Abstract
Background
Data obtained from screened newborns and from persons at known risk for Fabry disease suggest that this condition is much more common in Germany than previously assumed. Its clinical manifestations are very diverse, and its differential diagnosis is correspondingly broad. Thus, there is often a delay before the diagnosis of Fabry disease is established.
Methods
Selective literature search with special attention to studies of large groups of patients with respect to clinical manifestations, diagnostic evaluation, and treatment.
Results
The number of patients carrying the diagnosis of Fabry disease in Germany lies far below what would be expected from published prevalence figures from other countries. Angiokeratoma, acroparesthesia, hypertrophic cardiomyopathy, impaired sweating and corneal opacification (cornea verticillata) are typical manifestations of Fabry disease; many patients also have other, nonspecific complaints, such as gastrointestinal disturbances. It has been clearly shown that women can manifest the entire range of clinical manifestations. Studies involving large groups of patients have improved our understanding of hearing impairment and tinnitus in Fabry disease. Therapeutic trials are currently in progress to determine whether enzyme substitution can delay the occurrence of life-threatening sequelae such as progressive renal failure and cerebrovascular events.
Conclusions
Fabry disease is still underdiagnosed.
The average delay from the onset of symptoms to diagnosis is more than a decade. Treatment with human alpha-galactosidase A produced with genetic technology can improve most of the disease’s manifestations.
Keywords: lysosomal storage disease, Fabry disease, enzyme substitution, molecular medicine, diagnosis
Lysosomes are membrane bound organelles that contain 50 to 60 acid hydrolases and constitute a kind of cellular digestive tract. If one of these enzymes is missing, the lysosomal metabolism is interrupted and certain metabolites accumulate. Diseases that are due to a lysosomal enzyme deficiency are known as lysosomal storage diseases. Since lysosomes are present in most cells in the body, storage diseases manifest as multisystem pathologies.
One of the storage diseases is Fabry disease, which is due to X-linked, inherited alpha-galactosidase A deficiency (e1). As a result of the enzyme deficiency, the sphingolipid globotriaosylceramide (Gb3) accumulates in the lyosomes. Fabry disease is still regarded as a rare disease. However, studies of risk groups and prospective data collections from neonatal screening imply a much higher prevalence than hitherto assumed (Table 1). The reasons for the substantial variations in the prevalence rates are complex. The clinical course of Fabry disease is heterogeneous and variable, especially in women. The range of possible differential diagnoses is broad and may touch many medical subspecialties (Table 2). The risk of a delayed or incorrect diagnosis is correspondingly high. The time period from symptom onset to the correct diagnosis is long: 13 years in men and 17 years in women (e7).
Table 1. Prevalence rates of Fabry disease in different populations, 1999–2007.
| Method | Incidence | Source | |
| Retrospective analysis of diagnosed cases versus numbers of births | 1 : | 117 000 | Meikle et al. 1999 (e2) |
| Retrospective analysis of known causes of renal replacement therapy using dialysis | ~1 : | 16 000 | Thadhani et al. 2002 (e3) |
| Secondary screening of patients having renal replacement therapy using dialysis | 2 : | 1000 | Ichinose et al. 2005 (e4) |
| Secondary screening of patients having renal replacement therapy using dialysis | 1.6 : | 1000 | Kotanko et al. 2004 (e5) |
| Secondary screening of patients with cryptogenic stroke | 5: | 100 (Men) | Rolfs et al. 2005 (17) |
| 2.4: | 100 (Women) | ||
| Primary neonatal screening | 1 : | 3100 (Men) | Spada et al. 2006 (1) |
| Secondary screening of patients with hypertrophic cardiomyopathy | 1.8: | 100 (Men) | Monserrat et al. 2007 (e6) |
| 5: | 100 (Women) | ||
Table 2. Range of possible differential diagnoses.
