Abstract
CD59, a broadly expressed GPI anchored molecule, regulates formation of the membrane attack complex of the complement cascade. We previously demonstrated that mouse CD59 also down-modulates CD4+ T cell activity in vivo. In this study, we explored the role of CD59 on human CD4+ T cells. Our data demonstrate that CD59 is up regulated on activated CD4+ T cells and serves to down-modulate their activity in response to polyclonal and antigen- specific stimulation. The therapeutic potential of this finding was explored using T cells isolated from colorectal cancer patients. The findings were striking and indicated that blockade of CD59 significantly enhanced the CD4+ T cell response to two different tumor antigens. These data highlight the potential for manipulating CD59 expression on T cells for boosting weak immune responses, such as those found in individuals with cancer.
Keywords: Human, T cells, Tumour immunity
Introduction
CD59 is a GPI anchored glycoprotein expressed on all human tissues that inhibits the formation of the membrane attack complex in the complement (C) cascade (1). As well as regulating complement, CD59 influences the activity of CD4+ T cells. In vitro analyses of human T cells have shown that antibody cross-linking of CD59 promotes T cell activation, implying a costimulatory effect of CD59 on T cells (2). Our recent studies utilising CD59a –deficient mice (CD59a−/− mice) question the relevance of these findings since virus-specific CD4+ T cells from CD59a−/− mice exhibited enhanced proliferation and cytokine production compared to wild-type animals. This implies that, under physiological conditions, CD59 down-modulates CD4+ T cell responses(3), perhaps serving to limit T cell mediated immunopathology (4). With this in mind, we reasoned that blockade of CD59 would boost antigen-specific T cell responses thereby serving as a target for immunotherapeutic intervention for diseases characterized by weak immune responses such as cancer. To address whether this is indeed the case this study sought to to explore how CD59 impacts on human CD4+ T cells and to determine whether its blockade boosts immune responses in patients with colorectal cancer.
Materials and Methods
Generation of Fab fragments
Mapping studies have been described previously for various human CD59-specific antibodies (5). MEM-43 and HC-1 were selected based on their epitope binding regions. The CD59-specific antibodies, MEM-43 and HC-1 (both IgG2a) were incubated in digestion buffer (20 mM sodium phosphate, 10 mM EDTA, 20mM Cysteine HCl: pH 7.0) and immobilized papain (Pierce, Northumberland UK) at an enzyme to substrate ratio of 1/50 (w/w) for 5 hours at 37° C with constant mixing. Post incubation, the papain was separated from the antibodies by centrifugation. The Fc fragments were purified using a Hi-Trap protein A Sepharose column leading to a pure population of Fab fragments. This process was carried out using endotoxin free reagents under sterile conditions. The molecular weight and purity of the Fab fragments were evaluated using a 10% SDS-PAGE gel. Endotoxin levels (< 5 EU/ml) were assessed using the Limulus Amebocyte Lysate (Cambrex, New Jersey, USA) as described by the manufacturer.
Cell Preparation
Peripheral blood mononuclear cells and CD4+ T cells were purified from healthy donors and patients with colorectal cancer (CRC) as described earlier. (6) The study on CRC patients has been reviewed and approved by a local ethical committee.
Fluorescence staining
Samples were stained with fluorescently-labeled antibodies to CD4 (clone RPA-T4), CD25 (clone M-A251), CD45RA, CD45RO (clone UCHL1) and CTLA4 (clone BNI3) (BD Pharmingen, Oxford, UK), CD4 (clone S3.5) (Caltag, Bukinggham, UK), CD25 (clone 4E3) (Miltenyi Biotec, Surrey, UK) and CD69 (clone FN50) (eBioscience, San Diego, USA) All staining was performed in phosphate buffered saline (PBS), 2.5% FCS, 5 mM EDTA and intracellular staining was performed using the cytofix, cytoperm kit (BD Pharmingen, Oxford, UK). FOXP3 staining was performed using the FITC anti-human FOXP3 staining kit (clone PCH101) (71-5776 eBioscience, San Diego, USA). CD59 expression was detected using a biotinylated antibody (MEM-43) followed by SA-PerCPCy5.5. Lymphocytes were gated on forward and side scatter profiles. The Fab fragments of MEM-43 and HC-1 were tested for their ability to bind to CD59 by incubating both CD59+ve and CD59−ve U937 cells (U937CD59+ve and U937CD59−ve cells respectively) with the Fab fragments followed by fluorescently labeled secondary antibody (PE conjugated anti-IgG2a). All flow cytometric analsyis was performed on a FACSCalibur (BD Biosciences, Oxford, UK).
