Abstract
Background
Hemoglobin-based oxygen-carrying solutions (HBOC) provide emergency alternatives to blood transfusion to carry oxygen to tissues without the risks of disease transmission or transfusion reaction. Two primary concerns hampering the clinical acceptance of acellular HBOC are the occurrence of systemic and pulmonary vasoconstriction and the maintenance of the heme-iron in the reduced state (Fe+2). We recently demonstrated that pretreatment with inhaled nitric oxide prevents the systemic hypertension induced by HBOC-201 (polymerized bovine hemoglobin) infusion in awake mice and sheep without causing methemoglobinemia. However, the impact of HBOC-201 infusion with or without inhaled nitric oxide on pulmonary vascular tone has not yet been examined.
Methods
The pulmonary and systemic hemodynamic effects of breathing nitric oxide both before and after the administration of HBOC-201 were determined in healthy, awake lambs.
Results
Intravenous administration of HBOC-201 (12 ml/kg) induced prolonged systemic and pulmonary vasoconstriction. Pretreatment with inhaled nitric oxide (80 parts per million (ppm) for 1 h) prevented the HBOC-201-induced increase in mean arterial pressure, but not the increase of pulmonary arterial pressure, systemic vascular resistance, or pulmonary vascular resistance. Pretreatment with inhaled nitric oxide (80 ppm, 1 h) followed by breathing a lower concentration of nitric oxide (5 ppm) during and after HBOC-201 infusion prevented systemic and pulmonary vasoconstriction without increasing methemoglobin levels.
Conclusions
These findings demonstrate that pretreatment with inhaled nitric oxide followed by breathing a lower concentration of the gas during and after administration of HBOC-201 may enable administration of an acellular hemoglobin substitute without vasoconstriction while preserving its oxygen-carrying capacity.
Introduction
The development of hemoglobin-based oxygen carriers (HBOC) has been driven by several imperatives, such as the requirements for emergency field transfusion of large volumes of blood products, the prevalence of transfusion-transmitted diseases (HIV, Hepatitis B or C), and a shortage of blood donors.1 HBOCs might provide an alternative to blood transfusion due to their capacity to augment tissue oxygenation.2,3 Moreover, HBOCs offer the advantages of ready availability on the battlefield and a long shelf-life, without the risks of viral pathogens or the necessity for blood typing.4
One of the major safety concerns of HBOC products is systemic vasoconstriction.5 The vasoconstrictor effects of HBOCs may aggravate microcirculatory failure in splanchnic organs of patients with hemorrhagic shock.6 Systemic vasoconstriction may also contribute to the excess myocardial infarction and mortality seen in HBOC-treated patients, as reported in a recent meta-analysis of the available clinical trials data.7 HBOCs can also cause pulmonary vasoconstriction: studies of dogs, pigs, sheep and humans have shown a significant increase in pulmonary vascular resistance during hypovolemic resuscitation with HBOCs. 8–13
Several mechanisms have been proposed to explain HBOC-induced vasoconstriction. Winslow has proposed an “autoregulation theory” suggesting that enhanced plasma oxygen delivery by cell-free hemoglobin may trigger arteriolar vasoconstriction.14 Another hypothesis is that when hemoglobin tetramers are removed from their protective erythrocytic membranes, they diffuse through the vascular endothelium. The extravascular tetramer then binds nitric oxide synthesized by endothelial cells, thereby interrupting the vasodilator message to vascular smooth muscle cells and causing vasoconstriction.15 In a hemorrhagic shock model, microcirculatory recovery was greater after resuscitation with an HBOC with reduced nitric oxide-scavenging capacity than after resuscitation with a colloid or a first-generation hemoglobin solution.16 Our recent research report provides additional evidence that scavenging of endothelium-derived nitric oxide (synthesized by nitric oxide synthase 3) by cell-free tetrameric hemoglobin is the primary mechanism responsible for the vasoconstriction observed after the administration of HBOC.17
Another potential safety concern associated with administration of HBOCs is oxidative stress which may cause tissue injury.18 Plasma reductive capacity is required to maintain the infused HBOC in a reduced state (heme-Fe+2). Oxidation of hemoglobin results in the formation of methemoglobin (heme-Fe+3), which is unable to bind or deliver oxygen or nitric oxide and which can give rise to free radicals that have the potential to cause endothelial vascular injury.19,20
Recently, Minneci et al reported that in dogs, the systemic vasoconstriction induced by intravenous infusion of cell-free hemoglobin was prevented by concurrent breathing of nitric oxide (80 parts per million (ppm)).21 However, concurrent breathing of 80 ppm nitric oxide caused 85–90% of the circulating extracellular hemoglobin to be converted to methemoglobin after 1 h, disabling the oxygen-carrying capacity of the infused hemoglobin. We recently reported that inhalation of 80 ppm nitric oxide for 1 h before intravenous infusion of HBOC-201 (a cross-linked bovine hemoglobin), prevented the development of systemic hypertension without oxidizing the HBOC in two species (mice and sheep).17 In follow-up experiments, we observed that administration of HBOC-201 to awake lambs induced pulmonary vasoconstriction that could not be prevented by pretreatment with inhaled nitric oxide. In the current study, we sought to determine whether the pulmonary vasoconstriction induced by administration of HBOC-201 could be prevented by pretreatment with high doses of inhaled nitric oxide followed by breathing lower concentrations during and after administration of the HBOC. We report that pretreatment with inhaled nitric oxide (80 ppm, 1 h) followed by continuous inhalation of a low concentration of nitric oxide (5 ppm) during and after HBOC-201 infusion prevented the HBOC-induced pulmonary hypertension and decreased cardiac output without increasing extracellular or intracellular methemoglobin levels.
Materials and Methods
Animal preparation and hemodynamic measurements
This study was approved by the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, Massachusetts. Thirty-five Suffolk lambs (24.5±2.7 kg, mean±SD) were anesthetized with an intramuscular injection of ketamine hydrochloride (15 mg/kg; Hospira, Inc., Lake Forest, IL) as described previously.17 After emergence from general anesthesia, all lambs were allowed to recover for at least 2 h in a large-animal mobile restraint unit (Lomir, Malone, NY) before starting the study. Mean arterial pressure (MAP), mean pulmonary arterial pressure (PAP), central venous pressure, pulmonary arterial occlusion pressure, heart rate, and cardiac output were measured as described previously.22 Systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were calculated using standard equations.