| Organ (system) | Symptoms of Fabry disease | Possible differential diagnoses and/or misdiagnoses |
| Skin | Angiokeratoma | Fucosidosis, sialidosis, N-acetylgalactosamine deficiency, acral pseudolymphomatous angiokeratoma of childhood |
| Hypohidrosis/anhidrosis | Horner syndrome, therapy with topiramate, acetylcholine intoxication, ectodermal dysplasia | |
| Hyperhidrosis | Primary hyperhidrosis | |
| Lymphedema | Chronic venous insufficiency, rheumatic disorders | |
| Peripheral nervous system | (Neuropathic) pain | Rheumatic disorders, fibromyalgia, (cluster) headache, migraine, diabetic neuropathy, recurrent fever syndromes (for example, TRAPS), porphyria, uremic neuropathy, Guillain-Barré syndrome, hereditary neuropathy |
| Gastrointestinal tract | Abdominal pain, diarrhea, constipation, delayed intestinal passage | Gastritis, duodenal ulcer, celiac disease, gastrointestinal hemorrhage, Crohn’s disease, ulcerative colitis, diverticulitis, functional dyspepsia, irritable bowel syndrome, familial Mediterranean fever |
| Eyes | Cornea verticillata | Therapy with amiodarone, flecainide, tamoxifen; fucosidosis |
| Tortuositas vasorum | Diabetes mellitus, arterial hypertension, nephrotic syndrome, neurofibromatosis type 1, fibromuscular dysplasia, Rendu-Osler-Weber disease, velocardiofascial syndrome | |
| Uveitis | Rheumatic disorders (for example, juvenile idiopathic arthritis, ankylosing spondylitis), tubulointerstitial nephritis and uveitis syndrome (TINU), Behçet’s disease, sarcoidosis, Crohn’s disease | |
| Conjunctival aneurysms | Kawasaki syndrom,Diabetes mellitus | |
| Ears | Acute/chronic hearing loss | Apoplexy, multiple sclerosis, leopard syndrome |
| Tinnitus | Otosclerosis, borreliosis, sudden deafness, Menière’s disease, acoustic neurinoma | |
| Dizziness | Benign paroxysmal positional vertigo, Menière’s disease, vestibular neuritis,cerebellar/brain stem infarction | |
| Heart | Angina pectoris,myocardial infarction | Atherosclerosis |
| Palpitations | Atrial fibrillation, Wolf-Parkinson-White syndrome, hyperthyroidism, drug induced palpitations | |
| Cardiomyopathy | Mitochondriopathies, Long QT syndrome, myocarditis, Pompe disease, Neimann-Pick disease, hemochromatosis, Duchenne/Becker muscular dystrophy, neurofibromatosis type 1, systemic Lupus erythematodes, rheumatoid arthritis, dermatomyositis | |
| Valvular disorders | Endocarditis, rheumatic disorders, mucopolysaccharidoses | |
| Impaired variability of cardiac frequency | Arterial hypertension, mitral valve prolapse, diabetes mellitus, Sjögren syndrome, MELAS syndrome, obstructive sleep apnea | |
| Kidneys | Proteinuria/progressive renal failure | Diabetes mellitus, arterial hypertension, glomerulonephritis, systemic lupus erythematodes, hemolytic-uremic syndrome, gout, amyloidosis, diabetes mellitus, Schönlein-Henoch nephritis |
| Central nervous system | TIA, apoplexy, white matter lesions | Atherosclerosis, multiple sclerosis, mitochondriopathies, CADASIL |
TRAPS, TNF-receptor-associated periodic fever;
MELAS, mitochondrial encephalopathy, lactic acidosis, stroke-like symptoms;
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy
This review article shows the clinical diversity of Fabry disease and draws attention to this underdiagnosed and often forgotten pathology. A diagnostic algorithm is presented, as is an overview of therapeutic options.
We conducted a selective literature review, which took into account especially recent studies that describe results from larger patient cohorts or present completely new aspects of pathophysiology, clinical presentation, or treatment.
Clinical picture
The Box shows an overview of the range of possible complaints.
Box. Range of possible manifestations.
Neuropathic pain*1
Angiokeratoma*1
Disturbed sweating*1
Proteinuria, (progressive) renal failure, abdominal pain, diarrhea, constipation
Tinnitus, hearing loss
hypertrophic cardiomyopathy, cardiac arrhythmia, myocardial infarction
Corneal verticillata, Tortuositas vasorum
TIA, stroke, depression
*1 may be ubiquitous
Skin
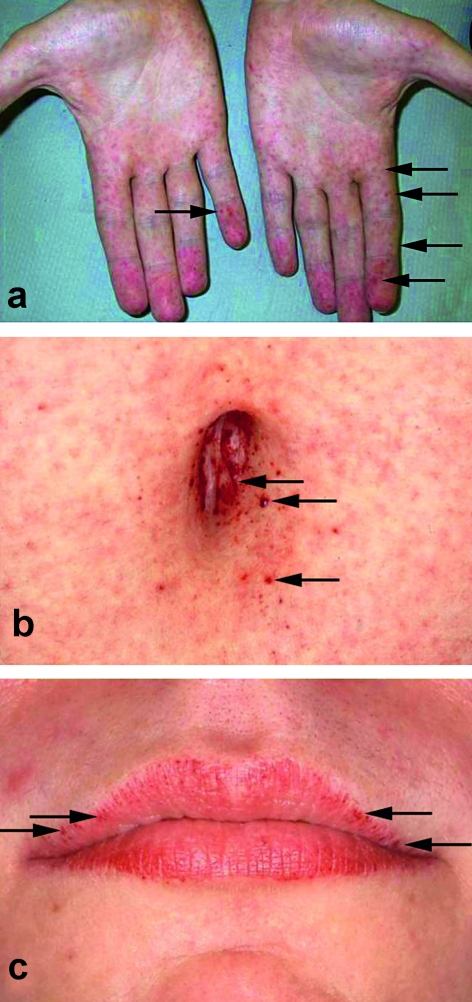
In children, the classic angiokeratoma (Figure 1) is found in only about 30% of those younger than 16. In adults, however, the pinhead sized, mostly singly positioned, reddish-brownish efflorescences (2) are seen in two thirds of male patients and more than a third of female patients. The diagnosis is, however, rarely made by a dermatologist (2). Typical locations are the fingertips, the bathing trunk area, the buttocks, and the periumbilical area, but angiokeratoma can also occur on the mucosa—for example, in the gastrointestinal tract (e8). Further skin manifestations are teleangiectasias, lymphedema, and sweating disturbances. In most cases, hypohidrosis is present (in 53% of men and 28% of women); more rarely, hyperhidrosis or anhidrosis (total absence of sweat secretion) (2). Another indication of Fabry disease may be intolerance to rising environmental temperatures.