Complement-mediated lysis
The complement inhibitory activity of intact MEM-43 and HC-1 antibodies and their Fab fragments was assessed in a C mediated lysis assay. Human erythrocytes, washed and resuspended at 1% in PBS, were sensitized with CD55 specific antibodies (100 μg/ml) for 40 minutes at 37° C. After extensive washing with C fixation diluent (CFD;Oxoid, Basingstoke, U.K. ) the erythrocytes were incubated with either MEM-43 or HC-1 antibodies or Fab fragments at 10μg/ml per 2× 07cells for 20 minutes on ice. Erythrocytes were washed by centrifugation and aliquoted at 2×106 cells per well in a 96-well plate. The erythrocytes were then incubated with various dilutions of normal human serum for 30 minutes at 37°C. Zero and 100% lysis controls were included in the assay. Plates were centrifuged and hemoglobin levels in the supernatants were measured (by absorbance at 412 nm) and values used as an index of lysis. Percent lysis was calculated as described earlier (7).
Activation of PBMCs
PBMCs at 2 ×10 6 cells/ml were activated using beads coated with both anti-CD3 and anti-CD28 antibodies (Dynal, UK) at a bead to cell ratio of 1:4, or purified protein derivative (PPD) from tuberculin at 2μg/ml (Statens Serum Institut, Copenhagen, Denmark). In some experiments PBMCs were CFSE (2μM) labeled prior to activation (3).
T cell proliferation assay
Purified CD4+ T cells or PBMCs, either CFSE labeled or unlabelled, were incubated with Fab fragments of anti-CD59 antibodies HC-1 and MEM-43 or control mouse IgG2a at 1 or 3 μg/ml for 20 minutes on ice. After washing, cells were stimulated with anti-CD3 antibodies in the presence of irradiated PBMCs (depleted of CD4+ T cells) as APCs and proliferation assessed by thymidine incorporation or dilution of CFSE. In some experiments instead of PBMCs, irradiated U937 cells that lack CD59 were used as APCs. To assess the response to recall antigens, PBMCs were stimulated with tetanus toxoid (TT) or PPD and proliferation assessed as above. Mitotic divisions, assessed by dilution of CFSE, were calculated as described by Yamazaki et al (8).
Cross linking studies
Purified CD4+ T cells were incubated with anti-CD59 antibodies (HC-1 or MEM-43 at 1μg/ml). After washing cells twice, 100μl of polyclonal rabbit anti-mouse IgG at 5μg/ml was added. Cells were further stimulated with anti-CD3 antibodies at 1μg/ml for 3 days and proliferation was assessed by pulsing cell cultures with 1 μCi/ well of thymidine for sixteen hours before harvesting.
IFN-γ ELISPOT assays
PBMC were incubated with Fab fragments of anti-CD59 antibodies HC-1 or MEM-43 and the frequency of T cells specific for recall and tumour associated antigens, carcinoembryogenic antigen (CEA) (9) and 5T4 (10) was determined by IFN-γ ELISPOT assay as described earlier (6). In some experiments the effect of Treg depletion on antigen-specific IFN- γ production was assessed in parallel assays. CD25hi cells were depleted by magnetic separation using CD25-specific microbeads as described previously (Miltenyi Biotec, Germany) (6).
