Abstract
Glutamate receptors are important target molecules of the acute effect of ethanol. We studied ethanol sensitivity of homomeric GluR-D receptors expressed in HEK293 cells, and examined whether recently discovered transmembrane AMPA receptor regulatory proteins (TARPs) affect ethanol sensitivity. Co-expression of the TARPs, stargazin and γ4, increased the time constant (τ-value) of current decay in the presence of agonist, thus slowing the onset of desensitization and increasing the steady-state current. Ethanol produced less inhibition of the peak current than the steady-state current for all types of the GluR-D receptors. In addition, ethanol concentration-dependently accelerated the rate of desensitization, measured as the τ-value of fast decay of peak current. This effect was enhanced with co-expression of TARPs. The recovery from desensitization was slowed down by co-expression of γ4, but ethanol did not affect this process in any GluR-D combination. The results support the idea that increased desensitization is an important mechanism in the ethanol inhibition of AMPA receptors, and indicate that co-expression of TARPs can alter this effect of ethanol.
Keywords: GluA4 receptors, Alcohol, Protein interactions, Recombinant receptors, Patch-clamp
Introduction
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors form a subclass of ionotropic glutamate receptors that are abundantly distributed throughout the brain (reviewed in Ozawa et al., 1998). They mediate excitatory currents during fast synaptic neurotransmission in most neurons of the brain. AMPA receptors also have an important role in learning and memory since their synaptic cell membrane expression is very plastic, as their membrane trafficking can be altered rapidly, e.g. in long-term potentiation (LTP) and depression (LTD) (Citri and Malenka, 2008). Native AMPA receptors are heterotetramers that are composed of GluR-A to D (GluR1–4; GluA1–4) subunits (Keinänen et al., 1990; Rosenmund et al., 1998). In heterologous expression systems all AMPA receptor subunits can form functional receptors as homomers.
Ethanol inhibits the function of ionotropic glutamate receptors (Lovinger et al., 1990; Dildy-Mayfield and Harris, 1992; Lovinger, 1993). N-methyl-D-aspartate (NMDA) receptors are considered to be the most important target among the glutamate receptors in acute actions of ethanol. NMDA receptors are inhibited by ethanol in synaptic transmission in brain slices, the threshold concentration being around 1–10 mM (Lovinger et al., 1990). Most of the published studies indicate that AMPA receptor-mediated excitatory postsynaptic currents are not markedly inhibited by ethanol in brain slice-preparations when the currents are evoked by electrical stimulation of afferent axons (e.g., Lovinger et al., 1990). To date AMPA receptors activated by presynaptically released glutamate have been shown to be inhibited only in the central amygdala and in the CA3 area of hippocampus of neonatal rats (Mameli et al., 2004; Zhu et al., 2007). In some experiments carried out using individual neurons or heterologously expressed recombinant receptors, both glutamate receptor subclasses have been reported to be similarly sensitive to ethanol (Dildy-Mayfield and Harris, 1992; Wirkner et al., 2000). In those studies, the agonist is applied over a relatively long time period, which produces desensitization of a substantial fraction of ion channels. Our previous study provided evidence that ethanol inhibition of AMPA receptors is strongly enhanced by receptor desensitization (Möykkynen et al., 2003).
Transmembrane AMPA receptor regulatory proteins (TARPs) are a relatively newly discovered protein family. Prototypic TARP, stargazin, was found by a spontaneous mutation in ataxic stargazer mouse line as a homologous protein to γ1calcium channel subunit (Letts et al., 1998). The role of stargazin in the regulation of trafficking and synaptic localization of AMPA receptors became evident when it was discovered that cerebellar granule cells of stargazer mice lacked functional AMPA receptors (Chen et al., 2000). Later TARPs were found to also enhance the function of AMPA receptors (Yamazaki et al., 2004). To date, six TARPs have been found, named γ2 (a.k.a. stargazin), γ3, γ4, γ5, γ7 and γ8 (Tomita et al., 2003; Kato et al., 2007; Kato et al., 2008). TARPs are probably required for synaptic expression of AMPA receptors in many, if not all, neurons, since they are ubiquitously expressed throughout the brain (Tomita et al., 2003) and synaptic AMPA receptor number is decreased in stargazer and various other TARP knock-out mice (Hashimoto et al., 1999; Rouach et al., 2005; Milstein et al., 2007; Menuz et al., 2008). TARPs profoundly affect the gating of AMPA receptors by decreasing desensitization and deactivation rates (Turetsky et al., 2005; Milstein et al., 2007; Kott et al., 2009). They also affect the pharmacology of AMPA receptors. They are reported to increase the efficacy of the partial agonist kainate and change the antagonists CNQX and DNQX to weak agonists (Menuz et al., 2007; Kott et al., 2009; Suzuki et al., 2008). Because TARPs are present in native AMPA receptor complexes and have a central role in the regulation of AMPA receptor function and expression, they can be considered as auxiliary AMPA receptor subunits.
