Abstract
Trypanosomiasis is arguably the most important disease of man and his domesticated animals in the tropics. There are few compounds available for its treatment. This has exacerbated the development of drug resistance. There is therefore urgent need to search for newer compounds to treat this important disease. Medicinal plants represent a potential source of the drugs. This paper reports a bioassay-guided study to search for possible biological activity (cytotoxic and trypanocidal) in two Ugandan medicinal plants. The methodology adopted was the so-called ‘ping-pong’ approach, involving phytochemical purification (column chromatography and preparative thin layer chromatography), alongside biological studies (cytotoxicity, antibacterial, trypanocidal and antifungal studies). Phytochemical investigations of Zanthoxylum chalybeum (seed) yielded a pure crystalline compound, 27-135D, which was characterized by 1HNMR as the alkaloid skimmianine. Studies on stem bark yielded three alkaloids 27-165A, 27-173A and 27-173B. All the above pure isolates, and the crude extracts of Z. chalybeum had neither biological activity nor cytotoxicity in the brine shrimp assay. A cytotoxic sesquiterpine, characterized as muzigadial, was isolated from W. ugandensis. It was highly toxic in the brine shrimp assay and also had in vitro trypanocidal activity against IL 3338 as well as IL1180; reference drug-resistant and drug-sensitive trypanosome strains respectively, comparable to diminazene aceturate and Geneticin (G418). Muzigadial also had antifungal activity against Candida albicans. It was concluded that the brine shrimp assay might be a useful predictor of trypanocidal activity of plant extracts and that muzigadial may be potentially valuable in the treatment of drug-resistant trypanosomosis.
Keywords: Muzigadial, Trypanocidal, Cytotoxicity, Brine shrimp, Antifungal, Antibacterial
Introduction
The species of W. ugandensis and Z. chalybeum are widely used in Uganda for the treatment of many diseases. The stem and roots of Warburgia ugandensis Sprague are used as expectorant, for the common cold and against fever, malaria, stomachache, constipation and diarrhea1, 2. In Uganda the stem bark of W. ugandensis is widely used as a general medicine for the treatment of various ailments including snakebites and measles and is a common component in a number of medicinal preparations3. The tree is now becoming one of the endangered species in the country4. Previous phytochemical studies on the plant have resulted in the isolation of several sesquiterpines5, 6,7 and insect anti-feedant agents6.
Natural products (such as fungi and the higher plants) have been known to be good sources of pharmacologically active compounds. The criteria for studies on particular natural products have been either the pre-existing traditional use of the plant in therapy (for example in case of quinine and the digitalis alkaloids as well as D-tubocurarine) or the search for chemicals in natural products with structural similarity to known pharmacologically active agents.
There are limitations to both approaches of studying natural products. In the first case (folkloric use) there is a lot of quackery and hucksterism associated with the use of higher plants, making it most times fruitless to investigate some claims. In the second case (chemical approach) the natural products have a vast array of compounds that are pharmacologically valueless, making it extremely tedious to pursue this approach.
The brine shrimp assay is a bench top assay that has utilized sea creatures (Artemisia salina) and has been found to be of great value in predicting pharmacological activity of compounds. It has the advantage that it is simple, no sterility is required and results are available in only 24 hours. Using this assay it has been possible to quickly identify compounds that have been screened for cytotoxic activity8.
We now report the results of bioassay-guided studies on the extracts of Z. chalybeum and W. ugandensis, using the brine shrimp lethality test. The aim of the study was to isolate and characterize the bioactive components of the two medicinal plants. Previous studies had indicated some antibacterial activity of W. ugandensis but at a very high concentration of the extracts3.
Materials and Methods
Plant Material
Z. chalybeum stem and seeds were collected from Kumi, eastern Uganda. W. ugandensis (stem) was collected from Mabira forest, Eastern Uganda. Botanists of the department of Botany, Makerere University and the Natural chemotherapeutic Laboratory by comparison with authentic samples kept there, identified the samples. Voucher specimens have been deposited at the department of Pharmacy, Makerere University, Kampala, Uganda.