Results
CD59 expression is increased on activated CD4+ T cells
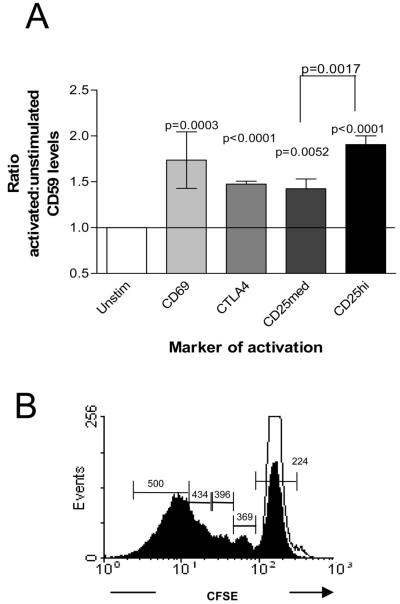
In order to understand how CD59 impacts on human CD4+ T cells, we first analyzed the pattern of CD59 expression amongst different populations of CD4+ T lymphocytes. For this purpose, PBMCs from healthy donors were phenotypically characterized using antibodies specific for T cell activation, regulation and memory markers. CD59 expression was found to be higher on freshly isolated CD4+ T cells expressing CD25 and Foxp3 as compared to CD4+ T cells negative for these markers (Table I). In accordance with a previous report, we also found that CD59 is expressed at a higher level on memory CD4+ T cells (CD45RO+) compared to naive T cells (CD45RA+) (11 and data not shown). These findings imply that CD59 expression is up-regulated on activated T cells and is most pronounced on highly differentiated T cells. To confirm this, CD59 expression was compared on PBMCs, either unstimulated or activated with anti-CD3 antibodies or recall antigens. Expression of CD59 increased upon stimulation in association with activation markers, CD69, CD25, CTLA4 and Foxp3 (Table I). The ratio of CD59 expression on activated versus unactivated cells was calculated and the increase was found to be statistically significant (Figure 1A). When CD59 expression was monitored on actively dividing CD4+ T cells using CFSE, its expression increased with successive cell divisions (Figure 1B).
Table:I.
CD59 expression on CD4+ T cells
| Unstimulated |
Anti-CD3+CD28 |
PPD |
||||
|---|---|---|---|---|---|---|
|
|
% of cells |
CD59 (MFI) |
% of cells |
CD59 (MFI) |
% of cells |
CD59 (MFI) |
| CD4+CD69− | 98.44 | 225 | 63.88 | 229 | 92.4 | 222 |
| CD4+CD69+ | 1.31 | 336 | 35.05 | 346 | 7.03 | 386 |
| CD4+CD25− | 73.73 | 225 | 50.24 | 209 | 72.20 | 217 |
| CD4+CD25med | 22.55 | 290 | 21.14 | 279 | 18.28 | 296 |
| CD4+CD25high | 1.24 | 330 | 26.89 | 406 | 6.69 | 309 |
| CD4+FoxP3− | 93.29 | 223 | 75.25 | 235 | 87.01 | 226 |
| CD4+FoxP3+ | 6.69 | 285 | 24.74 | 382 | 12.38 | 296 |
| CD4+CTLA4− | 98.1 | 225 | 78.98 | 241 | 94.85 | 222 |
| CD4+CTLA4+ | 1.02 | 330 | 18.69 | 362 | 3.97 | 342 |
PBMCs were either left unstimulated or stimulated with anti-CD3+CD28 beads or PPD. The percentage of cells within each subset is shown as well as the MFI of CD59 on each subset. Values are representative of four independent experiments performed using blood obtained from different individuals
Figure 1. CD59 expression is significantly increased on activated cells.
(A) The average ratio of CD59 expression on activated versus unactivated cells from four different individuals. The statistical significance indicated on the bar was calculated using a one sample t test. (B) Proliferation of CFSE labeled CD4+ T cells un-stimulated (open histogram) or stimulated with anti-CD3 + CD28 antibody-coated beads (filled histogram). Values on the histogram indicate MFI for CD59 expression on cells gated as indicated. The results are representative of four independent experiments performed using blood obtained from different individuals.