Biochemical measurements
Blood samples were collected before and after infusion of autologous whole blood or HBOC-201 as described previously.23 Plasma thromboxane B2 concentrations in plasma were determined with a Thromboxane B2 ELISA kit (Neogen Corporation, Lexington, KY).
Nitrate and nitrite levels in plasma were determined in 15 lambs with a nitrate/nitrite fluorometric assay kit (Cayman Chemical Company, Ann Arbor, MI).24
Hemoglobin and methemoglobin concentrations of whole blood and plasma were determined by the cyanomethemoglobin method measuring absorption at 540 nm and 630 nm with a spectrophotometer (Biomate 3, Thermoelectron Corporation, Waltham, MA).
Experimental protocol
Preparation of HBOC-201
HBOC-201 (12–14 g/dl, pH 7.6, methemoglobin 5%, containing 3% tetramer) is a preparation of glutaraldehyde-polymerized bovine hemoglobin in a buffered physiological solution of electrolytes and was obtained from the Biopure Corporation (Cambridge, MA).
Nitric oxide delivery
The tracheotomy tube was connected to a circuit consisting of a 3 L reservoir bag and a two-way non-rebreathing valve (Hans Rudolph, Kansas City, MO) to separate inspired gas from expired gas. Using volumetrically calibrated flowmeters (Cole-Parmer, Vernon Hills, IL), nitric oxide gas (900 ppm in nitrogen; Specialty Gases of America, Inc, Toledo, OH) was mixed with air and pure oxygen to obtain a final concentration of 80 ppm nitric oxide or 5 ppm nitric oxide both at FiO2=0.3. Nitric oxide concentration was measured with a chemiluminescense nitric oxide analyzer (Sievers, Model 280 Nitric Oxide analyzer, Boulder, CO) connected to the inspiratory limb of the two-way valve. Nitrogen dioxide and oxygen levels were continuously monitored. Exhaled gases were scavenged via a Venturi exhalation trap maintained at negative atmospheric pressure by the central vacuum system.
Effects of HBOC-201 and inhaled nitric oxide on systemic and pulmonary vascular resistance
Forty-eight hours before the experiments, each lamb was anesthetized with an intramuscular injection of ketamine (15 mg/kg), and 12 ml blood/kg of body weight was withdrawn via the jugular vein. The blood was collected in blood collection bags (Jorgensen Laboratories, Loveland, CO) containing sodium heparin (25 units/ml blood) and was stored at 4 °C for two days before re-infusion for the control group. On the day of the experiment, either autologous whole blood (control group) or HBOC-201 (12 ml/kg) was administered. During the experiments, the lambs were awake and breathed spontaneously while receiving an intravenous infusion of lactated Ringer solution (15 ml/kg/h). All hemodynamic measurements and blood samples were obtained at baseline and every 15–30 min.
Blood gases were measured with groups receiving either whole blood or HBOC-201 with a blood gas analyzer (Rapidlab 840, Chiron Diagnostics, Medfield, MA). Oxygen delivery and oxygen consumption were calculated as previously described.25
Four groups of lambs were studied. One group (n=6) received an intravenous infusion of autologous whole blood (warmed to 37 °C, 12 ml/kg over 20 min) while breathing at FiO2=0.3. A second group (n=5) received an intravenous infusion of HBOC-201 (12 ml/kg over 20 min) while breathing at FiO2=0.3. A third group (n=6) breathed 80 ppm nitric oxide at FiO2=0.3 for 1 h, followed by discontinuation of nitric oxide gas administration and infusion of HBOC-201 (12 ml/kg over 20 min) while breathing at FiO2=0.3. A fourth group (n=5) breathed 80 ppm nitric oxide at FiO2=0.3 for 1 h, followed by breathing 5 ppm nitric oxide at FiO2=0.3 for 2 h both during and after the infusion of HBOC-201 (12 ml/kg over 20 min). After 2 h, nitric oxide breathing was discontinued, and pulmonary and systemic hemodynamics were frequently monitored while lambs breathed at FiO2=0.30. Nitrate and nitrite levels in plasma were determined before and after breathing nitric oxide.
Effects of breathing increasing concentrations of nitric oxide on the intracellular and extracellular hemoglobin oxidation after HBOC-201 administration
Four additional lambs received an intravenous infusion of HBOC-201 (12 ml/kg over 20 min), followed by breathing sequential ascending concentrations of nitric oxide (0.5, 1, 2, 5, 10, 15, 30, 40, 60, and 80 ppm) for 15 min at each dose. Blood samples were taken for methemoglobin measurement in plasma and whole blood and for arterial blood gas tension and pH analysis (Rapidlab 840, Chiron Diagnostics, Medfield, MA) after breathing nitric oxide at each level.
Effects of nitric oxide breathing on hemodynamic measurements in awake lambs
Two additional lambs breathed nitric oxide (80 ppm, 1 h) followed by rapid discontinuation of nitric oxide gas, and then breathed at FiO2=0.3. MAP, PAP, pulmonary arterial occlusion pressure, central venous pressure, and heart rate were measured at baseline before nitric oxide breathing, and every 5 min after discontinuing nitric oxide breathing for 30 min.
Effects of an intravenous infusion of sodium nitrite on the systemic and pulmonary hemodynamic response to HBOC-201
In 4 additional lambs, sodium nitrite (1 mg/kg; dissolved in phosphate buffered saline) was infused at a rate of 1 ml/min for 5 min, followed by an intravenous infusion of HBOC-201 (12 ml/kg over 20 min) while breathing at FiO2=0.3. Hemodynamic measurements were recorded for 3 h. Nitrate and nitrite levels in plasma were determined before and after infusion of sodium nitrite.
Statistical analysis
All data are expressed as mean±SD. Statistical evaluations were performed with the Sigma Stat 3.0.1 program (Systat Software, Inc., San Jose, CA). A two-way repeated measures analysis of variance with interaction was used to compare data from different groups that were infused with HBOC-201 breathing air supplemented with or without nitric oxide, infused with HBOC-201 after a nitrite infusion, or infused with autologous whole blood while breathing air. Multiple group comparison analysis (plasma hemoglobin oxidation rate in sheep breathing increasing concentrations of nitric oxide) was performed by adding the Holm-Sidak procedure to the Student t test after analysis of variance. During the statistical analysis of our data, when the parametric assumption was violated (i.e., the normality test was failed), time and transfusion (whole blood, HBOC-201 supplemented with or without nitric oxide) were analyzed as variables with the Kruskall-Wallis test. A value of p<0.05 was considered significant.