Figure 1.
Angiokeratoma a) on the hands; b) periumbilically; c) on the lips, kindly provided by Dr Thomas Jansen, Bochum; reproduced from Beck M: "Fabry disease: clinical manifestations, diagnosis and therapy, 2nd ed. 2007" with permission from Oxford PharmaGenesis Ltd.
The cause is apparently impaired sympathetic innervation of the skin, as well as dysfunction of the sweat glands from deposition of storage material. The development of lymphedema is accompanied by obliteration, vascular ectasias, and/or increased permeability of the lymphatic vessels (e10, e12).
Pain
Male patients tend to experience acute, mostly burning, pain from the 14th year of life, and women from the age of 19 (3). Altogether, more than 70% of patients experience pain (3). In addition to the usual acroparesthesias, any region of the body may be affected. Some 15% to 30% of patients complain about neck pain and headache, and it is not always possible to distinguish such headaches from migraine or cluster headaches because imaging methods usually do not help to explain the pain. Physical activity, rising environmental or body temperatures, and concomitant illnesses may trigger pain crises. Foods such as coffee, meat, and alcohol may trigger pain, as can psychological stress. Not only acute pain, but also chronic neuropathic pain may develop.
The pain and disrupted sensation of vibration and temperature are due to stored sphingolipids in the dermal axons, which are mainly located in the thin myelinated A-δ fibers (e13).
Gastrointestinal symptoms
More than 50% of patients report gastrointestinal symptoms (4). Up to 50% of patients with Fabry disease complain of abdominal pain. The average age at which these symptoms manifest is 14 (4), which is also the age at which acroparesthesias set in (3). Both forms of pain manifestation are accompanied by the same histological changes to the neurons. Patients usually report switches from diarrhea to constipation, which may mimic irritable bowel syndrome (e14). No inflammatory changes are present; the symptoms are due to intestinal neuropathy with storage material on the smooth muscle cells, endothelial cells, and ganglial cells (e8). Meissner’s plexuses are vacuolized (e15). The result may be an early sensation of satiety and delayed intestinal passage (e8, e15, e16).
Eyes
Corneal opacities (corneal verticillata) have been described as almost pathognomonic for Fabry disease. As a rule, these changes do not lead to impaired vision (e17, e18). They occur in 75% of women and up to 90% of men with Fabry disease (5, e18). Corresponding corneal changes can be seen prenatally (e19) and are usually easy to detect in the ophthalmological examination using a slit lamp. In addition to treatment with amiodarone, Fabry disease is the most common cause of this form of corneal opacity (e20), and taking a simple medication history often helps clarify the cause.
Independently of corneal changes, up to 75% of men and 20% of women with Fabry disease develop tortuous retinal vessels (tortuositas vasorum) (5, e18). Corresponding changes have even been observed in infants and apparently become more frequent with age (5). Rarer ophthalmological symptoms include uveitis (e21, e22), closure of the central artery (e23), and aneurysmatic vessels in the conjunctiva (e18, e24).
Ears
Sensorineural hearing loss seems to be the most important ear manifestation of Fabry disease. Prevalence rates of acute hearing loss in Fabry disease, which develops within a few hours to days and is initially often reversible, range from 5% to 30% (6, 7, e25). Acute hearing loss is thus 60 times more common in Fabry disease than in the normal population; it affects twice as many men as women (7). Most patients experience slowly progressive hearing loss that permanently affects both ears and all frequencies (6, 7, e28). Onset of hearing loss is in the second decade of life in men and in the fourth decade in women. Hearing loss for higher frequencies develops more rapidly than for the lower frequencies (7). Remarkably, more than a third of patients in whom audiometric tests have shown hearing loss do not report any problems (7). Almost two thirds of women, but only slightly more than 40% of men, with Fabry disease experience tinnitus (6). Independently of the hearing loss or tinnitus, most patients with Fabry disease have damaged auditive functioning of the ear as well as a damaged vestibular organ (8). The most likely cause of the acute hearing loss are microvascular events (8). Chronic hearing loss, however, is usually the result of Gb3 accumulation in the audiovestibular ganglia and vessels of the cochlea and is therefore termed sensorineural (e26).
Progressive renal failure, cardiomyopathy, and myocardial infarction, as well as transient ischemic attacks (TIAs) and strokes reduce the survival time for male, untreated patients with Fabry disease to an average of 55 years, for women to 70 years (9).