Blockade of CD59 enhances CD4+ T cell responses
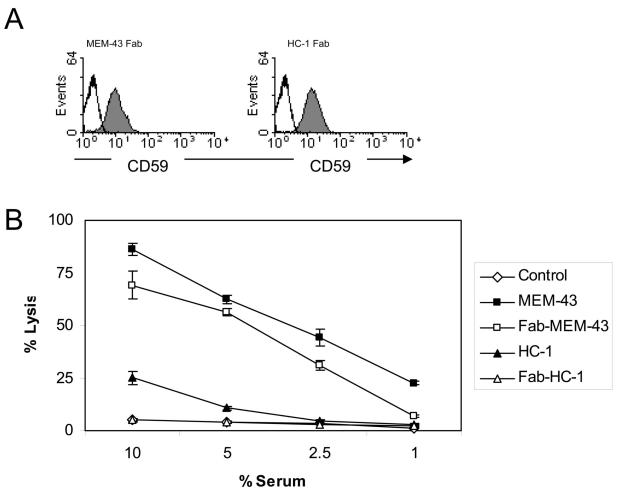
The functional significance of CD59 expression on CD4+ T cells was evaluated. As mentioned previously, we had previously found that CD59 down-modulates the activity of mouse T cells. To address whether CD59 impacts similarly on human CD4+ T cell activity, we reasoned that use of Fab fragments of CD59-specific antibodies, which would not induce cross linking, would enable us to compare the activity of CD4+ T cells expressing CD59 and those where CD59 is obscured by antibody binding. Two CD59-specific monoclonal antibodies, named MEM-43 and HC-1, were selected (9). These were selected due to their CD59-binding characteristics (5). MEM-43 binding causes significant inhibition of complement regulation by CD59 whereas HC-1 does not. Fab fragments of these antibodies, generated as described in materials and methods , were assessed for their ability to bind CD59 and to inhibit its complement regulating activity (Figures 2A – B). As expected, both Fab fragments bound CD59. (Figure 2A). To functionally test the Fab fragments, complement-mediated lysis of human erythrocytes was analyzed in the presence of the intact antibodies or the Fab fragments. As shown in Figure 2B, the Fab fragments behaved in a similar manner to the intact antibodies in that MEM-43-Fab inhibited the complement regulating activity of CD59 whereas HC-1-Fab did not. Overall therefore, the Fab fragments were deemed useful for use in experiments designed to address how CD59 impacts on CD4+ T cell activity.
Figure 2. Generation and characterization of Fab fragments.
(A) Binding of Fab fragments of CD59-specifc antibodies (MEM-43 and HC-1) was detected on CD4+ T cells by flow cytometry. (B) Haemolytic assay using intact and Fab fragments of CD59 specific antibodies MEM-43 and HC-1. Values shown are the mean ± SD in (triplicates)
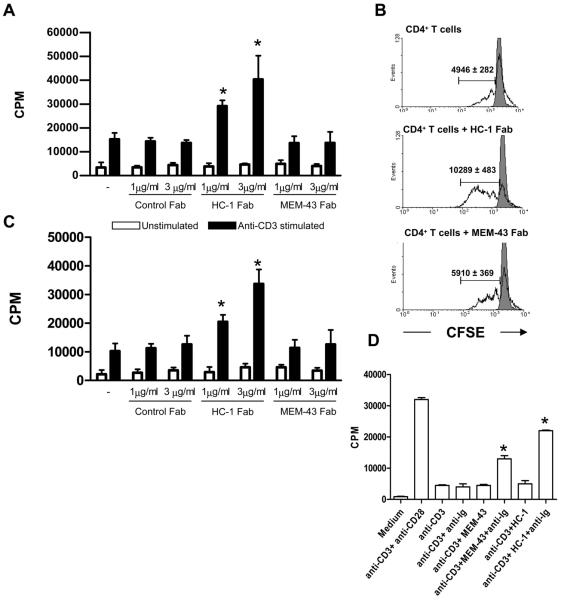
As mentioned above, we reasoned that use of Fab fragments, which would not induce cross linking of CD59, would enable us to evaluate the impact of blocking CD59 on activity of CD4+ T cells. When CD4+ T cells were incubated with the Fab fragments and subsequently stimulated with CD3-specific antibodies and APCs, those incubated with HC-1-Fab exhibited a significant and dose-dependent increase in proliferation in comparison with CD4+ T cells stimulated with anti-CD3 alone (Figure 3A). No effect was observed with MEM-43-Fab or the control Fab fragment (Figure 3A) When proliferation was assessed by CFSE dilution, more cell division was observed following incubation of the CD4+ T cells with HC-1-Fab whereas again, no effect of incubation with MEM-43-Fab was observed (Figure 3B). In experiments using CD59-ve U937 cells as APCs, the HC-1 Fab fragments increased the proliferation of CD4+ T cells in comparison to control cultures (Figure 3C). This experiment indicates that the modulatory effect of the HC-1-Fab is due to CD59 expressed on the responding CD4+ T cell.