Results
Effects of inhaled nitric oxide on systemic and pulmonary vascular resistance after challenge with HBOC-201
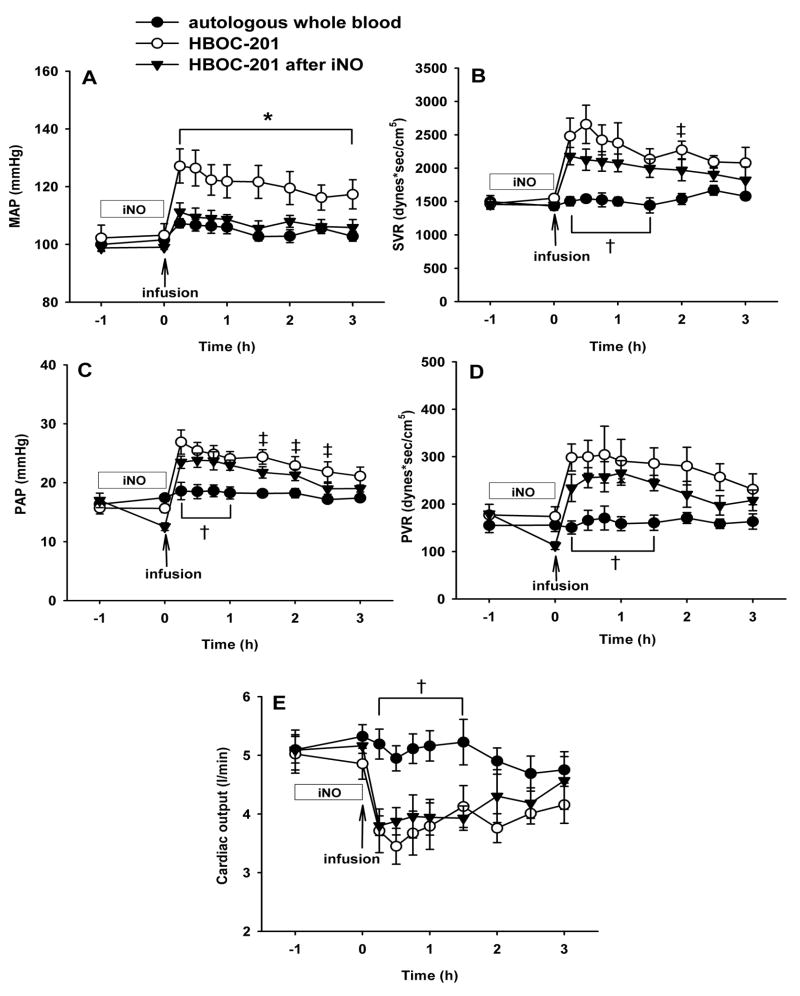
Infusion of autologous whole blood did not significantly alter MAP, PAP, SVR or PVR (fig. 1A–D). Infusion of whole blood did not alter oxygen delivery or oxygen consumption (data not shown). In contrast, MAP, PAP, SVR, and PVR were increased immediately after HBOC-201 infusion (12 ml/kg) in lambs breathing at FiO2=0.3 without added nitric oxide (P<0.05 differs versus autologous whole blood group). Administration of HBOC-201 did not significantly alter the oxygen delivery or consumption rate (data not shown). As we previously reported, pretreatment with inhaled nitric oxide (80 ppm, 1 h) blocked the systemic hypertensive effects of HBOC-201 challenge (P<0.05 differs from HBOC-201 without inhaled nitric oxide; fig. 1A). However, pretreatment with inhaled nitric oxide was unable to prevent the HBOC-201-induced acute systemic vasoconstriction (increased SVR, P<0.05 differs from autologous whole blood group; fig. 1B) or pulmonary vasoconstriction (increased PAP and PVR; P<0.05 differs from autologous whole blood group for both; fig. 1C,D). Cardiac output was lower in the group that received HBOC-201 infusion than in the group that received an autologous whole blood transfusion (P<0.05). Pretreatment with nitric oxide inhalation (80 ppm) did not prevent the reduction of cardiac output (P<0.05 differs versus HBOC-201 group with or without inhaled nitric oxide; fig. 1E). The decrease in cardiac output after infusion of HBOC-201 with or without nitric oxide pretreatment resulted from a lower heart rate since stroke volume did not differ between experimental groups (data not shown).
Fig. 1.
Mean arterial pressure (MAP; A), systemic vascular resistance (SVR; B), mean pulmonary arterial pressure (PAP; C), pulmonary vascular resistance (PVR; D), cardiac output (E) of awake lambs after an intravenous infusion of pre-warmed (37°C) autologous whole blood (12 ml/kg; n=6), intravenous HBOC-201 (n=5), or HBOC-201 (12 ml/kg) after pretreatment with breathing 80 ppm nitric oxide at FiO2=0.3 for 1 h (iNO; n=6). All lambs breathed at FiO2=0.3. *p<0.05 HBOC-201 differs from autologous whole blood and from HBOC-201 after inhaled nitric oxide, †p<0.05 autologous whole blood differs from HBOC-201 with or without pretreatment by inhaled nitric oxide, ‡p<0.05 HBOC-201 differs from autologous whole blood.
Effects of increasing nitric oxide concentrations on hemoglobin oxidation rate after HBOC-201 administration
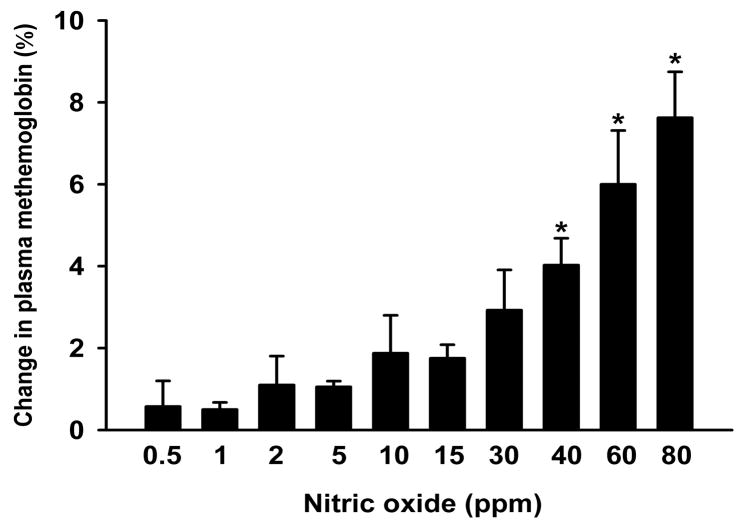
Since pretreatment with inhaled nitric oxide did not prevent the acute pulmonary hypertension induced in lambs by HBOC-201 infusion, we hypothesized that concurrent breathing of low levels of nitric oxide might block the HBOC-induced pulmonary hypertension without causing significant plasma methemoglobinemia. However, since it was known that breathing 80 ppm nitric oxide during HBOC administration induced marked plasma methemoglobinemia,21 we sought to identify a lower concentration of nitric oxide gas which when inhaled would dilate the pulmonary vasculature without inducing plasma methemoglobin formation. First, we studied lambs that received an infusion of HBOC-201, and plasma and whole blood methemoglobin levels were measured after breathing nitric oxide for 15 min at ascending concentrations from 0.5 to 80 ppm (fig. 2). We observed that the increase of plasma methemoglobin level was 1±0% after breathing 15 ppm nitric oxide for 15 min, 3±1% after breathing 30 ppm nitric oxide for 15 min, and 8±1% after breathing 80 ppm nitric oxide for 15 min. However, the increase of methemoglobin levels within red blood cells was only 1±0% after breathing at 80 ppm. Thus, continuously breathing nitric oxide at less than 30 ppm after HBOC-201 administration did not cause marked oxidation of either plasma or intracellular hemoglobin.