Purification and spectroscopy
Melting points were determined on a Koffler hot stage apparatus and a Thomas Hoover capillary melting point apparatus. Infrared spectra were run using Kbr discs on Perkin Elmer infrared spectrophotometer; Model 727B.1HNMR spectra were determined for solutions in deuteriochloroform and D2O with a Jeol 90MHz, tetramethylsilane as internal standard. Analytical TLC chromatograms were run on 0.2mm thick layer of silica gel, Merck. The products isolated were detected by UV fluorescence. Preparative layer chromatography was run on 1.0mm thick layer of silica gel. Column chromatography was performed on silica gel 60 (70–230 mesh) and sephadex LH-20.
Zanthoxylum chalybeum
a) Extraction and isolation of compounds from the seeds
Crushed seeds (100g) of the plant were soaked in ethanol, placed in a shaker for 24hr, filtered, concentrated under reduced pressure, to obtain 6.9g of the crude extract. Compound 27-135D crystallized out of the crude extract and was filtered off and re-crystallized in methanol. 1HNMR was done and also antimicrobial and brine shrimp assays using standard protocols8, 9. The mother liquor from above was chromatographed over silica gel column (flash) eluted with chloroform one first and then chloroform: methanol (9:1) to obtain three fractions.
b) Extraction and isolation of compounds from the stem bark
Stem bark of the plant (100g) was soaked in ethanol placed in a shaker overnight, filtered and concentrated under reduced pressure to obtain 7.6g of crude extract. The crude extract was chromatographed over silica gel column (flash) and eluted with Pet/EtoACand then EtoAC:MeOH mixtures of increasing polarity to obtain a total of 28 fractions. Fractions 21 and 22 (solvent systems EtoAC:MeOH(6:4) and EtOAC:MeOH (1:1), yielding a wghite crystalline compound 27-167A (20mg). Fraction 1, solvent system: EtOAC: MeOH (8:2) yielded an alkaloid, 27-165A. Fractions 2–6, solvent system: EtOAC:MeOH (1:1) yielded alkaloids 27-173A and 27-173B after purification using PTLCusing chloroform alone as the solvent system.
W. ugandensis
a) Extraction and isolation of compounds
The stem bark (100g) of W. ugandensis was soaked in EtOH, placed on a shaker for 24 hrs, filtered and concentrated to obtain the crude extract (13.2g). The crude extract 6.6g) was dissolved in 80% aqueous methanol (100ml) and extracted with petrol (100ml × 3). The petrol extract was dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure to obtain 0.48g of extract (27-163A). To the aqueous layer, distilled water (33.3 ml) was added and then extracted with chloroform (2× 100ml). The chloroform extract was dried over anhydrous Na2SO4, filtered concentrated to obtain 1.42g of extract (27-163B). The remaining aqueous extract was dried in the hood to obtain 2.40g of 27-163D. Standard procedures were used to study all fractions for antimicrobial9 and brine shrimp lethality8. Fraction 27-163A, which showed the highest activity, was applied on sephadex LH-20, eluted with hexane-C2HCl2 (1:4) and then hexane acetone (3:2) to obtain eight fractions. The first and the second fractions were blank on analytical TLC were hence discarded. The second fraction (27-169A) which showed the highest activity was further purified on PTLC (solvent system, benzene: petrol: EtOAC [3:9:1] and benzene: petrol: EtOAC [3:4:3] to obtain compounds 27-179J, 27-179K, 27-179L, 27-179M and 27-179A which showed the highest activity in the brine shrimp test.
b) 24- hour growth inhibition assay
Samples of continuously growing trypanosome cultures were diluted to a density of 4 × 105 trypanosomes per ml. Standard procedures10 were adopted for in vitro trypanocidal studies. Diminazene aceturate (Berenil) was used as a known positive control. Muzigadial was the test compound. The compounds were dissolved in distilled water (Berenil) and DMSO (Muzigadial) at a concentration of 1 mg/ml and serial tenfold dilution made. 1 ml of the trypanosomes (TC3338 and IL1180) was dispensed per well of a 24 well plate (Costar) and 10–40 µl of varying concentrations of the drugs administered in duplicate. The cultures were incubated at 37°C in 4.5% CO2 in air for 24 hrs. Control cultures (without drugs) were incubated under the same condition but with distilled water (or DMSO). A cell count was done on a Modified Neubaur Haemocytometer after 24 hrs.