Figure 3. Blocking CD59 on CD4+ T cells increases the immune response.
(A) Proliferation of CD4+ T cells stimulated with anti-CD3 antibodies in the presence of irradiated PBMCs as APCs and Fab fragments of antibodies to CD59 (HC-1 or MEM-43) or control Fab fragments. (B) Proliferation of CFSE labeled CD4+ T cells when stimulated with anti-CD3 antibodies in the presence of irradiated PBMCs as APC and either HC-1 or MEM-43 Fab fragments. Values (mean of triplicates) in the histograms indicate the mitotic divisions under the respective conditions. (C) Proliferation of CD4+ T cells stimulated with anti-CD3 antibodies in the presence of irradiated CD59-ve U937 cells and either HC-1 or MEM-43 Fab fragments. (D) Proliferation of CD4+ T cells stimulated with anti-CD3 antibodies and cross-linked anti-CD59 antibodies. Statistical significance (* = p< 0.05) was evaluated using the Student's t test. The results are representative of four independent experiments performed using blood obtained from different individuals.
In order to understand how the intact MEM-43 and HC-1 antibodies function when used for cross-linking CD59 on CD4+ T cells, we examined the CD4+ T cell response when CD59 was cross-linked using these intact antibodies. In accordance with previous publications, cells incubated with CD3-specific antibodies and either cross-linked MEM-43 or HC-1, showed enhanced proliferation as compared to cells incubated with CD3-specific antibodies alone (Figure 3D).
Blocking CD59 on CD4+ T cells increases response to recall antigens
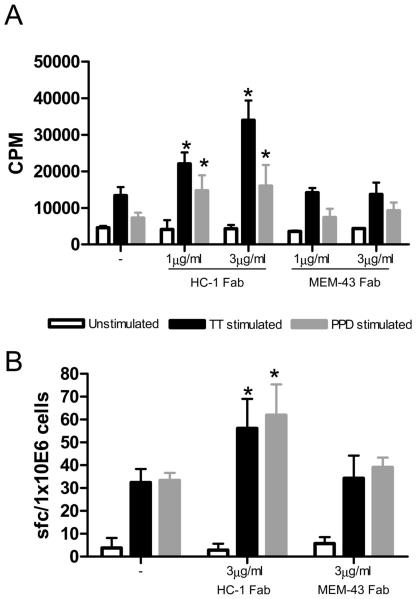
We next sought to understand how blocking CD59 affects proliferation and cytokine production following antigen-specific stimulation of CD4+ T cells. Using the Fab fragments described above, we found that T cells incubated with titrated amounts of HC-1-Fab, proliferated significantly more than cells stimulated in the presence or absence of MEM-43 Fab fragments (Figure 4A). Incubation with HC-1-Fab in the absence of antigen had no effect on T cell proliferation ruling out a non-specific effect of the Fab fragment. ELISPOT assays were subsequently performed to assess the impact of CD59 on IFN-γ production. The results of these experiments paralleled those observed in the proliferation assays since blocking CD59 with HC-1-Fab (but not MEM-43-Fab or control Fabs) increased the number of IFN-γ producing cells in an antigen-dependent manner (Figure 4B). Collectively these data, which show that blocking CD59 on T cells can enhance both antigen-specific proliferation and cytokine production, imply that CD59 normally serves to down modulate antigen-specific CD4+ T cell responses.
Figure 4. Blocking CD59 increases the CD4+ T cell response to recall antigens.