Fig. 2.
Changes in plasma methemoglobin level after breathing nitric oxide at ascending concentrations for 15 min at each nitric oxide level, all after infusion of HBOC-201 (12 ml/kg) in awake lambs (n=4). *p<0.05 differs from the changes in methemoglobin level induced by breathing 0.5 ppm nitric oxide for 15 min.
Effects of continuously breathing a low level of nitric oxide during and after HBOC-201 administration on systemic and pulmonary vascular resistance
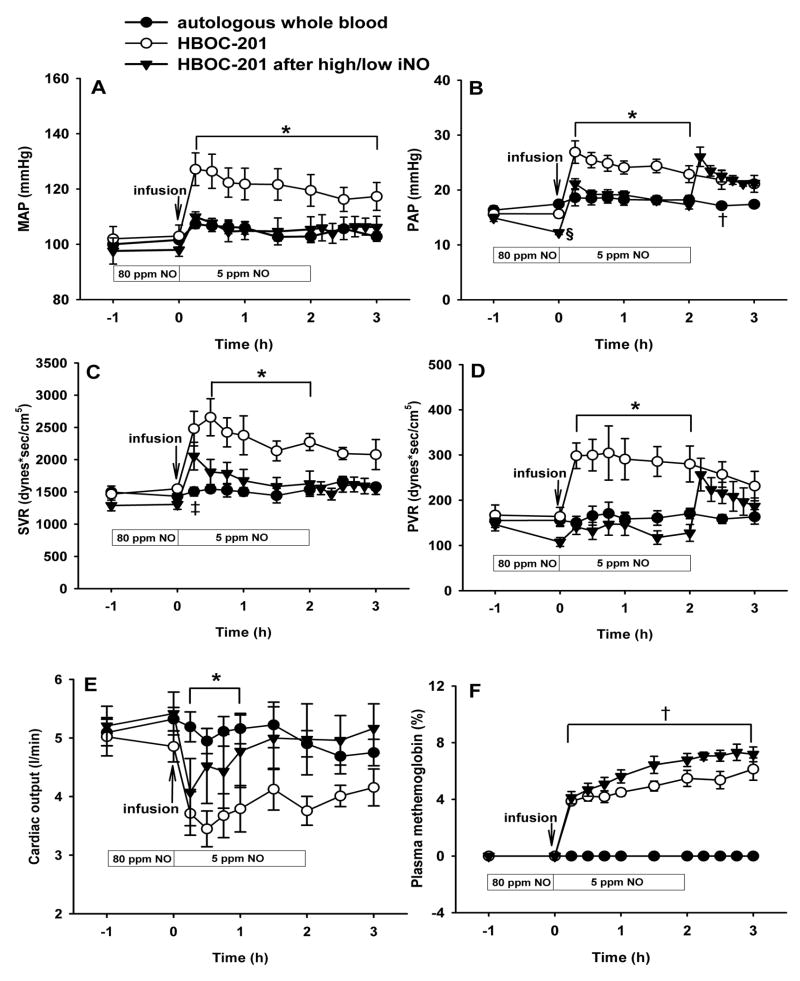
To examine whether breathing a low concentration of nitric oxide during and after HBOC-201 infusion could block the acute pulmonary hypertension induced by HBOC administration, we pretreated lambs with 80 ppm nitric oxide breathing for 1 h, followed by infusion of HBOC-201 (12 ml/kg) while continuously breathing 5 ppm nitric oxide for 2 h. This two-level combination of nitric oxide breathing prevented the changes in systemic and pulmonary vascular resistance induced by HBOC-201 (P<0.05 HBOC-201 differs from autologous whole blood and from HBOC-201 after inhaled nitric oxide; fig. 3A–D). However, after 2 h, when nitric oxide breathing was acutely discontinued, the PAP and PVR immediately increased (fig. 3B and D), but there were no effects on MAP or SVR. After infusion, cardiac output was lower in the group that received HBOC-201 than in the group that received autologous whole blood. After pretreatment with 80 ppm nitric oxide followed by continuously breathing 5 ppm nitric oxide, the HBOC-201-induced decrease in cardiac output was markedly attenuated (P<0.05 differs versus HBOC-201 group without inhaled nitric oxide; fig. 3E). After administration of HBOC-201, plasma methemoglobin levels in the group breathing at 80 ppm and then 5 ppm nitric oxide did not increase beyond the levels observed in the HBOC-201 group breathing at FiO2=0.3 without nitric oxide (P=NS; fig. 3F).
Fig. 3.
Mean arterial pressure (MAP; A), mean pulmonary arterial pressure (PAP; B), systemic vascular resistance (SVR; C), pulmonary vascular resistance (PVR; D), Cardiac output (E), and plasma methemoglobin concentration (F) of awake lambs after infusion of autologous whole blood (n=6), intravenous HBOC-201 (n=5), or HBOC-201 (12 ml/kg) after pretreatment by breathing 80 ppm nitric oxide for 1 h followed by continuously breathing 5 ppm nitric oxide for 2 h (high/low nitric oxide, n=6). *p<0.05 HBOC-201 differs from autologous whole blood and from HBOC-201 after high/low inhaled nitric oxide, †p<0.05 autologous whole blood differs from HBOC-201 with or without high/low inhaled nitric oxide, ‡p<0.05 HBOC-201 differs from autologous whole blood, §p<0.05 differs from autologous whole blood and HBOC-201.