We expressed the stargazin and γ4 TARP proteins with the GluR-D flip (GluR-Di) AMPA receptor subunit in HEK293 cells in order to investigate the effect of TARPs on ethanol inhibition of glutamate-induced currents. We chose the prototypical stargazin (γ2) and γ4, which has a strong effect on desensitization (Korber et al., 2007). γ4 is also heavily expressed during brain development (Tomita et al., 2003), which makes it a good expression partner for GluR-D, also strongly expressed during that period (Zhu et al., 2000). We used electrophysiology to test whether ethanol inhibited the ion currents and affected desensitization of GluR-D to a similar extent with or without the TARPs. This study revealed a novel role of TARPs in the action of ethanol.
Materials and Methods
DNA constructs
The generation of expression plasmids for rat GluR-D flip and flop variants has been described previously (Pasternack et al., 2002; Coleman et al., 2006). The generation of L505Y point-mutated GluR-D flip was done in pFastbac1 by overlap extension PCR with mutagenic primers, and the mutant cDNA was then transferred to pcDNA3.1(−) vector for mammalian cell expression. All PCR regions were sequenced. Stargazin and γ4 were human clones and obtained as a kind gift from John L. Black III (Mayo Medical School, Rochester, MN, USA).
Cell culture and transfection
HEK293 cells were cultured in DMEM supplemented with 10% fetal calf serum and 2 mM L-glutamine and 1% penicillin-streptomycin solution. Immediately before transfection the cells were replated at a density of 2 × 105 cells per millilitre into 35 mm culture dish. Cells were transfected using the calcium phosphate precipitation method (2 μg AMPA receptor and TARP plasmids and 0.7 μg EGFP DNA per 35 mm dish) (Coleman et al., 2003). The medium was changed 18 h after the transfection, and the cells were used for patch-clamp experiments on the following day. In each transfection, both the GluR-D plasmid alone and GluR-D plus TARP plasmids were transfected in order to minimize the variation between the transfection conditions. The co-transfection with pEGFP-C1 plasmid helped the detection of transfected cells by green fluorescent protein (GFP) fluorescence.
Patch clamp electrophysiology
Whole-cell patch-clamp recordings were made from EGFP-positive HEK293 cells with an Axopatch 200B amplifier, Clampex 8.2 software and a Digidata 1322A analog to digital converter (Molecular Devices, Sunnyvale, CA, USA), or with an EPC 9/2 double patch-clamp amplifier and pulse v 8.80 software (HEKA electronic, Lambrecht, Germany) (Möykkynen et al., 2003). Electrodes were pulled from borosilicate glass capillaries (World Precision Instruments, Stevenage, UK) and had a resistance of 4–6 MΩ when filled with internal solution containing (in mM): N-methyl-D-glucamine 100; CH3SO3H 100; CsF 40; MgCl2 10; HEPES 10; EGTA 5, pH 7.4. The cells were continuously perfused with a recording solution containing (in mM): NaCl 150; KCl 2.5; CaCl2 2.5; MgCl2 1; HEPES 10; D-glucose 10, pH 7.4. L-Glutamate (Sigma-Aldrich, St. Louis, MO, USA) and ethanol (Altia, Rajamäki, Finland) were dissolved in the recording solution. They were applied to the cells using a stepper motor driven applicator (Warner instrument, Hamden, CT, USA). AMPA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was from Tocris Bioscience (Bristol, UK). Recordings were made at a sampling rate of 10 kHz and lowpass bassel-filtered at 1 kHz. Each glutamate application was done twice and traces were averaged. Data were analysed using Clampfit 10.2 (Molecular Devices, Sunnyvale, CA, USA) and the statistical analyses were carried out using Prism 3.0 software (GraphPad, San Diego, CA, USA) and SPSS 15.0.1 for Windows (SPSS Inc., Chicago, IL, USA). The decay phase of the currents was fit with a single exponential function in Clampex 10. Original values were analyzed with repeated measures two-way ANOVA with Dunnett’s Multiple Comparison Test to find out which EtOH concentrations significantly affected glutamate-evoked peak and steady-state currents. Normalized values were analyzed with two-way ANOVA and Bonferroni post hoc test to find the significance of the differences between GluR-D alone and GluR-D co-expressed with TARPs or between peak and steady-state currents in ethanol inhibition. Data are given as arithmetic averages ± SD in the Result section, and as arithmetic averages ± SEM in the figures. The statistical tests and significances are given in figure legends. Each expression with stargazin, γ4 or L505Y GluR-D included their own set of GluR-D controls to facilitate comparison to the control condition and to ensure consistency of receptor expression and pharmacology.