Cardiovascular system
On average, more than 50% of patients with Fabry disease have cardiac symptoms at the age of 36 (10). Some 33% of women and more than 50% of men with Fabry disease—more with advancing age—who do not receive treatment develop progressive left ventricular hypertrophy (LVH). Conversely, a secondary screening study for left ventricular cardiomyopathy identified Fabry disease in 15 of 508 patients (11). In addition to cardiomyopathy, other cardiac symptoms are common, such as a shortened PR interval, a negative T wave, and a high amplitude. Up to 20% of male and female patients experience cardiac arrhythmias (10, e27); this may also affect children (10). Clinically relevant valvular disorders are present in 15% of patients (12). In spite of the relative frequency of problems associated with angina pectoris, patients with Fabry disease rarely experience myocardial infarctions due to stenosis (e28). Cardiac symptoms do not manifest only in adults but also affect children and adolescents. Manifest left ventricular hypertrophy was found in 7 out of 20 children; the remaining patients had a left ventricular mass above the 75th percentile of healthy children (12). Left ventricular functional disorders can be detected before the myocardium thickens (e29). In childhood, impaired variability of heart frequency may be observed, which is indicative of involvement of the parasympathetic and sympathetic nervous system (12).
Pathophysiologically, storage of Gb3 in myocardial cells, cells of the conduction system (e30), and smaller coronary vessels has been found (e31).
Kidneys
Proteinuria is regarded as the earliest sign of clinically relevant renal involvement in Fabry disease; it is found in 10% of all children younger than 18 years who have Fabry disease. In individual cases, this may occur at the age of 2 years (14). At age 35 it is present in about half of male patients. By age 47, half of all untreated male patients with Fabry disease have developed terminal renal failure. Similar data are also available for female patients (e28), and in individual cases, renal failure develops in adolescence (15). Macroscopic changes to the kidneys are seen in 50% of adults with Fabry disease—for example, as renal cysts—and their prevalence seems to increase with age (e33, e34).The cause of renal failure in Fabry disease is glomerular damage (14, 15). Renal biopsies in children have shown that lipid storage in podocytes and glomerular, interstitial, or vascular changes develop even before clinical renal functional impairment occurs (14).
Central nervous system
The most severe neurological complications in Fabry disease are TIA and (ischemic) stroke. Almost 25% of patients experience a cerebrovascular event over the course of their disease (mean age in men, 34 years; in women, 54 years) (16). Clinical precursors may be hearing loss, dizziness, migraine, and diplopic images. It must be born in mind that not only are patients with Fabry’s disease at increased risk of stroke, but that Fabry’s disease must be excluded as a possible cause in any patient aged under 55 who suffers a stroke (17).
In 50% of patients aged 33 to 47 years, the central nervous system shows unspecific changes in the form of white matter lesions, in half of these in combination with gray matter lesions (e35). Pathophysiologically, CNS areas with white matter lesions have a lower cerebral glucose metabolism than areas without such changes. There are also indications that the white matter lesions are caused by an imbalance of regional cerebral blood circulation and glucose metabolism (e36). A recent study has shown 87% precision for cranial MR-angiography of the basiliar artery with an increased diameter, which helps distinguish patients with Fabry disease from their peers of the same age (Fellgiebel et al. Neurology 2009; 72: 63–8).
Other complaints
A multisystem disease that is accompanied by chronic pain, that has a far too long diagnostic latency period, and that is associated with a substantially lower life expectancy is necessarily also accompanied by an increased risk of depression (e37). The quality of life of untreated patients with Fabry disease is obviously greatly reduced compared with the normal population (e38).
Diagnosis
A suspected diagnosis of Fabry disease has to be deduced from the individual clinical picture. This is crucial for affected patients; the range of possible differential diagnoses is wide (Table 2). In case of doubt, Fabry disease should be included in the range of possible diagnoses that need clarifying in all patients with atypical clinical courses, uncertain diagnoses, or an unclear clinical picture.
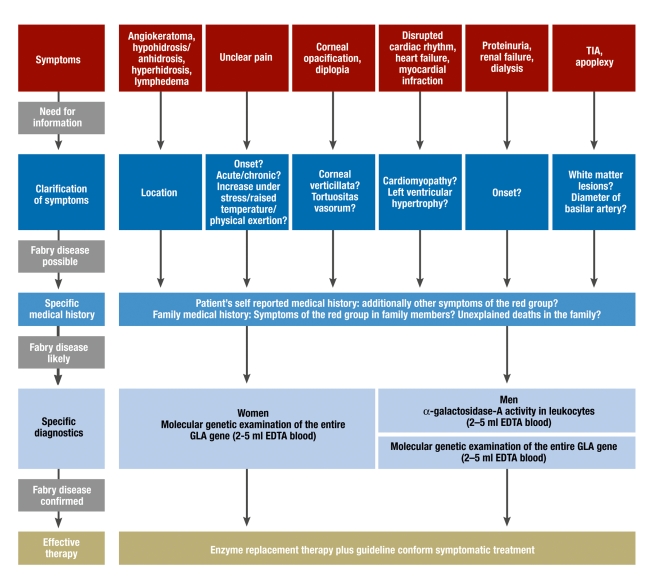
It should be noted that measuring enzyme activity—in affected men, typically <1%—often yields false positive or false negative results in women. The randomized X inactivation is responsible for this; as a result of this, the healthy or faulty GLA gene is switched on or off in each cell, independently of each other and at random. Women with suspected Fabry disease therefore need to undergo a molecular genetic examination with complete sequencing of the GLA gene. Such an examination costs from 70 euros. In principle, histopathological diagnostic tests are possible, but these have been rendered less important by the relatively simple enzyme tests and molecular diagnosis. A diagnostic flow chart for patients with Fabry disease is shown in Figure 2. Additionally, prenatal diagnosis is possible, especially by using chorionic villus sampling (e39). After the diagnosis has been made, patients should immediately be referred to human genetic counseling.