Results
Zanthoxylum chalybeum (seed)
Compound 27-135D was a light brownish crystal, which crystallized from methanol. The 1HNMR (90MHz, CDCl3, TMS internal standard) had features typical of skimmianine. The three singlets (3H, each) at δ4.03,4.12 and 4.4 are due to three methoxy groups. Two doublets indicate the two-furano protons at δ 7.0 and 7.57 and the two doublets at δ7.2 and 7.9 indicate the presence of two ortho aromatic protons at C6 and C5 respectively. The melting point was determined on a Koffler hot stage apparatus to be 176–178°C, uncorrected. Comparison was made by superimposition of 1H NMR spectrum over authentic spectra of those previously isolated from Techlea nobilis11. Both the crude extract and the pure compound 27-135D did not show antimicrobial (anti-bacterial and antifungal) activity in the paper disc assay at concentrations of 200µg/ml. There was also no cytotoxicity on the brine shrimps.
Zanthoxylum chalybeum (stem bark)
Compound 27-165A was a yellow amorphous compound demonstrated to be an alkaloid by spraying with Dragendorf reagent on analytical TLC12. The 1HNMR features; TMS internal standard, CDCl3, were: singlets at δ8.1, 6.7, 6.6, 5.9,4.4 and 4.6; triplet at δ5.3 and a multiplet at 1.0. Compound 27-173A was whitish and also elicited a positive Dragendorf test. The 1HNMR (TMS, internal standard, CDCl3) had the following features: singlets at δ6.7, 5.3,5.6,5.0,2.1 and 1.0. Compound 27-173B was a whitish, amorphous compound, elicited a positive alkaloid test on analytical TLC. Its 1HNMR showed the following features: δ 5.3, 4.4, 4.2,3.9,3.6,2.7, 2.4, 2.3, 2.0,1.3, and 0.9. Compound 27-167A was a white crystalline compound. In the flame test it gave off black soot indicating it was an organic compound. Paper chromatography with reference sugars gave a negative test. It had a melting; point of 188–189°C. The compound was insoluble in methanol, chloroform, DMSO and acetone, but soluble in water. The 1HNMR in D2O did not however elicit any signal. IR (KBr) spectrum had a broad band at 3400cm−1 indicating the presence of hydroxyl groups. It was not possible to assign any specific structures to these alkaloids on the basis of this evidence. Further phytochemical studies were not deemed necessary since they did not show any interesting biological activity. The crude as well as the pure compounds above did not show any antimicrobial activity in the paper disc assay. Neither did they show cytotoxicity in the brine shrimp assay.
Warburgia ugandensis
Of the fractions on which antimicrobial and brine shrimp lethality was done, the following were active: Fractions 27-179A, 27-179H and 27-179K had antimicrobial activity (but only against C. albicans and not on the bacteria tested [S. aureus and E. coli]). Fraction 27-179M had the highest antimicrobial activity and also had 100% kill on shrimps at concentrations of 50µg/ml and 100µg/ml. Compound 27-179L had completely no antimicrobial activity, but had a very high cytotoxic effect on the brine shrimps. Compound 27-179H had both a high antimicrobial and cytotoxic effect. The 1HNMR of the isolates did not show any similarities to the compounds occurring in Warburgia as described in the literature6, 13. There were no signals in the aromatic region, but very many in the aliphatic region. The compounds are therefore not Warbuganal, Muzigadial, or Warburgin.