Results
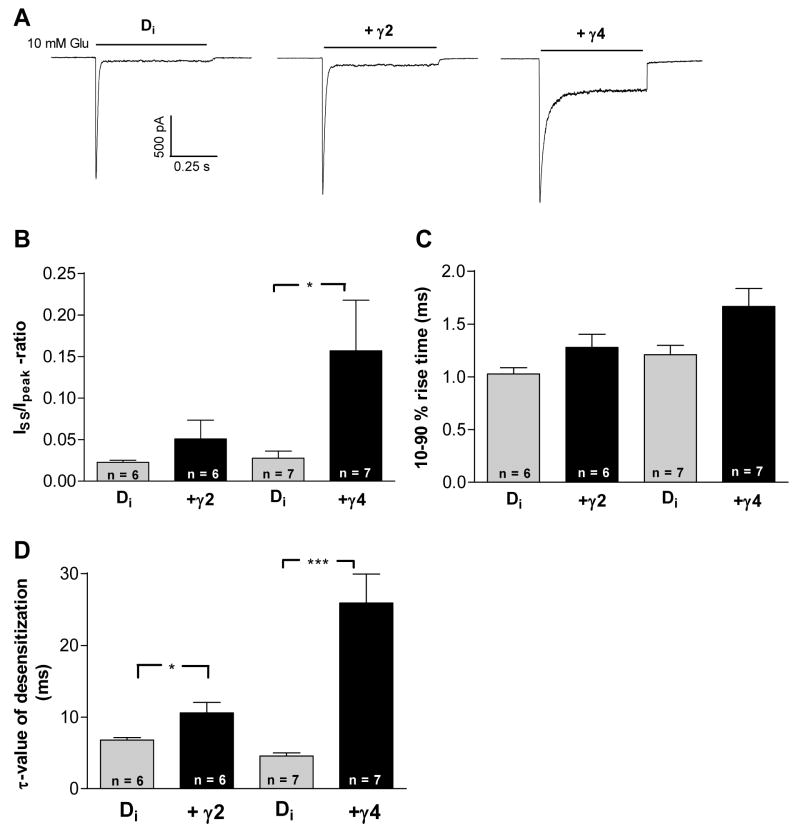
In order to study the ethanol effect on AMPA receptor currents a saturating concentration of glutamate (10 mM) was applied to the HEK293 cells for 1–2 s. Glutamate applications to the cells evoked a current that had a distinct peak component followed by a steady-state component (Fig. 1A). Co-application of 50 μM of CNQX with 10 mM glutamate blocked the current completely confirming that the current was mediated by AMPA receptors (data not shown). TARP expression did not affect the peak current amplitude, but the ratio of steady-state and peak currents was higher in TARP co-transfected cells (Fig. 1B). Co-transfection with TARPs tended to slightly slow down the 10 to 90 % rise-time (Fig. 1C). The τ-values of desensitization in the control GluR-D receptors were 6.7 ± 0.74 ms (n = 6) and 4.6 ± 1.1 ms (n = 7) and in the stargazin or γ4 co-expressed receptors 10.6 ± 3.5 ms (n = 6) and 25.8 ± 10.6 ms (n = 7), respectively (Fig. 1D). TARP co-expression thus slowed down the desensitization process and the onset of steady-state current.
Fig. 1.
