Abstract
Objective
Homeobox genes are transcriptional regulators that orchestrate embryonic development. The HOXA13 gene is responsible for the development of the vagina and regulates extracellular matrix (ECM) constituents. We hypothesized that vaginal expression of HOXA13 may be decreased in women with pelvic organ prolapse (POP) and sought to determine if hypoestrogenism affects its expression.
Design
Biopsy specimens were obtained from the anterior apex of the vagina from women with and without POP. Immunohistochemistry and real time PCR were used to determine HOXA13 expression in premenopausal controls, premenopausal women receiving leuprolide acetate, and in premenopausal and postmenopausal women with POP.
Results
HOXA13 was expressed in all specimens. HOXA13 expression was 14 fold lower in premenopausal women with prolapse than premenopausal controls (P<0.001). In both POP groups, HOXA13 expression was lower than the leuprolide group (p=<0.001). There were no differences in HOXA13 expression between premenopausal controls and specimens from women treated with leuprolide acetate (p= 1.0) or between the premenopausal and postmenopausal POP group (p= 1.0).
Conclusion
Vaginal HOXA13 expression is diminished in women with POP compared to women with normal support. In women with POP, expression of HOXA13 was not affected by menopause. Expression of HOXA13 was also not affected by exposure to leuprolide acetate, suggesting that estrogen and HOXA13 work through separate pathways in the ECM metabolism of the vagina. Understanding genetic predispositions to developing POP may identify younger patients at risk who may benefit from preventative strategies such as weight loss or smoking cessation and not necessarily from estrogen therapy.
Introduction
Pelvic organ prolapse (POP), is the result of weakened endopelvic fascia causing herniation of the uterus, bladder, bowel and/or rectum into the vaginal canal.1 The prevalence of pelvic organ prolapse has been shown to affect up to 30–50% of the population.2 It is also projected that over the next 30 years, the number of women seeking treatment for prolapse will double.3 In addition to the rising numbers of women seeking treatment for POP, up to 30% of patients who have surgical repair develop recurrent POP, leading investigators to search for a better understanding of the underlying mechanisms of this disorder.4
The pathophysiology of POP is thought to be multifactorial. Clincal risk factors for POP have been classically delineated as child birth, aging, menopause and increasing BMI.1, 5–7 More recent investigations have revealed that women with POP have decreased vaginal and uterosacral smooth muscle content and extracellular matrix abnormalities, including changes in the dynamic balance in collagen content and expression of metalloproteinases in the vaginal extracellular matrix.8–14 However, little is known regarding regulation of the ECM proteins and pathophysiological pathways associated with POP.
Estrogen has been shown to increase fibroblast proliferation, to positively affect collagen production and to decrease expression of matrix metalloproteinases.15–18 In postmenopausal women, collagen subtypes are altered in the endopelvic fascia, and hormones have been shown to restore the biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats.19,20 Therefore, the influence of menopause on the ECM has been studied looking to determine pathways that estrogen may be involved in the development of prolapse.
Homeobox (HOX) genes are evolutionarily conserved genes encoding transcription factors that regulate mammalian embryonic growth and development of the urogenital tract. The HOXA cluster genes mediate segmental differentiation of the paramesonephric duct into morphologically distinct organs of the female reproductive tract.21 These genes have been shown to persist in the adult reproductive tract and maintain plasticity of the genital tract during times of hormonal and structural changes that occur during the menstrual cycle and in pregnancy.21–23 Previously, we have shown that HOXA11 is responsible for the development of the uterosacral ligament and is decreased in women with POP.14 We have also shown that HOXA11 regulates collagen type III and MMP2 expression in vitro.14
HOXA13 is responsible for the development of the upper vagina.21 Mutations in this gene cause the hand-foot-genital syndrome which includes an array of vaginal structural abnormalities.24–27 Microarray studies have shown that HOX genes regulate several ECM components.28, 29 Since HOXA13 is a regulatory gene of the vagina, we hypothesized that alterations of expression of this gene may be involved in the development of POP. In this study, we determined that vaginal HOXA13 expression is decreased in women with POP. We also showed that in women with POP, the expression of HOXA13 is not affected by menopause. We have also shown that expression of HOXA13 is not altered in hypoestrogenic women receiving leuprolide acetate. These findings suggest that estrogen and HOXA13 work in separate pathways in ECM metabolism of the vagina.