Figure 2.
Flow chart from (unspecific) symptoms to therapy of Fabry disease
Treatment and conclusions
Since 2001, two preparations have been licensed for the causal treatment of Fabry disease in the European Union. Both gene technologically produced alpha-galactosidase A variants are based on human DNA, but they are produced in different ways and have different glycosylation patterns (e40). The treatment is entirely safe; both preparations are administered as infusions every fortnight (22, 23). Differences exist with respect to dosage (0.2 mg/kg for agalsidase alfa and 1.0 mg/kg for agalsidase beta) and infusion time (40 minutes independent of body weight for agalsidase alfa and 15 mg/h for agalsidase beta). Especially in the first 3 months of treatment, adverse medication effects may occur, which should be classed primarily as allergic reactions. Further to headaches, hot flushes, and a raised temperature, patients may develop nausea and vomiting, flushing, and chills. After primary treatment of these problems (stopping the infusion; glucocorticoids, H1-receptor blockers, and, if required, H2-receptor blockers), the infusion can, in all experience, be continued.
The therapy has to be continued for a patient’s entire lifetime; the costs are substantial, amounting to 250 000 euros per patient per year, and are independent of which of the two preparations patients choose after receiving comprehensive information. Since this is the only causal therapeutic option for Fabry disease, the German health insurers cover the costs, and the prescription and administration of the therapy are classed as an additional position that does not affect the practice’s drug budget.
Table 3 provides an overview of the positive effects of enzyme replacement therapy as described thus far. To enable better understanding, we classified the references by type of study design. Obviously, it has been possible for only very few studies to be conducted in a double blind, randomized, and controlled fashion, as patients with a known diagnosis of Fabry disease were given causal therapy after the preparations had become licensed. The available information about the long term treatment with enzyme replacement therefore comes mostly from cohort studies that were developed from the two available patient registries, or from open extension studies of the phase III trials. Nonetheless, it is clear that patients with Fabry disease benefit from enzyme replacement. Further to an improved quality of life, the function of the vital organs improved significantly, or the progression of the disease was halted. However, even 8 years after enzyme replacement therapy for Fabry disease has been introduced, many therapeutic questions remain unanswered—for example, whether the treatment is able to prevent relevant organ manifestations and reduce mortality due to Fabry disease. These and other questions are currently the subject of clinical research.
Table 3. Positive effects reported for enzyme replacement therapy (ERT) in Fabry’s disease.
| Organ (system) | Symptoms | Effect with enzyme replacement therapy | Study design | Source |
| Nervous system | Pain | Pain reduction | Double blind, randomized, controlled | 23 |
| Cohort study | 3 | |||
| Peripheral neuropathy | Improved function of peripheral nerves | Open controlled study | e45 | |
| Improved function of peripheral nerves | Open extension study of a double blind, randomized, controlled trial | e46 | ||
| Increased regional blood circulation in the CNS | Reversible | Double blind, randomized, controlled + 12 months open extension study | e47 | |
| Gastrointestinal tract | Abdominal pain | Reduction | Cohort study | 4 |
| Diarrhea, constipation | Tendency toward normalization of bowel movement | Cohort study | 4 | |
| Skin | Sweating | Normalization of sweating | Open extension study of a double blind, randomized, controlled trial | e46 |
| Ear | Hearing impairment, hearing loss | No progression Improvement in less severe hearing loss | Open controlled study | 8 |
| Double blind, randomized, controlled | 19 | |||
| Disturbances of equilibrium | Regression | Open controlled study | 8 | |
| Kidneys | Renal failure | Gb3 storage in glomeruli dissolved | Double blind, randomized, controlled | 22 |
| Creatinine clearance improved; decrease in number of abnormal glomeruli | Double blind, randomized, controlled | 23 | ||
| Fall in GFR is stopped | Open controlled study | e41 | ||
| Cohort study | e42 | |||
| Creatinine values fall | Cohort study | 25 | ||
| Heart | Cardiomyopathy | Regression | Double blind, randomized, controlled | 20 |
| No progression | Cohort study | e43 | ||
| Regression | Open controlled study | e44 | ||
| Disrupted variability of cardiac frequency | Normalization | Open controlled study | 21 | |
| Angina pectoris, myocardial infarction | Gb3 storage in endothelial cells dissolved | Double blind, randomized, controlled | 22 | |
| Quality of life | Improvement | Cohort study | 25 |
Key messages.
Fabry disease is a severe multisystem disorder that starts in childhood and takes a chronic course.
Progressive renal failure, advancing cardiomyopathy, and cerebrovascular events substantially reduce life expectancy in men and women.
Recent studies have suggested a much higher incidence of Fabry disease than hitherto assumed.
Therapy with gene technologically produced human alpha-galactosidase A is safe and effective.