The effect of TARPs on kinetics of GluR-Di receptors expressed in HEK293 cells. (A) Representative traces of GluR-Di, GluR-Di+γ2 (stargazin) and GluR-Di+γ4 receptors illustrate the slowing of speed of onset and the reduction in degree of desensitization by TARP expression. (B) Steady-state/peak current –ratio is higher with TARP co-expression, indicating that TARPs inhibit desensitization. Both TARP co-expressions had their own control cells. * p < 0.05 (Mann-Whitney-test). (C) 10–90 % rise time of the peak current is slightly, albeit non-significantly higher with TARP co-expressions (p = 0.13 with γ2 and p = 0.087 with γ4, Student’s t-test). (D) TARP co-expression increased the τ-value of desensitization estimated by a single exponential fit. * p < 0.05, *** p < 0.001 (Mann-Whitney-test).
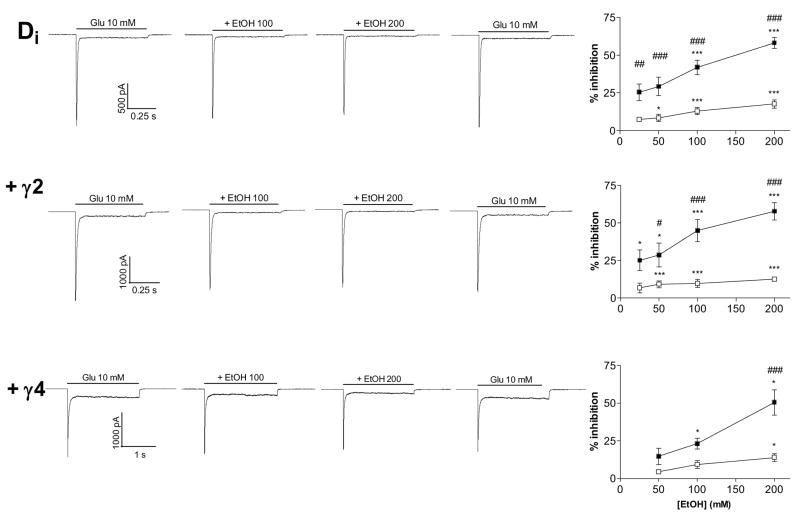
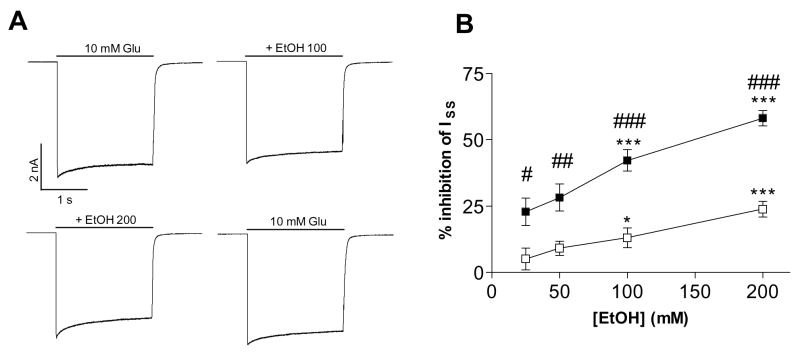
Ethanol inhibited glutamate-currents mediated by the GluR-Di AMPA receptors both with and without the TARPs, in a concentration-dependent manner. The steady-state current was inhibited more than the peak current (Fig. 2). The inhibition was reversible and repeatable, as the 10-mM glutamate-evoked currents returned to the control level after ethanol washout. Ethanol inhibition of both peak and steady state currents was similar in all GluR-Di receptors with and without TARPs. The results are in concordance with those of our previous study with acutely isolated neurons (Möykkynen et al., 2003).
Fig. 2.