Effects of acute nitric oxide withdrawal on the normal lamb
To determine whether there was rebound pulmonary vasoconstriction after discontinuing nitric oxide breathing in normal lambs (that did not receive HBOC-201),26 we obtained pulmonary and systemic hemodynamic measurements before and after the acute withdrawal of nitric oxide (80 ppm, 1h; online supplement fig. 1). Our results demonstrate that there is no change of MAP, PAP, SVR and PVR after discontinuing nitric oxide breathing in healthy lambs. (See Supplemental Digital Content Figure 1, MAP, PAP, SVR, and PVR of normal awake lambs before and after inhaling nitric oxide.)
Plasma thromboxane B2 levels after HBOC-201 infusion
Thromboxane B2 is the principle metabolite of the powerful endogenous vasoconstrictor thromboxane A2.23 It has been previously shown that severe acute pulmonary hypertension in lambs can be induced by infusion of thromboxane analogs as well as by the endogenous release of thromboxane A2 after activation of complement.27 To determine whether or not HBOC-201 infusion induced pulmonary hypertension via synthesis and release of thromboxane metabolites by pulmonary intravascular macrophages28, we measured plasma thromboxane B2 concentration before and after infusion of HBOC-201. Thromboxane B2 levels did not differ before and after HBOC-201 infusion (data not shown).
Effects of sodium nitrite infusion on the systemic and pulmonary vascular resistance response to HBOC-201
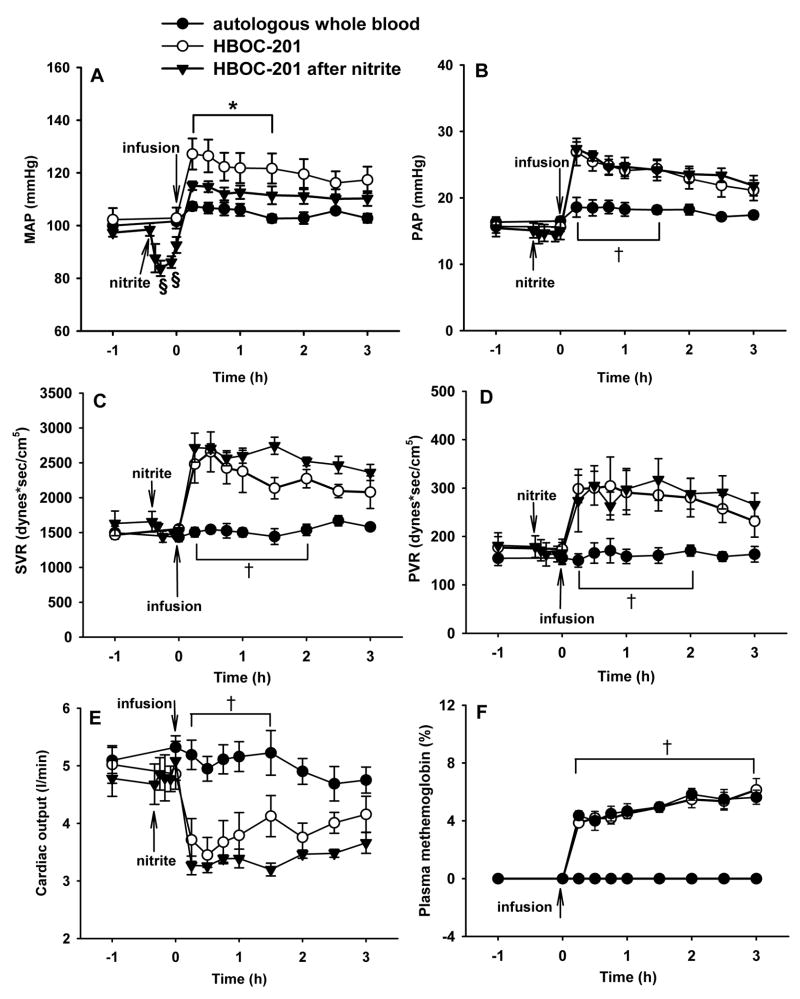
Breathing nitric oxide leads to an accumulation of nitric oxide metabolites including nitrate and nitrite.29,30 Nitrite can be converted back to nitric oxide via nitrite reductases including deoxyhemoglobin.31 To examine whether nitrite administration could prevent the pulmonary hypertension caused by HBOC-201 infusion, sodium nitrite (1 mg/kg) was administered intravenously as an infusion before HBOC-201 was infused (fig. 4). The nitrite infusion transiently lowered the MAP from baseline 99±2 to 84±3 mmHg (P=0.008), but had no effect on PAP or cardiac output (fig. 4A, B, and E). Nitrite infusion attenuated the systemic hypertension induced by intravenous infusion of HBOC-201 (fig. 4A), but nitrite did not prevent the increases of PAP, PVR, or SVR or the decrease in cardiac output (P<0.05 differs versus autologous whole blood group; fig. 4B–E). Plasma methemoglobin levels did not differ in lambs receiving HBOC-201 alone and lambs receiving HBOC-201 after a nitrite infusion (fig. 4F).
Fig. 4.
Mean arterial pressure (MAP; A), mean pulmonary arterial pressure (PAP; B), systemic vascular resistance (SVR; C), pulmonary vascular resistance (PVR; D), cardiac output (E), and plasma methemoglobin concentration (F) of awake lambs after infusion of 37°C autologous whole blood (12 ml/kg) (n=6), after intravenous HBOC-201 (n=5), or HBOC-201 (12 ml/kg) after pretreatment with a sodium nitrite infusion (1 mg/kg over 5 min; n=4). *p<0.05 HBOC-201 differs from autologous blood and from HBOC-201 after nitrite, †p<0.05 autologous whole blood differs from HBOC-201 with or without nitrite infusion, ‡p<0.05 HBOC-201 after nitrite differs from autologous whole blood, §p<0.05 differs from before nitrite administration.