Methods
Acquisition of Human Tissue
All experiments were performed with approval of the Yale Human Investigation Committee. Vaginal specimens were collected between September 2005 and July 2007, from women undergoing hysterectomy or anterior vaginal repair at our institution. Prior to their surgery, a pelvic examination was performed to evaluate for the presence of POP. Vaginal prolapse was graded according to the pelvic organ prolapse quantification system advocated by the International Continence Society.30 Women with stage II anterior wall prolapse or greater were assigned to the POP group. Data regarding age, menopausal status, hormone use, and parity were recorded. Women with previous pelvic surgery, hormone use, and pessary use prior to surgery were excluded from the study.
At the time of surgery, 3–5mm biopsies were taken from the midline anterior vaginal cuff for consistency. Specimens were divided in half. One section was promptly placed in RNAlater (Ambion, Inc.) and stored at −80 °C until extraction of RNA was performed. The other half was placed in 10% formalin for analysis using immunohistochemistry. Six specimens of the premenopausal POP group and 3 specimens from the postmenopausal POP group were very small and in these cases, the tissue was processed for RNA extraction only.
Immunohistochemistry
Slides were prepared from 14 vaginal specimens obtained from premenopausal patients with POP, 22 from postmenopausal patients with POP, 6 from premenopausal control patients and 5 from patients with normal pelvic support who received leuprolide acetate. Formalin fixed specimens were embedded in paraffin, sectioned (5 μ thickness) and fixed to glass slides. Immunohistochemistry was performed using a protocol that has been previously described.14 HOXA13 antibody (made in rabbit, #ab26084, Abcam, Inc, Cambridge, MA) in a 1: 500 concentration as the primary antibody. Rabbit IgG as the primary antibody were used as negative controls. Duplicate slides of the primary antibody in addition to the negative controls were prepared for each specimen. Photographs of the slides were taken at a magnification of 60× under the same lighting conditions using a Kodak DC290 Zoom digital camera (Eastman Kodak Company, Rochester, NY). Two observers evaluated the slides for intenstity of staining.
RNA extraction
A total of 56 vaginal specimens were evaluated by real time PCR in this study; 20 vaginal specimens obtained from premenopausal patients with POP, 25 from postmenopausal patients with POP, 6 from premenopausal control patients and 5 from patients with normal pelvic support who received leuprolide acetate. Specimens in RNAlater for RNA extraction were thawed on ice and placed in 1ml of TRIzol per 100mg tissue (Invitrogen Corporation). Tissue was homogenized on ice and total RNA was isolated using methodology described by the manufacturer. The optical density (OD) of the RNA was measured using SmartSpex 300 (Bio-Rad Laboratories, Inc.) at 260nm to determine the concentration. One μg of high purity RNA (OD 260/280 ratios 1.8–2.0) wasreverse-transcribed using the Eppendorf Mastercycler (Eppendorf of North America) and the Bio Rad iScript cDNA synthesis kit using conditions previously described.14
The mRNA expression of vaginal HOXA13 was determined using real time RT-PCR. The PCR primers used for amplification were: 5′-ATGCCTGGCTACCTGGATATGC-3′ for the HOXA13 sense, 5′-GGGCAGAGTGGACTTCCAGAGGT-3′ for the HOXA13 antisense,. 5′-AGAGGGAAATCGTGCGTGAC-3′ for the beta actin sense and 5′-CAATAGTGACCTGGCCGT-3′ for the beta actin antisense.. The PCR reaction was carried out in a MyiQ Real-Time PCR detection system (Bio-Rad Laboratories, Inc.). The melting peak of each sample was routinely determined by melting curve analysis to ascertain that only the expected products had been generated. Negative controls were run in the absence of reverse transcriptase. All specimens were run in duplicate and the experiments were repeated twice.