Ethanol inhibition of GluR-Di receptor currents. GluR-Di receptors were inhibited by ethanol (EtOH) with or without the co-expression of stargazin (γ2) and γ4 TARPs to a similar extent. Representative traces show the effects of 100 and 200 mM ethanol on 10-mM glutamate-evoked currents. Graphs on the right panel show the results of ethanol inhibition of the peak and steady-state currents. Steady-state currents (■) are inhibited more than the peak currents (□) in all receptors. Repeated measures ANOVA found that EtOH inhibition differed between the peak and steady state currents (F1,8–10 > 5.7, p < 0.05) and that EtOH had a dose effect (F4,32–40 = 4.9, p < 0.01) in each receptor. # p < 0.05, ## p < 0.01, ### p < 0.001 for the significance of the difference between the peak and steady-state currents (Bonferroni test). * p < 0.05, *** p < 0.001 for the difference from the control current (Dunnett’s test). (n = 4–7)
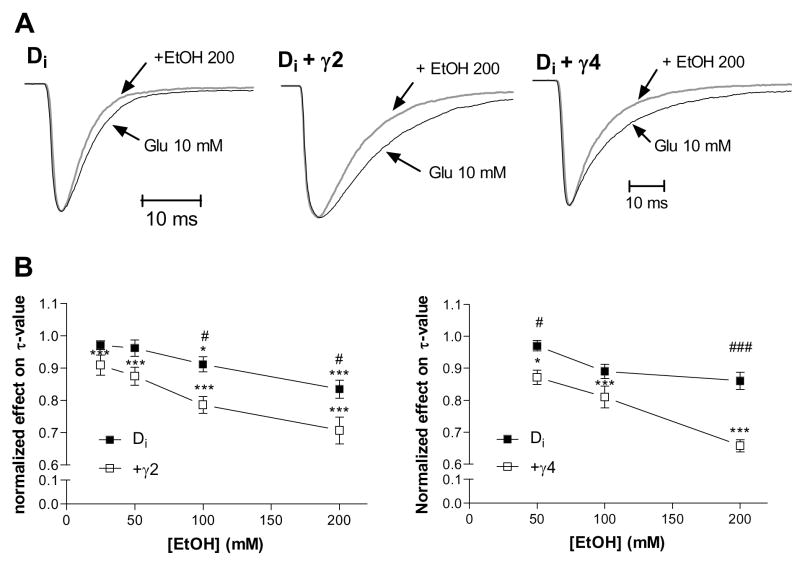
Ethanol decreased the τ-value of desensitization for GluR-Di. Co-expression of stargazin or γ4 further increased the effect of ethanol on desensitization (Fig. 3A and B). The highest ethanol concentration tested (200 mM) reduced the τ-value by 16.5 ± 4.9 % (n = 5) in the control receptors, and by 29.3 ± 9.3 % (n = 6) in the stargazin co-expressed receptors. Ethanol (200 mM) lowered the τ-value by 14.4 ± 5.8 % (n = 5) and 34.2 ± 5.0 % (n = 7) with and without γ4 co-expression, respectively.
Fig. 3.
The effect of ethanol on the τ-value of desensitization. Panel (A) depicts how ethanol (EtOH) speeded up the rate of desensitization in recording traces for all GluR-Di receptors. The peak amplitudes of the traces have been scaled to the level of 10 mM glutamate control current. (B) TARP co-expression increased EtOH’s effect on desensitization (γ2: F1,10 = 12.3, p < 0.01; γ4: F1,10 = 38.2, p < 0.001; Bonferroni test: # p < 0.05; ## p < 0.01; ### p < 0.001 for the significance of difference from GluR-Di+TARP). The effect of EtOH on desensitization was dose dependent (γ2: F4,40 = 36.6, p < 0.001; γ4: F3,30 = 34.3, p < 0.001; Dunnett’s test: * p < 0.05, *** p < 0.001). (n = 5–7)
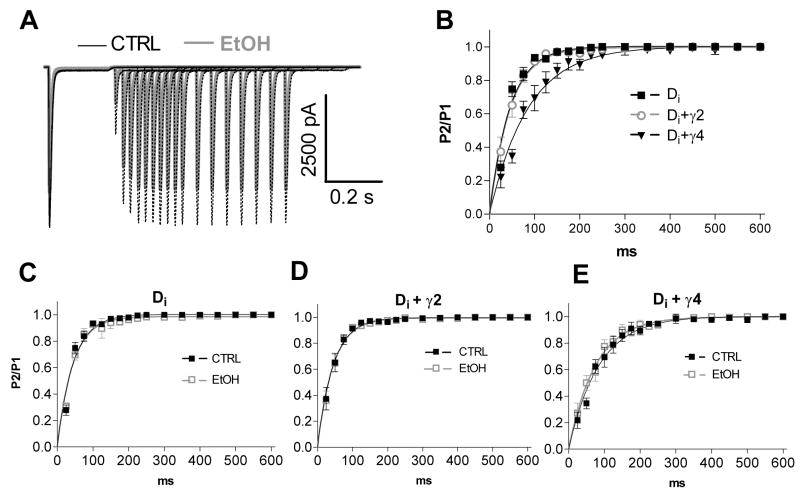
We also studied the recovery from desensitization by applying two 200-ms pulses of 10-mM glutamate to the cells with variable intervals ranging from 25 to 600 ms. With this application protocol, the current evoked by the second pulse had a smaller amplitude as compared to that produced by the first one with short interpulse intervals, before it was fully recovered from desensitization (Fig. 4A). The peak current amplitude of the second pulse was divided by that of the first one giving the P2/P1-ratio. The ratios were plotted against the pulse intervals, and the points fitted to a single exponential equation, giving then τrec-values, the time constants of recovery from desensitization. The recovery from desensitization for the GluR-Di was not altered by the co-expression of stargazin, but γ4 significantly prolonged it (Fig. 4B). The τrec-values were 31 ms (95% confidence interval 27–37 ms, n = 7), 31 ms (29–33 ms, n = 7) and 59 ms (53–66 ms, n = 5) for GluR-Di alone, GluR-Di+stargazin and GluR-Di+γ4, respectively. In contrast to our previous observation in isolated hippocampal neurons (Möykkynen et al., 2003), ethanol did not affect recovery from desensitization (Fig. 4C, D and E).