Fabry disease should be included in the list of differential diagnoses in unclear pathologies and those that take an atypical course.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Dr Hoffmann and Professor Mayatepek have received unlimited, project bound, research support from Shire Germany GmbH, one of the manufacturers of recombinant human alpha-galactosidase A. Dr Hoffmann has also received honoraria for lectures from Shire Germany GmbH and Genzyme Ltd.
References
- 1.Spada M, Pagliardini S, Yasuda M, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orteu CH, Jansen T, Lidove O, et al. Fabry disease and the skin: data from FOS, the Fabry outcome survey. Br J Dermatol. 2007;157:331–337. doi: 10.1111/j.1365-2133.2007.08002.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann B, Beck M, Sunder-Plassmann G, Borsini W, Ricci R, Mehta A. Nature and prevalence of pain in Fabry disease and its response to enzyme replacement therapy—a retrospective analysis from the Fabry Outcome Survey. Clin J Pain. 2007;23:535–542. doi: 10.1097/AJP.0b013e318074c986. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann B, Schwarz M, Mehta A, Keshav S. Gastrointestinal symp-toms in 342 patients with Fabry disease: prevalence and response to enzyme replacement therapy. Clin Gastroenterol Hepatol. 2007;5:1447–1453. doi: 10.1016/j.cgh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 2007;91:210–214. doi: 10.1136/bjo.2006.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegemann S, Hajioff D, Conti G, et al. Hearing loss in Fabry disease: data from the Fabry Outcome Survey. Eur J Clin Invest. 2006;36:654–662. doi: 10.1111/j.1365-2362.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- 7.Ries M, Kim HJ, Zalewski CK, et al. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain. 2007;130:143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palla A, Hegemann S, Widmer U, Straumann D. Vestibular and auditory deficits in Fabry disease and their response to enzyme replacement therapy. J Neurol. 2007;254:1433–1442. doi: 10.1007/s00415-007-0575-y. [DOI] [PubMed] [Google Scholar]
- 9.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38:750–760. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linhart A, Kampmann C, Zamorano JL, et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 11.Monserrat L, Gimeno-Blanes JR, Marin F, et al. Prevalence of fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2007;50:2399–2403. doi: 10.1016/j.jacc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 12.Kampmann C, Wiethoff CM, Whybra C, Baehner FA, Mengel E, Beck M. Cardiac manifestations of Anderson-Fabry disease in children and adolescents. Acta Paediatr. 2008;97:463–469. doi: 10.1111/j.1651-2227.2008.00700.x. [DOI] [PubMed] [Google Scholar]
- 13.Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry Disease in 352 Pediatric Patients in the Fabry Registry. Pediatr Res. 2008;645:50–55. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- 14.Tondel C, Bostad L, Hirth A, Svarstad E. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–776. doi: 10.1053/j.ajkd.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Branton MH, Schiffmann R, Sabnis SG, et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81:122–138. doi: 10.1097/00005792-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Buechner S, Moretti M, Burlina AP, et al. Central nervous system involvement in anderson-fabry diease: A clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008;791:249–254. doi: 10.1136/jnnp.2008.143693. [DOI] [PubMed] [Google Scholar]
- 17.Rolfs A, Bottcher T, Zschiesche M, et al. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–1796. doi: 10.1016/S0140-6736(05)67635-0. [DOI] [PubMed] [Google Scholar]
- 19.Hajioff D, Enever Y, Quiney R, Zuckerman J, Mackermot K, Mehta A. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. J Inherit Metab Dis. 2003;26:787–794. doi: 10.1023/B:BOLI.0000009948.86528.72. [DOI] [PubMed] [Google Scholar]
- 20.Hughes DA, Elliott PM, Shah J, et al. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153–158. doi: 10.1136/hrt.2006.104026. [DOI] [PubMed] [Google Scholar]
- 21.Ries M, Gupta S, Moore DF, et al. Pediatric Fabry disease. Pediatrics. 2005;115:e344–e355. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- 22.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 23.Schiffmann R, Kopp JB, Austin HA, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 24.Moore DF, Scott LT, Gladwin MT, et al. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001;104:1506–1512. doi: 10.1161/hc3801.096352. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann B, de Lorenzo Garcia A, Mehta A, Beck M, Widmer U, Ricci R. FOS European Investigators. Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: data from FOS (Fabry Outcome Survey). J Med Genet. 2005;42:247–252. doi: 10.1136/jmg.2004.025791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Desnick RJ, Ioannou YA, Eng CM. alpha-galactosidase A deficiency: Fabry disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3733–3810. [Google Scholar]
