems counterintuitive given the well-documented role of IL-1β in sickness behavior. However, the present results in young adult mice are entirely consistent with reports by others where IL-1ra was given ICV and LPS given peripherally. For example, ICV-injected IL-1ra did not attenuate the depressive effect of LPS on social exploration and body weight (Bluthe et al., 1992), activation of the HPA axis (Dunn, 2000) or elevated cerebral norepinephrine metabolism in young mice (Dunn, 1992, 2000). In this model the inability of IL-1ra to inhibit sickness behavior is mostly attributed to the fact that LPS induces multiple proinflammatory cytokines with redundant properties so inhibition of a single cytokine is not sufficient. Indeed, one study showed that LPS-induced sickness behavior was blocked only if IL-1β, IL-6, and tumor necrosis factor-α were antagonized simultaneously (Swiergiel and Dunn, 1999). The current data show that although central IL-1ra was not able to abrogate the effects of LPS on adult animals, it did attenuate the depression in both locomotor and social behavior as well as elevated hippocampal and hypothalamic IL-1β mRNA in aged animals. It is important to note that IL-1ra did not entirely block sickness behavior in old mice but instead restored it to a level that one might suggest is physiological and not pathological. These findings suggest that IL-1ra affects adult and aged mice differently and that centrally mediated effects of IL-1β account for a significant part of LPS-induced sickness behavior in aged mice.
The underlying reason for this is unknown but there are several possibilities. We previously showed that aged mice are more vulnerable to the behavioral deficits associated with IL-1β (Nelson et al., 1999). In the same way, the aged brain may be more responsive to the protective effects of IL-1ra. IL-1β and the type I IL-1 receptor (IL-1R1) are expressed at relatively low levels in the brain under physiological conditions (Lynch and Lynch, 2002). However, under pathophysiological conditions there is a marked elevation in the level of both molecules (Friedman, 2001; Giulian and Lachman, 1985). In addition, Lynch et al. (2002) demonstrated that the age-related increase in IL-1β is accompanied by an increase in IL-1R1. Thus it is reasonable to presume that the elevated level of IL-1β, and the concomitant increase in its receptor, creates an environment in the aged brain whereby IL-1ra has more potential to provide protection against IL-1β signaling.
In conclusion, the present study demonstrates that several LPS-induced behavioral deficits in aged animals can be effectively ameliorated by centrally administered IL-1ra. Thus, in providing evidence of a direct role for brain IL-1β in LPS-induced sickness behavior, the present findings can be extended to suggest that inhibiting the central actions of IL-1β may be useful for minimizing behavioral complications in older individuals with a peripheral infection.
Acknowledgments
This research was supported by NIH grants AG16710 and MH069148 (to R.W.J.). J.A. is supported by a National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award 5T32 DK59802 from the NIDDK to the Division of Nutritional Sciences at the University of Illinois.
Footnotes
5.1. Disclosure Statement
The authors declared no actual or potential competing interests. The experimental procedures involving animals were consistent with PHS guidelines and approved by the campus IACUC.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. Jama. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ. The role of interleukin-1 and tumor necrosis factor alpha in the neurochemical and neuroendocrine responses to endotoxin. Brain Res Bull. 1992;29:807–812. doi: 10.1016/0361-9230(92)90148-q. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of the IL-1 receptor antagonist on the IL-1- and endotoxin-induced activation of the HPA axis and cerebral biogenic amines in mice. Neuroimmunomodulation. 2000;7:36–45. doi: 10.1159/000026418. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp Neurol. 2001;168:23–31. doi: 10.1006/exnr.2000.7595. [DOI] [PubMed] [Google Scholar]
- Giulian D, Lachman LB. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985;228:497–499. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- Johnston M, Wakeling A, Graham N, Stokes F. Cognitive impairment, emotional disorder and length of stay of elderly patients in a district general hospital. Br J Med Psychol. 1987;60 (Pt 2):133–139. doi: 10.1111/j.2044-8341.1987.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Lynch MA. The age-related increase in IL-1 type I receptor in rat hippocampus is coupled with an increase in caspase-3 activation. Eur J Neurosci. 2002;15:1779–1788. doi: 10.1046/j.1460-9568.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Nelson KP, Marks NL, Heyen JR, Johnson RW. Behavior of adult and aged mice before and after central injection of interleukin-1beta. Physiol Behav. 1999;66:673–679. doi: 10.1016/s0031-9384(98)00339-4. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. The roles of IL-1, IL-6, and TNFalpha in the feeding responses to endotoxin and influenza virus infection in mice. Brain Behav Immun. 1999;13:252–265. doi: 10.1006/brbi.1999.0565. [DOI] [PubMed] [Google Scholar]
- Wofford JL, Loehr LR, Schwartz E. Acute cognitive impairment in elderly ED patients: etiologies and outcomes. Am J Emerg Med. 1996;14:649–653. doi: 10.1016/S0735-6757(96)90080-7. [DOI] [PubMed] [Google Scholar]