At a concentration of 1mg/ml and less (10µg/well) of both Muzigadial and diminazene, there was no trypanocidal effect on both IL3338 and IL1180. But at 40 µg/well there was 100% kill by muzigadial (both IL3338 and IL1180). At 40 µg/well there was significantly higher effect of Berenil on IL1180 than on IL3338. G418 (Geneticin) elicited 100% kill on both IL1180 and IL3338 at 10µg per well. At 1µg per well IL3338 showed significantly more resistance to G418 than IL1180.
Discussion
In vivo assays usually provide a relatively reliable method of determining the drug sensitivity of trypanosome isolates14. Assays in either bovine or murine hosts involve infection, usually of blood stream forms. Performing such tests however is sometimes made difficult in that livestock are expensive. Moreover, the animals to be used for the work have to be trypanosome naïve, which is often difficult to ensure. The use of laboratory mice has the advantage of being relatively inexpensive with respect to cost of animals, housing and maintenance. However, their main limitations are the inability of some trypanosome strains to grow in mice, and the necessity to extrapolate the results from the murine to bovine hosts. Mouse assays are considered to provide only a broad indication of the level of sensitivity of a trypanosome population14. It would appear from this study that the brine shrimp assay might be a useful predictor of trypanocidal activity in natural products. This might hold future promise with respect to cost.
While the water and ethanol extracts of W. ugandensis showed some antibacterial activity in the agar well diffusion assay (Fig. 1), muzigadial did not. This suggests that the antibacterial activity of W. ugandensis derives from some other as yet unidentified component of the plant. This requires further detailed phytochemical investigation.
Fig 1.
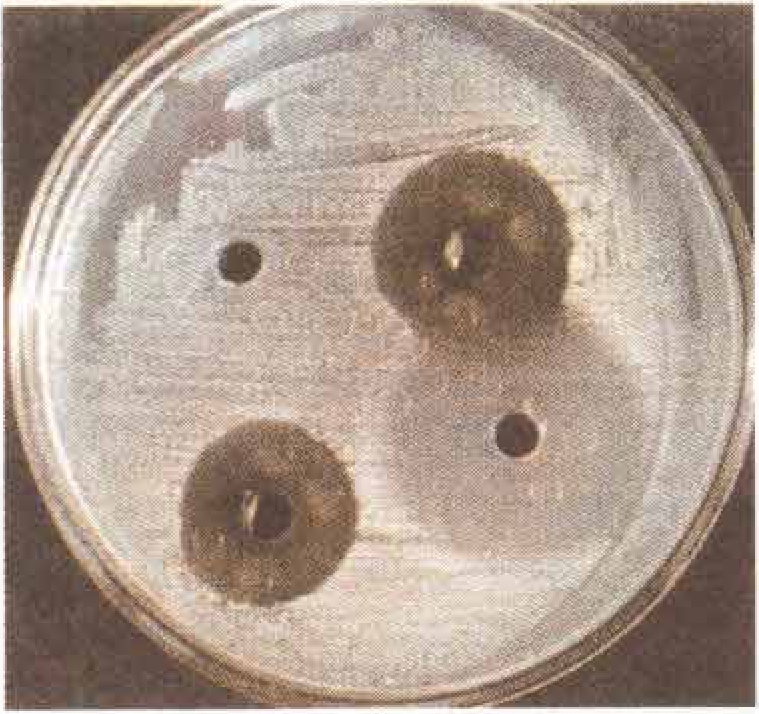
Antibacterial activity of W.ugandensis extracts against Escherischia coli (agar well diffusion assay)
Further studies are also required to demonstrate whether muzigadial might have in vivo trypanocidal activity. If this should be confirmed, muzigadial should be a candidate for development into a trypanocidal drug, especially since it seems to be active against the drug resistant IL3338.