- e2.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- e3.Thadhani R, Wolf M, West ML, et al. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61:249–255. doi: 10.1046/j.1523-1755.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- e4.Ichinose M, Nakayama M, Ohashi T, Utsunomiya Y, Kobayashi M, Eto Y. Significance of screening for Fabry disease among male dialysis patients. Clin Exp Nephrol. 2005;9:228–232. doi: 10.1007/s10157-005-0369-4. [DOI] [PubMed] [Google Scholar]
- e5.Kotanko P, Kramar R, Devrnja D, et al. Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol. 2004;15:1323–1329. doi: 10.1097/01.asn.0000124671.61963.1e. [DOI] [PubMed] [Google Scholar]
- e6.Monserrat L, Gimeno-Blanes JR, Marin F, et al. Prevalence of fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2007;50:2399–2403. doi: 10.1016/j.jacc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- e7.Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur J Clin Invest. 2004;34:236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- e8.O’Brien BD, Shnitka TK, McDougall R, et al. Pathophysiologic and ultrastructural basis for intestinal symptoms in Fabry’s disease. Gastroenterology. 1982;82:957–962. [PubMed] [Google Scholar]
- e9.Yamamoto K, Sobue G, Iwase S, Kumazawa K, Mitsuma T, Mano T. Possible mechanism of anhidrosis in a symptomatic female carrier of Fabry’s disease: an assessment by skin sympathetic nerve activity and sympathetic skin response. Clin Auton Res. 1996;6:107–110. doi: 10.1007/BF02291231. [DOI] [PubMed] [Google Scholar]
- e10.Lao LM, Kumakiri M, Mima H, et al. The ultrastructural characteris-tics of eccrine sweat glands in a Fabry disease patient with hypohidrosis. J Dermatol Sci. 1998;18:109–117. doi: 10.1016/s0923-1811(98)00032-2. [DOI] [PubMed] [Google Scholar]
- e11.Schiffmann R, Floeter MK, Dambrosia JM, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28:703–710. doi: 10.1002/mus.10497. [DOI] [PubMed] [Google Scholar]
- e12.Amann-Vesti BR, Gitzelmann G, Widmer U, Bosshard NU, Steinmann B, Koppensteiner R. Severe lymphatic microangiopathy in Fabry disease. Lymphat Res Biol. 2003;1:185–189. doi: 10.1089/153968503768330229. [DOI] [PubMed] [Google Scholar]
- e13.Dutsch M, Marthol H, Stemper B, Brys M, Haendl T, Hilz MJ. Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol. 2002;19:575–586. doi: 10.1097/00004691-200212000-00011. [DOI] [PubMed] [Google Scholar]
- e14.Hoffmann B, Keshav S. Gastrointestinal symptoms in Fabry disease: everything is possible, including treatment. Acta Paediatr Suppl. 2007;96:84–86. doi: 10.1111/j.1651-2227.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- e15.Cable WJ, Kolodny EH, Adams RD. Fabry disease: impaired autonomic function. Neurology. 1982;32:498–502. doi: 10.1212/wnl.32.5.498. [DOI] [PubMed] [Google Scholar]
- e16.Argoff CE, Barton NW, Brady RO, Ziessman HA. Gastrointestinal symptoms and delayed gastric emptying in Fabry’s disease: response to metoclopramide. Nucl Med Commun. 1998;19:887–891. doi: 10.1097/00006231-199809000-00009. [DOI] [PubMed] [Google Scholar]
- e17.Ohkubo H. Several functional and fluorescein fundus angiographic findings in Fabry’s disease. Ophthalmologica. 1988;196:132–136. doi: 10.1159/000309889. [DOI] [PubMed] [Google Scholar]
- e18.Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A. Oph-thalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Experiment Ophthalmol. 2005;33:164–168. doi: 10.1111/j.1442-9071.2005.00990.x. [DOI] [PubMed] [Google Scholar]
- e19.Tsutsumi A, Uchida Y, Kanai T, Tsutsumi O, Satoh K, Sakamoto S. Corneal findings in a foetus with Fabry’s disease. Acta Ophthalmol (Copenh) 1984;62:923–931. doi: 10.1111/j.1755-3768.1984.tb08444.x. [DOI] [PubMed] [Google Scholar]
- e20.Kono JO, Podskarbi T, Shin Y, Lanzl I. Oligosymptomatic cornea verticillata in a heterozygote for Fabry disease: a novel mutation in the alpha-galactosidase gene. Cornea. 2003;22:175–177. doi: 10.1097/00003226-200303000-00020. [DOI] [PubMed] [Google Scholar]
- e21.Shen YD, Yang CM, Huang JS. Fabry disease manifesting as chronic uveitis—treated with enzyme replacement therapy. Eye. 2007;21:431–432. doi: 10.1038/sj.eye.6702517. [DOI] [PubMed] [Google Scholar]
- e22.Elstein D, Altarescu G, Zimran A. Uveitis and Fabry disease. Eye. 2007;21:448–449. doi: 10.1038/sj.eye.6702625. [DOI] [PubMed] [Google Scholar]
- e23.Andersen MV, Dahl H, Fledelius H, Nielsen NV. Central retinal artery occlusion in a patient with Fabry’s disease documented by scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh) 1994;72:635–638. doi: 10.1111/j.1755-3768.1994.tb07193.x. [DOI] [PubMed] [Google Scholar]
- e24.Spaeth GL, Frost P. Fabry’s disease. Its ocular manifestations. Arch Ophthalmol. 1965;74:760–769. doi: 10.1001/archopht.1965.00970040762005. [DOI] [PubMed] [Google Scholar]