Gene expression levels were standardized by calculating mRNA ratios relative to the house-keeping gene beta actin. The difference in the average cycle threshold (ΔCt) between the housekeeping gene (beta actin) and our gene of interest, HOXA13, was determined. The formula 1/2ΔCt was used to compare relative values of RNA.
Statistics
Descriptive data are expressed as mean ± SEM and percentages. Parametric demographic data of the subjects were compared between groups using the unpaired Student’s t- test. The results of relative mRNA expression between the groups were compared using a one-way analysis of variance (ANOVA) with the Bonferoni correction. Statistical analysis was performed with Excel software (Microsoft Office 2003) and Sigma Stat. A P value less than 0.05 was considered statistically significant.
Results
Demographic background of subjects
The cohort in this study population was predominantly white (91.3%). The remainder of the participants consisted of women of African American (6.6%) and Hispanic (2.1%) ancestry. To evaluate the influence of estrogen status on HOXA13 expression, premenopausal controls were compared to premenopausal women receiving leuprolide acetate (a GnRH agonist) and premenopausal women with POP were compared to postmenopausal women with POP.
None of the postmenopausal women with POP were taking hormone replacement therapy. In the premenopausal POP group, 9 women had stage II anterior vaginal wall prolapse and 5 women had stage III prolapse. In the postmenopausal POP group, 12 women had stage II anterior vaginal wall prolapse and 10 had stage III prolapse. No one in the study had stage IV disease. All women in the POP group underwent surgery for symptomatic prolapse. All of the women with POP had urinary incontinence and none of the controls had this condition.
For those patients who received leuprolide acetate, one patient received one dose, 2 patients received 2 doses, and 2 patients received 3 doses prior to their surgery. All patients received the last dose 3–4 weeks before their hysterectomy.
There were no differences in age, parity or BMI between the premenopausal controls compared the premenopausal POP group (Table 1) and the premenopausal controls compared to the patients receiving leuprolide acetate (data not shown). The postmenopausal POP was significantly older than the premenopausal POP group, but did not differ in regards to parity or BMI (data not shown).
Table 1.
Demographic background of premenopausal controls and premenopausal prolapse subjects
| Variable | Premenopausal Control | Premenopausal POP | P value |
|---|---|---|---|
| Age (years) | 40.6 + 3.5 | 45.6 + 5.7 | 0.09 |
| Parity | 1.7 + 1.6 | 2.38 + 1.0 | 0.34 |
| BMI | 28.1 + 6.6 | 27.6 + 6.8 | 0.90 |
Vaginal HOXA13 expression is lower in women with POP and not affected by menopause or exposure to leuprolide acetate
Immunohistochemistry demonstrated expression of HOXA13 in the vagina in patients with and without prolapse, however, the positive immunoreactivity of intranuclear staining was consistently less pronounced in the prolapse group compared to controls (Figure 1). The immunoreactivity of the premenopausal and postmenopausal POP specimens was similar to each other, as were the immunoreactivity of the premenopausal controls and the leuprolide specimens. (Figure 1A–D).
Figure 1.