In conclusion, W. ugandensis has been shown to have compounds with interesting biological activity. The in vitro trypanocidal activity seems to be attributable to the sesquiterpene muzigadial, which does not have antibacterial activity. There seems to be a need to protect the tree since nearly all field observations suggested that most of the trees encountered in the forest had been extensively debarked (Fig. 2), supporting earlier suggestions that it may be an endangered species4. Fortunately, the tree is sometimes grown by villagers near homesteads (Fig. 3), probably due to its perceived medicinal value.
Fig 2.
Warbugia ugandensis: some of the trees are grown in the plantations near homesteads.
Fig 3.
Warbugia ugandensis - most forest trees often extensively debarked like this.
Acknowledgements
The GTZ (Germany) veterinary project, Faculty of Veterinary medicine, Makerere University supported this work. Some of the phytochemical studies were done in the chemistry department, Addis Ababa University under the NAPRECA-IPICS supported exchange scheme. Trypanosome culture training was acquired at the International Laboratory for Research on Animal Diseases (ILRAD).
References
- 1.Watt JM, Breyer-Brandwijk MG, editors. Medicinal and poisonous plants of Southern and Eastern Africa. 2nd Ed. Edinburgh and London: 1962. p. 1523. [Google Scholar]
- 2.Kokwaro JW, editor. Medicinal plants of East Africa. Kampala, Nairobi, Dar-Es-Salaam: East African Literature Bureau; 1976. p. 215. [Google Scholar]
- 3.Olila D. A study of the antimicrobial activities of Zanthoxylum chalybeum and Warburgia ugandensis-Ugandan medicinal plants. Uganda: Makerere University; 1993. p. 155. MSc Thesis. [Google Scholar]
- 4.Odyek-Olwa, Olila D, Albrecht C, Dagne E. Muzigadial, a cytotoxic sesquiterpene from Warburgia ugandensis; Proceedings of 4th NAPRECA symposium, Antananarivo, Madagascar, Sept. 19th–23rd 1993; pp. 107–108. [Google Scholar]
- 5.Brooks CJW, Draffan GH. Sesquiterpenoids of Warburgia spp II: Ugandensolide and ugandensidial (cinnamodial) Tetrahedron. 1969;25:2887–2898. [Google Scholar]
- 6.Kubo I, Miura I, Pettei MJ, Lee YW, Pilkiewicz F, Nakanishi K. Muzigadial and Warbuganal: potent antifungal, antiyeast and African army worm antifeedant agents. Tetrahedron letters. 1977;1:4553–4556. [Google Scholar]
- 7.Kioy D, Gray AI, Watermann PG. Sesquiterpenoids of Warburgia spp. Phytochemistry. 1990;29:3535. [Google Scholar]
- 8.McLaughlin JL. Brine shrimp and crown gall tumors: Simple bioassays for the discovery of plant antitumor agents; Proceedings of the N.H.I. Workshop, Bethesda; 1988. pp. 22–24. [Google Scholar]
- 9.Chabra SC, Shao JF, Mshiu EN, Uiso FC. Antifungal activity among traditionally used herbs in Tanzania. The Dar Medical Journal. 1982;1:68–73. [Google Scholar]
- 10.Kaminsky R, Brun R. A drug incubation infectivity test for assessing resistance in trypanosomes. Acta Tropica. 1993;54:279–289. [Google Scholar]
- 11.Abiy Y, Dagne E. Alkaloids of Techlea nobilis. Phytochemistry. 1988;27:651–653. [Google Scholar]
- 12.Farnsworth NR, Euler KL. Medicinal plants in therapy. Lloydia. 1982;25:186–194. [Google Scholar]
- 13.Ceo D, Gray AI, Waterman PG. Sesquiterpenoids of Warburgia spp. Phytochemistry. 1990;29:3535–3538. [Google Scholar]
- 14.Sones KR, Njogu AR, Holmes PH. Assessment of sensitivity of T. congolense to isometamidium chloride: comparison of tests using cattle and mice. Acta Tropica. 1988;45:153–164. [PubMed] [Google Scholar]