- e25.Germain DP, Avan P, Chassaing A, Bonfils P. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet. 2002;3 doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Schachern PA, Shea DA, Paparella MM, Yoon TH. Otologic histopathology of Fabry’s disease. Ann Otol Rhinol Laryngol. 1989;98:359–363. doi: 10.1177/000348948909800509. [DOI] [PubMed] [Google Scholar]
- e27.Shah JS, Hughes DA, Sachdev B, et al. Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry disease. Am J Cardiol. 2005;96:842–846. doi: 10.1016/j.amjcard.2005.05.033. [DOI] [PubMed] [Google Scholar]
- e28.Chimenti C, Hamdani N, Boontje NM, et al. Myofilament degradation and dysfunction of human cardiomyocytes in Fabry disease. Am J Pathol. 2008;172:1482–1490. doi: 10.2353/ajpath.2008.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Weidemann F, Breunig F, Beer M, et al. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
- e30.Frustaci A, Chimenti C. Images in cardiovascular medicine. Cryptogenic ventricular arrhythmias and sudden death by Fabry disease: prominent infiltration of cardiac conduction tissue. Circulation. 2007;116:e350–e351. doi: 10.1161/CIRCULATIONAHA.107.723387. [DOI] [PubMed] [Google Scholar]
- e31.Elliott PM, Kindler H, Shah JS, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart. 2006;92:357–360. doi: 10.1136/hrt.2004.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Whybra C, Kampmann C, Willers I, et al. Anderson-Fabry disease: clinical manifestations of disease in female heterozygotes. J Inherit Metab Dis. 2001;24:715–724. doi: 10.1023/a:1012993305223. [DOI] [PubMed] [Google Scholar]
- e33.Ries M, Bettis KE, Choyke P, et al. Parapelvic kidney cysts: a distinguishing feature with high prevalence in Fabry disease. Kidney Int. 2004;66:978–982. doi: 10.1111/j.1523-1755.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- e34.Glass RB, Astrin KH, Norton KI, et al. Fabry disease: renal sonographic and magnetic resonance imaging findings in affected males and carrier females with the classic and cardiac variant phenotypes. J Comput Assist Tomogr. 2004;28:158–168. doi: 10.1097/00004728-200403000-00002. [DOI] [PubMed] [Google Scholar]
- e35.Crutchfield KE, Patronas NJ, Dambrosia JM, et al. Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology. 1998;50:1746–1749. doi: 10.1212/wnl.50.6.1746. [DOI] [PubMed] [Google Scholar]
- e36.Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R. White matter lesions in Fabry disease occur in „prior“ selectively hypometabolic and hyperperfused brain regions. Brain Res Bull. 2003;62:231–240. doi: 10.1016/j.brainresbull.2003.09.021. [DOI] [PubMed] [Google Scholar]
- e37.Cole AL, Lee PJ, Hughes DA, Deegan PB, Waldek S, Lachmann RH. Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherit Metab Dis. 2007;30:943–951. doi: 10.1007/s10545-007-0708-6. [DOI] [PubMed] [Google Scholar]
- e38.Miners AH, Holmes A, Sherr L, Jenkinson C, MacDermot KD. Assessment of health-related quality-of-life in males with Anderson Fabry Disease before therapeutic intervention. Qual Life Res. 2002;11:127–133. doi: 10.1023/a:1015009210639. [DOI] [PubMed] [Google Scholar]
- e39.Desnick RJ. Prenatal diagnosis of Fabry disease. Prenat Diagn. 2007;27:693–694. doi: 10.1002/pd.1767. [DOI] [PubMed] [Google Scholar]
- e40.Lee K, Jin X, Zhang K, et al. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13:305–313. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- e41.Feriozzi S, Schwarting A, Sunder-Plassmann G, West M, Cybulla M. Agalsidase alfa slows the decline in renal function in patients with Fabry disease. Am J Nephrol. 2008;29:353–361. doi: 10.1159/000168482. [DOI] [PubMed] [Google Scholar]
- e42.Schwarting A, Dehout F, Feriozzi S, Beck M, Mehta A, Sunder-Plassmann G. Enzyme replacement therapy and renal function in 201 patients with Fabry disease. Clin Nephrol. 2006;66:77–84. [PubMed] [Google Scholar]
- e43.Kovacevic-Preradovic T, Zuber M, et al. Anderson-Fabry disease: long-term echocardiographic follow-up under enzyme replacement therapy. Eur J Echocardiogr. 2008;9:729–735. doi: 10.1093/ejechocard/jen129. [DOI] [PubMed] [Google Scholar]
- e44.Weidemann F, Breunig F, Beer M, et al. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
- e45.Hilz MJ, Brys M, Marthol H, Stemper B, Dütsch M. Enzyme replacement therapy improves function of C-, Adelta-, and Abeta-nerve fibers in Fabry neuropathy. Neurology. 2004;62:1066–1072. doi: 10.1212/01.wnl.0000118207.84514.40. [DOI] [PubMed] [Google Scholar]
- e46.Schiffmann R, Floeter MK, Dambrosia JM, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28:703–710. doi: 10.1002/mus.10497. [DOI] [PubMed] [Google Scholar]
- e47.Moore DF, Altarescu G, Ling GS, et al. Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke. 2002;33:525–531. doi: 10.1161/hs0202.102601. [DOI] [PubMed] [Google Scholar]