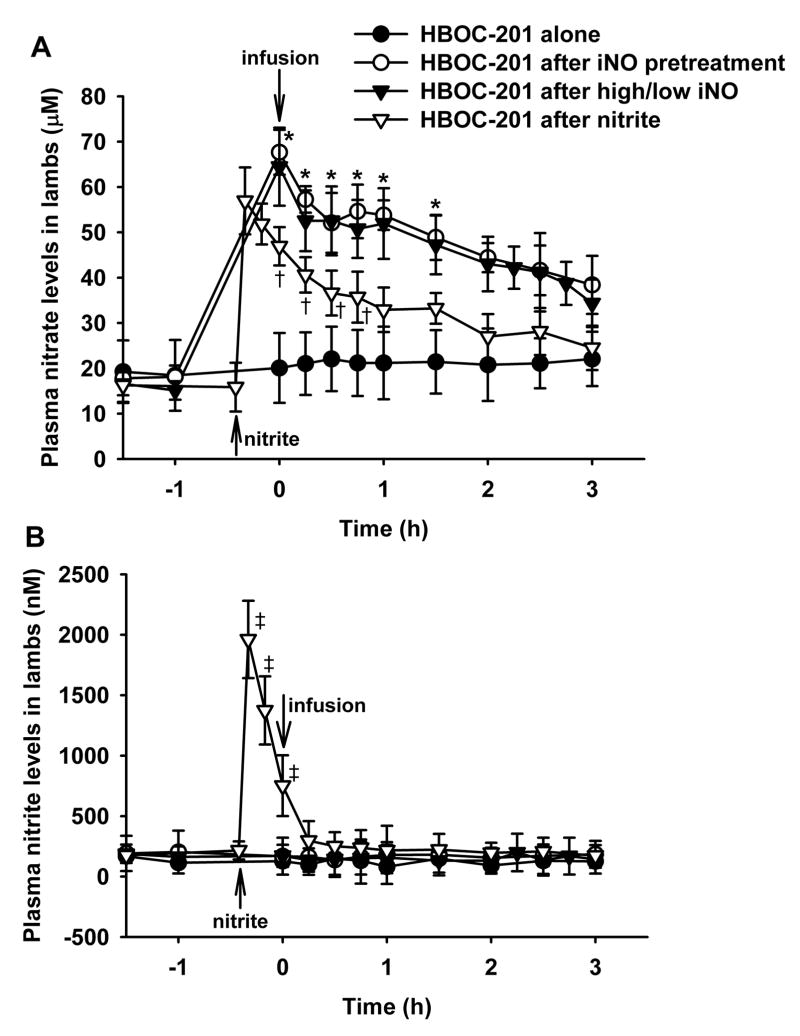
Plasma nitrate and nitrite levels before and after HBOC-201 infusion
Nitrate and nitrite levels were measured in plasma samples taken from lambs pretreated with inhaled nitric oxide, from lambs treated with two levels of inhaled nitric oxide (80 ppm, 1 h before HBOC infusion and 5 ppm thereafter), and from lambs receiving a nitrite infusion (fig. 5). Plasma nitrate levels did not change in lambs receiving HBOC-201 alone, but dramatically increased in lambs breathing nitric oxide and in lambs given a nitrite infusion (P<0.05 groups differs versus HBOC-201 alone group; fig. 5A). On the other hand, plasma nitrite levels did not change in sheep breathing nitric oxide, but levels were markedly increased in the lambs given intravenous nitrite before HBOC-201 administration. After the sodium nitrite infusion, the increase in plasma nitrite levels was transient, and nitrite concentrations returned to the baseline levels by 15 min after HBOC-201 infusion (fig. 5B).
Fig. 5.
Plasma nitrate (A) and nitrite (B) levels in awake lambs after infusion of intravenous HBOC-201 alone (n=3), intravenous HBOC-201 with pretreatment by inhaled nitric oxide (80 ppm, 1 h; n=3), intravenous HBOC-201 with pretreatment by inhaled nitric oxide (80 ppm, 1 h) followed by continuously breathing nitric oxide (5 ppm, 2 h; n=5; high/low iNO), or intravenous HBOC-201 after nitrite infusion (n=4). *p<0.05 HBOC-201 with pretreatment by inhaled nitric oxide and HBOC-201 with high/low inhaled nitric oxide differ from HBOC-201 alone, †p<0.05 HBOC-201 after nitrite bolus differs from HBOC-201 alone, ‡p<0.05 HBOC-201 after nitrite bolus differs from its own baseline.
Discussion
In this study, we report that the intravenous administration of HBOC-201 caused marked pulmonary and systemic vasoconstriction and decreased cardiac output in awake lambs. Pretreatment with inhaled nitric oxide or intravenous nitrite did not prevent HBOC-induced pulmonary vasoconstriction or decreased cardiac output. We identified a low concentration of inhaled nitric oxide which, when administered simultaneously with HBOC after pretreatment with 80 ppm nitric oxide for 1 hour, prevented the increase in PVR and decrease in cardiac output. The combination of pretreatment with 80 ppm nitric oxide followed by concurrent administration of a low concentration of inhaled nitric oxide (5 ppm) did not cause a significant increase of plasma methemoglobin levels.
Inhaled nitric oxide is a selective pulmonary vasodilator that has been used to treat pulmonary hypertension in babies and adults, as well as to prevent chronic lung diseases associated with prematurity.32 Accumulating evidence suggests that inhaled nitric oxide may have systemic effects on coagulation, inflammation, recovery from ischemia/reperfusion injury, and vascular tone, although the precise mechanisms responsible for these systemic effects are controversial.32–34 In a recent study, we reported that pretreatment with 80 ppm inhaled nitric oxide for 1 h prevented the systemic hypertension induced by subsequent infusion of HBOC-201 in both mice and sheep without causing plasma methemoglobinemia.17 Surprisingly, in the present study, despite pretreatment with inhaled nitric oxide, HBOC-201 infusion in the awake lamb caused acute pulmonary hypertension and a major reduction of cardiac output (see fig. 1). Our studies with HBOC-201 infusion are consistent with the hemodynamic changes in various species reported by others.10,12,13,35 The increase of systemic and pulmonary arterial pressure and elevation of systemic and pulmonary vascular resistance with a deceased cardiac output indicate a diffuse vasoconstrictor effect of HBOC-201 infusion. Although we found ample evidence of pulmonary vasoconstriction after HBOC infusions in animal studies,8–12,17 we are unaware of studies measuring pulmonary hemodynamics while infusing HBOC-201 or other HBOCs in healthy human volunteers. It has been recently reported that the PAP significantly increased after HBOC-201 infusion in patients with an acute coronary syndrome undergoing percutaneous coronary interventions.36 Therefore, we sought a method to prevent the pulmonary vasoconstriction associated with infusion of HBOC-201.