(A) Proliferation of T cells in response to recall antigens TT and PPD in the presence of Fab fragments of MEM-43 and HC-1. (B) IFN-γ response to recall antigens in the presence or absence of Fab fragments of MEM-43 and HC-1.. The bars indicate the number of spot forming cells enumerated in an ELISPOT assay for IFN-γ. Statistical significance (* = p< 0.05) was evaluated using the Student's t test. The results are representative of four independent experiments performed using blood obtained from different individuals
Blocking CD59 on CD4+ T cell increases the immune response to tumor antigens
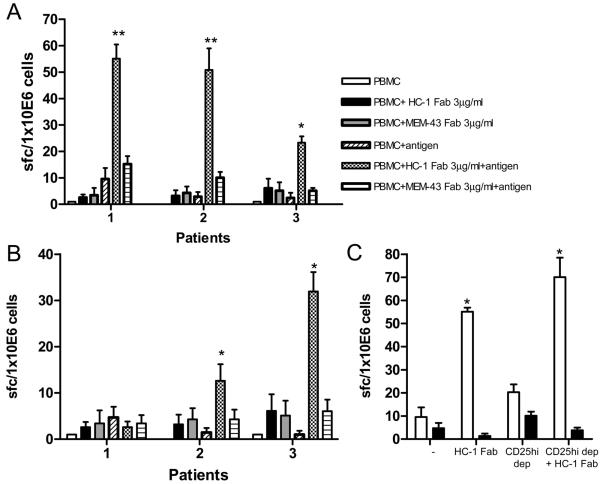
We next examined whether blockade of CD59 could be used to enhance CD4+ T cell responses generally found to be weak in nature, such as those measured in patients with colorectal cancer (CRC). For this purpose, we examined responses to two tumor-associated antigens (CEA and 5T4) using PBMC from the CRC patients in ELISPOT assays. In the presence of HC-1 Fab fragments, there was a significant and antigen-specific increase in the frequency of antigen-specific IFN-γ producing cells compared to cells incubated with MEM-43-Fab or medium alone (Figure 5 A and B). Thus blockade of CD59 can boost the CD4+ T cell response to tumor antigens in these patients.
Figure 5. Blocking CD59 increases the IFN-γ response to tumour antigens in CRC patients.
IFN-γ production in response to tumor antigens CEA (A) and 5T4 (B) in the presence and absence of Fab fragments of antibodies to CD59. The results are representative of three independent experiments with blood obtained from different individuals. (C) IFN-γ response to tumor antigens in PBMCs depleted and undepleted for CD4+CD25hi cells in the presence and absence of Fab fragments of antibodies to CD59. Statistical significance (* = p< 0.05, ** = p < 0.01) was evaluated using the Student's t test.
Since we previously found that the expression of CD59 is higher on CD4+ T cells expressing high levels of Foxp3, we examined whether the effects of CD59-blockade by the HC-1-Fab were mediated by inhibiting the activity of regulatory T cells. This was also of interest in light of our previous findings that Tregs inhibit 5T4-and CEA-specific CD4+ T cell responses in CRC patients ((6) and data not shown). Should HC-1-Fab act by blocking the inhibitory effects of Tregs, incubation with the Fab fragments would be expected to have no effect on the frequency of CD4+ T cells producing IFN-γ in cultures depleted of Tregs. However, as shown in Figure 6C, we found that, although depletion of CD4 + CD25hi cells did enhance responses, incubation with HC-1-Fab increased the number of antigen-specific IFN-γ producing cells even in cultures depleted of these regulatory cells. Collectively these data indicate that CD59 limits antigen-specific T cell responses in a manner that is independent of Tregs (Figure 5C).
Discussion
Following our previous studies in the mouse (3,4), we set out to investigate whether CD59 impacts on the activity of human CD4+ T cells. These investigations revealed that CD59 expression is increased on activated and memory T cells and that its expression influences both CD4+ T cell proliferation and cytokine production in response to stimulation with cognate antigen. These findings indicate that the responsiveness of both central memory cells (measured in proliferation assays) and effector memory cells (measured in ELISPOT assays) is increased following blockade of CD59.