Fig. 4.
Recovery from desensitization in the presence of 100 mM ethanol. (A) Overlapping traces show the responses of GluR-Di receptors to two 200-ms pulses of 10-mM glutamate with variable intervals in the absence and presence of 100 mM ethanol (EtOH). (B) Recovery from desensitization of GluR-Di receptors with and without co-expression of TARPs. The plot shows the amplitude of the peak current of the second pulse relative to the amplitude of the peak current of the first pulse (P2/P1) as a function of pulse interval. The GluR-Di+γ4 receptors had slower recovery than the other receptors. The data for GluR-Di receptor alone (C), GluR-Di+γ2 (D) and GluR-Di+γ4 (E) in the absence and presence of 100 mM ethanol indicate that ethanol did not affect the recovery from desensitization. (n = 5–7)
Ethanol inhibition is markedly decreased in the non-desensitizing point-mutated L497Y GluR-Ai receptors expressed in HEK293 cells (Möykkynen et al., 2003). We wanted to test whether ethanol inhibition is also decreased with the analogous point mutation constructed in GluR-Di receptors. Application of 10 mM glutamate evoked a current which had a small but distinct peak current (appr. 9 % higher than steady state current) indicating that the L505Y GluR-Di receptors still undergo desensitization to some extent. Ethanol inhibited both the peak and steady state currents of L505Y GluR-Di equally, and, therefore, only the steady state current was used in further analysis. The steady state current of L505Y GluR-Di receptors was significantly less inhibited by ethanol than the steady state current of wild type GluR-Di receptors (200 mM ethanol inhibited L505Y only by 23.9 ± 7.1 %, n = 6 and wild type by 58.1 ± 7.2 %, n = 6) (Fig. 5B).
Fig. 5.
Ethanol inhibition of L505Y point-mutated GluR-Di receptor. (A) The extent of desensitization is markedly decreased in L505Y receptors as compared to wild-type GluR-Di, as can be seen from the representative recording traces. Horizontal bar indicates the 10 mM glutamate application in the absence and presence of 100 mM ethanol. (B) The steady-state currents of L505Y receptors (□) are less sensitive to EtOH than those of wild-type GluR-Di receptors (■). Repeated measures ANOVA: F1,9 = 7.8, p < 0.05. # p < 0.05, ## p < 0.01, ### p < 0.001 for the significance of the difference in ethanol inhibition between the wild-type and point-mutant L505Y GluR-Di receptors (Bonferroni test). * p < 0.05, *** p < 0.001 for the significance of the difference between the each ethanol concentration and control current (Dunnett’s test). (n = 8 for L505Y and 10–12 for wild-type)
Discussion
In the present study, the effects of ethanol on the function of AMPA-type glutamate receptors containing GluR-D subunits were studied without and with two members of the transmembrane AMPA receptor regulatory protein TARP family in HEK293 cells. We found that the function of GluR-D was inhibited by ethanol with and without the co-expression of TARPs, the steady-state current being inhibited more than the peak current. Ethanol also decreased the τ-value of desensitization and co-expression of TARPs enhanced this effect. The results indicate that TARPs make the receptors more susceptible to ethanol inhibition, because they slow the desensitization process, and thus increase the exposure of the receptors to the effect of ethanol on desensitization. The results from this study support our hypothesis that the ethanol inhibition of AMPA receptor is mediated partly, if not wholly, by increased receptor desensitization.