After infusing HBOC-201 into lambs, we studied the effects of inhaling increasing concentrations of nitric oxide. Surprisingly, we found that extracellular hemoglobin was resistant to oxidation by inhaled nitric oxide when the gas was breathed at levels below 30 ppm (see fig. 2). Then, we evaluated whether nitric oxide if continuously inhaled at low levels would prevent the systemic and pulmonary vasoconstriction induced by HBOC-201 administration. We learned that pretreatment by breathing nitric oxide (80 ppm, 1 h) followed by breathing a low concentration of nitric oxide (5 ppm) during and after HBOC-201 challenge prevented the development of both systemic and pulmonary vasoconstriction in awake lambs (see fig. 3).
Our results suggest that continued breathing of low levels of nitric oxide will be necessary to reverse the persistent nitric oxide scavenging caused by HBOC-201 administration in the pulmonary circulation. Acute withdrawal of nitric oxide breathing in our lambs, even 2 h after HBOC-201 infusion caused acute rebound pulmonary vasoconstriction (see fig. 3). In contrast, sudden withdrawal of nitric oxide breathing in a normal sheep (without circulating HBOC-201) does not cause any hemodynamic alterations (see Supplemental Digital Content Figure 1). The vasoconstriction observed after the sudden withdrawal of inhaled nitric oxide following HBOC-201 infusion is reminiscent of the rebound pulmonary hypertension occurring in adults with acute respiratory distress syndrome and children after cardiac surgery upon sudden withdrawal of nitric oxide inhalation. Slow reduction of the concentration of inhaled nitric oxide has been successful in preventing rebound pulmonary hypertension.37 It is conceivable that slow weaning of inhaled nitric oxide may prevent rebound pulmonary hypertension in patients treated with HBOCs.
We considered the possibility that infusing HBOC-201, a foreign antigen, might induce pulmonary hypertension by activating complement with the elaboration of pulmonary thromboxane A2, a potent endoperoxide vasoconstrictor.23 We measured plasma thromboxane B2 levels after HBOC-201 infusion. The levels remained low and were not different between animals receiving a control transfusion of autologous blood and those demonstrating HBOC-201-induced pulmonary hypertension (data not shown). These findings suggest that thromboxane generation does not contribute to the pulmonary hypertension associated with HBOC-201 administration.
In a comparison with inhaled nitric oxide, an intravenous infusion of a dose of sodium nitrite sufficient to induce systemic hypotension, followed by HBOC-201 administration attenuated the development of systemic hypertension, but did not prevent the development of acute pulmonary vasoconstriction (see fig. 4). Serial measurements of nitrate and nitrite levels in plasma revealed that infusion of sodium nitrite increased levels of both metabolites (see fig. 5). In comparison, breathing nitric oxide elevated only the plasma nitrate level, but not the plasma nitrite level (see fig. 5). These results suggest that the prevention of HBOC-induced vasoconstriction by nitric oxide inhalation is not attributable to the generation of nitrite.
The awake lamb is a commonly-used model for pulmonary circulatory studies of the human newborn, as it has a muscularized pulmonary arterial circulation. Thus, the lamb may be excessively susceptible to pulmonary vasoconstriction due to HBOC infusion. However, the pulmonary circulation of the human newborn is also muscularized as is that in adults with chronic pulmonary hypertension or other lung vascular diseases. It is conceivable that newborns and adults with pulmonary vascular diseases may be quite susceptible to vasoconstriction from HBOC infusion. We previously reported that very high levels of inhaled nitric oxide were required to partially reverse thromboxane induced pulmonary hypertension.27 In this study, the ability of low concentrations of nitric oxide breathing to completely prevent HBOC-201 pulmonary hypertension suggests that nitric oxide scavenging by HBOC causes this pulmonary hypertension (and not thromboxane A2 release). High doses of inhaled nitric oxide can cause methemoglobinemia and may damage the lung and perhaps other organs.38 On the other hand, each year thousands of infants with hypoxic respiratory failure and pulmonary hypertension are treated with inhaled nitric oxide, usually commencing at 20–80 ppm for at least several hours.39 Since most of these newborns survive without renal or respiratory failure, it is unlikely that short term breathing of such doses of nitric oxide gas is highly toxic. However, the current study is not a study of a hemorrhagic shock model. We chose to study a “top-load” model without hypotension or shock in order to isolate and examine the hemodynamic effects of the agent that we infused (HBOC-201). Nonetheless, before clinical use of inhaled nitric oxide with HBOC to treat hemorrhage and shock, additional animal studies should be undertaken.
In conclusion, we report that pretreatment of lambs with inhaled nitric oxide, followed by continuously breathing a low concentration of nitric oxide, can prevent the pulmonary and systemic vasoconstriction induced by HBOC-201 administration without causing any significant elevation of plasma methemoglobin levels. This promising combination therapy merits further evaluation as a method to enable HBOC administration without vasoconstriction in patients with acute traumatic anemia.
Supplementary Material
Supplemental Digital Content Figure 1: Mean arterial pressure (MAP; A), pulmonary arterial pressure (PAP; B), systemic vascular resistance (SVR; C), and pulmonary vascular resistance (PVR; D) of normal awake lambs (n=3) before and after inhaling nitric oxide (80 ppm, 1 h) at FiO2=0.3. Hemodynamic parameters did not change after ceasing to breathe nitric oxide.
Acknowledgments
The authors would like to thank Hui Zheng, Ph.D., Assistant Professor in Medicine at Harvard Medical School, Massachusetts General Hospital Biostatistics Center, Boston, Massachusetts, for statistical assistance and help with data analysis.
Sources of Financial Supporta; This work was supported by US Public Health Service Grant HL-42397 to Warren M. Zapol from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Summary Statement: Pretreatment with inhaled nitric oxide followed by continuous breathing of a low concentration of nitric oxide prevents systemic and pulmonary vasoconstriction induced by HBOC-201 administration in sheep without causing methemoglobinemia.