The latter point was elucidated using both intact antibodies and Fab fragment of CD59-specific antibodies. Intact antibodies and their corresponding Fab fragments affect their target cells in distinct ways. Whilst CD59-specific Fab fragments simply block access to a portion of the target molecule, cross-linked intact antibodies facilitate clustering of molecules that may not normally occur under physiological activation conditions. Hence whilst cross-linking by both CD59-specific antibodies, HC-1 and MEM-43, resulted in enhanced CD4+ T cell proliferation, only one of the Fab fragments (HC-1) enhanced proliferation. Overall this implies that cross-linking induces proliferation regardless of the antibody binding site whereas increased proliferation by CD59 blockade depends on the binding site of the antibody. The difference when CD59 is blocked with two different Fab fragments (HC-1 and MEM-43) could be explained by the fact that these antibodies bind to non-identical epitopes on CD59 (5), suggesting that HC-1 but not MEM-43 binds to the active site of CD59 responsible for modulation of CD4+ T cell activity. Use of the HC-1-Fab enhanced T cell proliferation even when CD59-ve cells were used as APCs thereby indicating that the modulatory effect of CD59 is due to its expression on CD4+ T cells. It is also noteworthy that our findings in both mouse and human indicate that the down-modulatory effect of CD59 is observed only when APCs are present in proliferation assays implying the presence of an as yet unidentified ligand expressed on the surface of APCs that binds CD59 on CD4+ T cells resulting in delivery of a negative signal to the responding T cells. With this in mind, it is possible that HC-1-Fab but not MEM-43-Fab inhibits this interaction.
In summary, this study demonstrates that CD59 expression is up-regulated on activated CD4+ T cells and that blockade of CD59 enhances CD4+ T cell responses. These data support the hypothesis that, as in the mouse, CD59 influences human CD4+ T cell activity by exerting a down-modulatory effect. Whilst the mechanism through which CD59 mediates this effect remains to be elucidated, the data presented here highlight the potential utility of inhibiting CD59. Currently there is a great deal of interest in adoptive cell transfer as a method of treating patients with cancer (12). In vitro expanded autologous CD4+ T cell clones specific for the tumour-associated antigen NY-ESO-1 when adoptively transferred, showed durable clinical remission (13), supporting an anti-tumour role for CD4+ T cells. Manipulation of these cells through blockade or silencing of CD59 expression may represent a novel means of refining the responsiveness of antigen-specific CD4+ T cell responses for the purpose of improving the efficacy of adoptive immunotherapy.
Abbreviations used in this paper
- CRC
Colorectal cancer
- PPD
Purified protein derivative
- TT
Tetanus toxoid
Footnotes
The work was funded by a Wellcome trust project grant awarded to AMG and BPM that supports BS (ref no: 079115). AMG is an MRC senior research fellow (ref no: G117/488). MPL is a recipient of Wellcome trust prize studentship (ref no: 073055). BPM is funded by Wellcome trust programme grant (ref no: 068590)
References
- 1.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol. Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Korty PE, Brando C, Shevach EM. CD59 functions as a signal-transducing molecule for human T cell activation. J. Immunol. 1991;146:4092–4098. [PubMed] [Google Scholar]
- 3.Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. J. Immunol. 2005;175:7098–7102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longhi MP, Williams A, Wise M, Morgan BP, Gallimore A. CD59a deficiency exacerbates influenza-induced lung inflammation through complement-dependent and -independent mechanisms. Eur. J. Immunol. 2007;37:1266–1274. doi: 10.1002/eji.200636755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodian DL, Davis SJ, Morgan BP, Rushmere NK. Mutational analysis of the active site and antibody epitopes of the complement-inhibitory glycoprotein, CD59. J. Exp. Med. 1997;185:507–516. doi: 10.1084/jem.185.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS. ONE. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baalasubramanian S, Harris CL, Donev RM, Mizuno M, Omidvar N, Song WC, Morgan BP. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J. Immunol. 2004;173:3684–3692. doi: 10.4049/jimmunol.173.6.3684. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J. Clin. Lab. Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 10.Woods AM, Wang WW, Shaw DM, Ward CM, Carroll MW, Rees BR, Stern PL. Characterization of the murine 5T4 oncofoetal antigen: a target for immunotherapy in cancer. Biochem. J. 2002;366:353–365. doi: 10.1042/BJ20020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terstappen LW, Nguyen M, Lazarus HM, Medof ME. Expression of the DAF (CD55) and CD59 antigens during normal hematopoietic cell differentiation. J. Leukoc. Biol. 1992;52:652–660. doi: 10.1002/jlb.52.6.652. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]