In this study, ethanol inhibited the glutamate-evoked currents of GluR-Di receptors to the same extent as in previous studies the currents mediated by native AMPA receptors (Wirkner et al., 2000; Möykkynen et al., 2003). The steady-state current was inhibited more than the peak current for all GluR-D receptors. The co-expression with TARPs did not affect the differential ethanol sensitivity of the peak and steady-state current components. Interestingly, we observed that ethanol accelerated the onset of desensitization, revealed as shortening of the τ-value of desensitization, and this effect was further enhanced in receptors co-transfected with TARPs. This finding provides direct evidence for the idea that ethanol increases desensitization of AMPA receptors. Previously, increased desensitization was suggested to play a role in ethanol inhibition on the basis of indirect evidence: blocking the desensitization by cyclothiazide and using non-desensitizing point-mutated GluR-Ai receptors expressed in HEK293 cells reduced the ethanol inhibition (Möykkynen et al., 2003).
Important molecular domains for AMPA receptor opening and desensitization are the ligand binding domain and a linker domain between the ligand binding domain and the channel pore (Armstrong and Gouaux, 2000; Sun et al., 2002; Horning and Mayer, 2004; Armstrong et al., 2006). The ligand binding domain forms a clamshell-like binding cleft as a combination of two extracellular S1 and S2 domains of AMPA receptors (Stern-Bach et al., 1994). When an agonist binds to the binding cleft, the cleft is thought to close, which stretches the linker domain causing pressure on the channel pore area. The pressure then conveys to channel opening or desensitization. TARPs make physical contact with the AMPA receptor ligand binding domain at the first extracellular loop (Tomita et al., 2007). At least two different mechanisms for TARP effects on AMPA receptor gating have been suggested (reviewed in Milstein and Nicoll, 2008). TARPs may increase the binding cleft closure or they might interact directly with the linker domain. Both of these mechanisms could increase the pressure on the channel pore region mediated by the linker domain. It is not known how ethanol interacts with AMPA receptors at the molecular level. There are, however, data suggesting that ethanol has a binding pocket in AMPA receptors. Alcohols exhibit a cutoff phenomenon for AMPA receptor inhibition. The potency of n-alcohols to inhibit AMPA receptors function grows with increasing carbon chain length, but only up to heptanol (Akinshola, 2001). This most likely means that ethanol has a binding pocket within a receptor domain that is accessible via a hydrophobic milieu, like lipid cell membrane. Ethanol binding pocket may, therefore, be located in close proximity to the pore-forming regions of the AMPA receptors. Regarding desensitization of the AMPA receptor, ethanol and TARPs have opposite effects: TARPs slow and decrease desensitization, whereas ethanol speeds up and increases it. Ethanol may, therefore, influence the channel pore region hindering the opening of the channel and favoring the desensitized state.
In a previous study (Möykkynen et al., 2003), we reported that ethanol lengthens the time needed for the AMPA receptors to recovery from desensitization in isolated hippocampal neurons. Here, we did not observe any effect of ethanol on recovery from desensitization in recombinant homomeric GluR-D receptors. The recovery from desensitization of these receptors was much faster than that of receptors in acutely isolated neurons, indicating that the conditions or receptor properties between the experiments differ substantially. The difference may reflect the differences in AMPA receptor and TARP subunits expressed in native neurons as compared to HEK293 cells. It should be noted that τrec-values measured in this study for GluR-Di receptors are higher than those previously reported for this same subunit expressed in HEK 293 cells (Grosskreutz et al., 2003). However, ultra-fast agonist application method was used in this previous study along with excised outside-out membrane patches, giving both GluR-D flip and flop splice variants extremely fast τrec-values of around 3 ms. Our agonist delivery and washout conditions were slower, which might explain the differences. Loss of cytoskeletal interactions in outside-out membrane patches leading to faster recovery kinetics might also contribute to these differences. In the present study, co-expression of stargazin did not affect recovery from desensitization, but γ4 expression slowed recovery. Interestingly, an opposite effect of stargazin on recovery from desensitization of GluR-A receptors expressed in HEK293 cells has been reported (Priel et al., 2005), indicating that the effects of TARPs on AMPA receptors might be receptor subunit selective. Our observation that the other TARP studied here, γ4, slows the recovery from desensitization, also suggests that the effects of TARPs on AMPA receptor function depend on the TARP subunit in question.