References
- 1.Kresie L. Artificial blood: an update on current red cell and platelet substitutes. Proc (Bayl Univ Med Cent) 2001;14:158–61. doi: 10.1080/08998280.2001.11927754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buehler PW, Alayash AI. All hemoglobin-based oxygen carriers are not created equally. Biochim Biophys Acta. 2008;1784:1378–81. doi: 10.1016/j.bbapap.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Moore EE, Johnson JL, Cheng AM, Masuno T, Banerjee A. Insights from studies of blood substitutes in trauma. Shock. 2005;24:197–205. doi: 10.1097/01.shk.0000180075.76766.fe. [DOI] [PubMed] [Google Scholar]
- 4.Reid TJ. Hb-based oxygen carriers: are we there yet? Transfusion. 2003;43:280–7. doi: 10.1046/j.1537-2995.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- 5.Raat NJ, Liu JF, Doyle MP, Burhop KE, Klein J, Ince C. Effects of recombinant-hemoglobin solutions rHb2.0 and rHb1.1 on blood pressure, intestinal blood flow, and gut oxygenation in a rat model of hemorrhagic shock. J Lab Clin Med. 2005;145:21–32. doi: 10.1016/j.lab.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 6.von Dobschuetz E, Hutter J, Hoffmann T, Messmer K. Recombinant human hemoglobin with reduced nitric oxide-scavenging capacity restores effectively pancreatic microcirculatory disorders in hemorrhagic shock. Anesthesiology. 2004;100:1484–90. doi: 10.1097/00000542-200406000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieter H, Hagen G, Waschke KF, Kohler A, Wenneis B, Bruckner UB, van Ackern K. Isovolemic hemodilution with a bovine hemoglobin-based oxygen carrier: effects on hemodynamics and oxygen transport in comparison with a nonoxygen-carrying volume substitute. J Cardiothorac Vasc Anesth. 1997;11:3–9. doi: 10.1016/s1053-0770(97)90243-3. [DOI] [PubMed] [Google Scholar]
- 9.Hess JR, MacDonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol. 1993;74:1769–78. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 10.Cohn SM, Zieg PM, Rosenfield AT, Fisher BT. Resuscitation of pulmonary contusion: effects of a red cell substitute. Crit Care Med. 1997;25:484–91. doi: 10.1097/00003246-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Chavez FA, Huerta S, Brown R, York GB, Minei JP. Resuscitation from hemorrhagic shock comparing standard hemoglobin-based oxygen carrier (HBOC)-201 versus 7.5% hypertonic HBOC-201. J Trauma. 2007;63:1113–9. doi: 10.1097/TA.0b013e3181561157. [DOI] [PubMed] [Google Scholar]
- 12.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol. 1995;79:236–42. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 13.Levy JH, Goodnough LT, Greilich PE, Parr GV, Stewart RW, Gratz I, Wahr J, Williams J, Comunale ME, Doblar D, Silvay G, Cohen M, Jahr JS, Vlahakes GJ. Polymerized bovine hemoglobin solution as a replacement for allogenic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124:35–42. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 14.Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003;5:508–17. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 15.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–97. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Hermann J, Corso C, Messmer KF. Resuscitation with recombinant hemoglobin rHb2.0 in a rodent model of hemorrhagic shock. Anesthesiology. 2007;107:273–80. doi: 10.1097/01.anes.0000270756.11669.64. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Phamacol Exp Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 19.Harrington JP, Gonzalez Y, Hirsch RE. Redox concerns in the use of acellular hemoglobin-based therapeutic oxygen carriers: the role of plasma components. Artif Cells Blood Substit Immobil Biotechnol. 2000;28:477–92. [PubMed] [Google Scholar]
- 20.Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol. 2002;30:39–51. doi: 10.1081/bio-120002726. [DOI] [PubMed] [Google Scholar]
- 21.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weimann J, Ullrich R, Hromi J, Fujino Y, Clark MW, Bloch KD, Zapol WM. Sildenafil is a pulmonary vasodilator in awake lambs with acute pulmonary hypertension. Anesthesiology. 2000;92:1702–12. doi: 10.1097/00000542-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Morel DR, Lowenstein E, Nguyenduy T, Robinson DR, Repine JE, Chenoweth DE, Zapol WM. Acute pulmonary vasoconstriction and thromboxane release during protamine reversal of heparin anticoagulation in awake sheep. Evidence for the role of reactive oxygen metabolites following nonimmunological complement activation. Circ Res. 1988;62:905–15. doi: 10.1161/01.res.62.5.905. [DOI] [PubMed] [Google Scholar]
- 24.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–6. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 25.Prielipp RC, MacGregor DA, Butterworth JF, 4th, Meredith JW, Levy JH, Wood KE, Coursin DB. Pharmacodynamics and pharmacokinetics of milrinone administration to increase oxygen delivery in critically ill patients. Chest. 1996;109:1291–301. doi: 10.1378/chest.109.5.1291. [DOI] [PubMed] [Google Scholar]
- 26.Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–7. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 27.Fratacci MD, Frostell CG, Chen TY, Wain JC, Jr, Robinson DR, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator of heparin-protamine vasoconstriction in sheep. Anesthesiology. 1991;75:990–9. doi: 10.1097/00000542-199112000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Staub NC. Pulmonary intravascular macrophages. Annu Rev Physiol. 1994;56:47–67. doi: 10.1146/annurev.ph.56.030194.000403. [DOI] [PubMed] [Google Scholar]
- 29.Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–87. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–84. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 32.Bloch KD, Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–48. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–60. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaston B. Summary: systemic effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3:170–2. doi: 10.1513/pats.200506-049BG. [DOI] [PubMed] [Google Scholar]
- 35.Olofsson C, Ahl T, Johansson T, Larsson S, Nellgard P, Ponzer S, Fagrell B, Przybelski R, Keipert P, Winslow N, Winslow RM. A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol-modified Hemoglobin (Hemospan®) in patients undergoing major orthopedic surgery. Anesthesiology. 2006;105:1153–63. doi: 10.1097/00000542-200612000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Serruys PW, Vranckx P, Slagboom T, Regar E, Meliga E, de Winter RJ, Heyndrickx G, Schuler G, van Remortel EAM, Dube GP, Symons J. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. Eurointervention. 2008;3:600–9. doi: 10.4244/eijv3i5a108. [DOI] [PubMed] [Google Scholar]
- 37.Koh E, Niimura J, Nakamura T, Yamakage H, Takahashi H. Long-term inhalation of nitric oxide for a patient with primary pulmonary hypertension. Jpn Circ J. 1998;62:940–2. doi: 10.1253/jcj.62.940. [DOI] [PubMed] [Google Scholar]
- 38.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. Br Med J. 2007;334:779–86. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Figure 1: Mean arterial pressure (MAP; A), pulmonary arterial pressure (PAP; B), systemic vascular resistance (SVR; C), and pulmonary vascular resistance (PVR; D) of normal awake lambs (n=3) before and after inhaling nitric oxide (80 ppm, 1 h) at FiO2=0.3. Hemodynamic parameters did not change after ceasing to breathe nitric oxide.