This study shows for the first time directly that ethanol accelerates the desensitization of AMPA receptors and that co-expression of TARPs with AMPA receptors further enhances this acceleration. The findings of this study relate to the situation in the native brain, because TARPs are considered to assemble with AMPA receptors in many brain areas and neuronal types (Hashimoto et al., 1999; Tomita et al., 2003; Rouach et al., 2005; Milstein et al., 2007; Menuz et al., 2008). Desensitization might have little effect on neurotransmission in mature synapses because of the fast glutamate clearance from the synaptic cleft (Clements et al., 1992). However, desensitization probably plays a role in some distinct brain areas, where synaptic currents decay at the rate of desensitization rather than deactivation (Trussell et al., 1993; Barbour et al., 1994; Otis et al., 1996; Maguire, 1999). In addition, it seems that during brain development, desensitization becomes more important in shaping the decay of excitatory postsynaptic currents as shown in mossy fiber-cerebellar granule cell synapses (Wall et al., 2002). Ethanol concentrations used in the present study (25–100 mM) were relatively high, but still reachable during alcohol intoxication. The acceleration of receptor desensitization shown in this study may therefore decrease AMPA receptor function in intact brain and contribute to ethanol intoxication.
Acknowledgments
This study was supported by the Finnish Cultural Foundation, the Finnish Foundation for Alcohol Studies, the Sigrid Jusélius Foundation, as well as the Division of Intramural Clinical and Basic Research of the National Institute on Alcohol Abuse and Alcoholism, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinshola BE. Straight-chain alcohols exhibit a cutoff in potency for the inhibition of recombinant glutamate receptor subunits. Br J Pharmacol. 2001;133:651–658. doi: 10.1038/sj.bjp.0704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinänen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SK, Möykkynen T, Cai C, von Ossowski L, Kuismanen E, Korpi ER, Keinänen K. Isoform-specific early trafficking of AMPA receptor flip and flop variants. J Neurosci. 2006;26:11220–11229. doi: 10.1523/JNEUROSCI.2301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Comparison of ethanol sensitivity of rat brain kainate, DL-α-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1992;262:487–494. [PubMed] [Google Scholar]
- Grosskreutz J, Zoerner A, Schlesinger F, Krampfl K, Dengler R, Bufler J. Kinetic properties of human AMPA-type glutamate receptors expressed in HEK293 cells. Eur J Neurosci. 2003;17:1173–1178. doi: 10.1046/j.1460-9568.2003.02531.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, γ-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Korber C, Werner M, Kott S, Ma ZL, Hollmann M. The transmembrane AMPA receptor regulatory protein γ4 is a more effective modulator of AMPA receptor function than stargazin (γ2) J Neurosci. 2007;27:8442–8447. doi: 10.1523/JNEUROSCI.0424-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott S, Sager C, Tapken D, Werner M, Hollmann M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins γ2, γ3, γ4, and γ8. Neuroscience. 2009;158:78–88. doi: 10.1016/j.neuroscience.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci Lett. 1993;159:83–87. doi: 10.1016/0304-3940(93)90804-t. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G. Rapid desensitization converts prolonged glutamate release into a transient EPSC at ribbon synapses between retinal bipolar and amacrine cells. Eur J Neurosci. 1999;11:353–362. doi: 10.1046/j.1460-9568.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Menuz K, O’Brien JL, Karmizadegan S, Bredt DS, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J Neurosci. 2008;28:8740–8746. doi: 10.1523/JNEUROSCI.1319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits α-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pasternack A, Coleman SK, Jouppila A, Mottershead DG, Lindfors M, Pasternack M, Keinänen K. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J Biol Chem. 2002;277:49662–49667. doi: 10.1074/jbc.M208349200. [DOI] [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP γ-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O’Hara PJ, Heinemann SF. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kessler M, Arai AC. The fast kinetics of AMPA GluR3 receptors is selectively modulated by the TARPs γ4 and γ8. Mol Cell Neurosci. 2008;38:117–123. doi: 10.1016/j.mcn.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Robert A, Howe JR, Usowicz MM. The speeding of EPSC kinetics during maturation of a central synapse. Eur J Neurosci. 2002;15:785–797. doi: 10.1046/j.1460-9568.2002.01910.x. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]