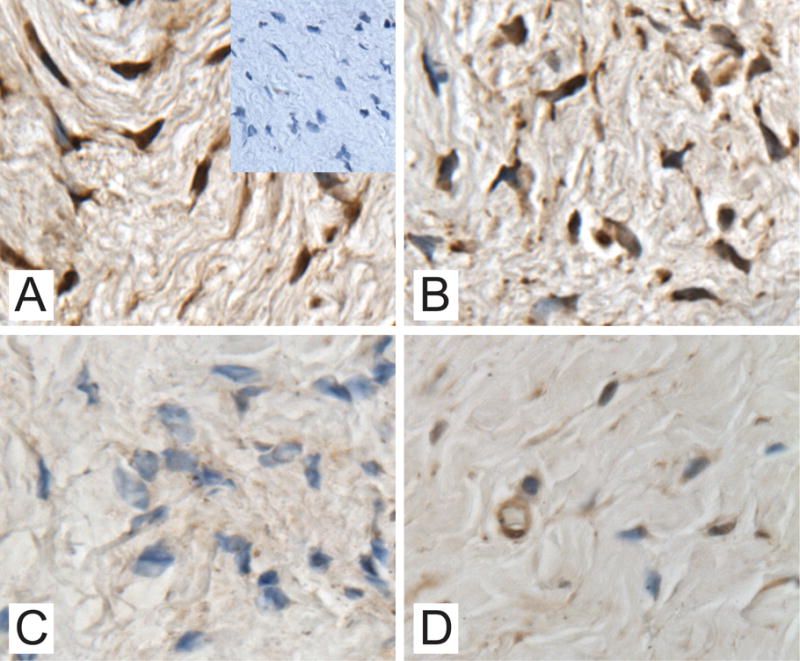
Immunostaining of HOXA13 in vaginal specimens obtained from a patient with normal pelvic support with inlay of immunostaining with rabbit IgG as the negative control (1A.), a patient with normal pelvic support treated with leuprolide acetate (1B.), a premenopausal patient with POP (1C.) and a postmenopausal patient with POP (1D.) (magnification 60x)
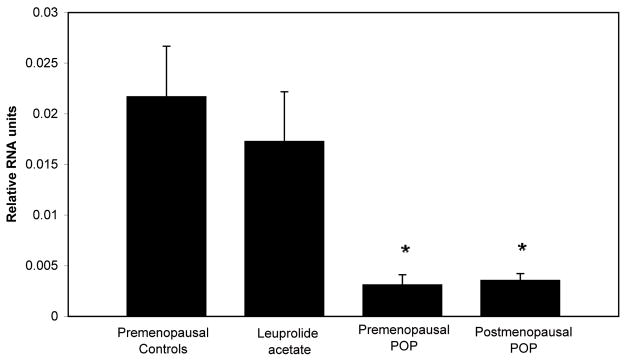
To quantitatively evaluate the vaginal expression of HOXA13 in each group of subjects, we used real time RT PCR. We found that the relative mRNA expression of HOXA13 of premenopausal women with POP was 14 fold lower compared to premenopausal controls (p<0.001), despite similarities in age, parity, and BMI between the groups. Vaginal expression of HOXA 13 was also significantly lower in postmenopausal POP specimens compared to controls (p<0.001). Additionally, both the premenopausal and postmenopausal POP specimens had lower expression of HOXA13 compared to the specimens obtained from women receiving leuprolide acetate. (p<0.001 for both groups). There were no differences in vaginal expression of HOXA13 between premenopausal controls and specimens obtained from women exposed to leuprolide acetate (p=1.0) or between specimens obtained from premenopausal women with POP and postmenopausal women with POP (p=1.0), (Figure 2.).
Figure 2. Comparison of HOXA13 mRNA expression in vaginal specimens.
Premenopausal controls n=6, Leuprolide acetate n=5, Premenopausal POP n=20, Postmenopausal POP n=25. Comparisons were made after normalization with beta actin. * indicates that expression of vaginal HOXA13 in groups is significantly different from the expression in the premenopausal controls and from specimens obtained from women treated with leuprolide acetate.
Discussion
The present study demonstrates that HOXA13 expression is decreased in the vagina of women with POP, and that expression of this gene is not affected by menopause or treatment with leuprolide acetate. Interestingly, the expression of HOXA13 was lower in the premenopausal patients with POP compared to postmenopausal patients with POP, although this was not statistically significant. In premenopausal patients, inducing a hypoestrogenic state with a GnRH agonist did not affect HOXA13 expression.
Williams et al. demonstrated that HOXA13 regulates many genes involved in the ECM, including some that have been shown to be altered in women with POP mentioned above.29 Their data revealed that expression of HOXA13 led to up regulation of several collagen types, which confer both the tensile strength of the vagina and basement membrane constituents. Additionally, genes in the glycoprotein family were also upregulated by HOXA13 expression including, fibulin, fibrillin 1,laminins 2 and 3, and osteonectin.29 These genes are involved in the intricate networking of elastic fibers, basement membrane, microfibrils, and proteoglycan aggregates.31–36 Mutations in this family of genes (filbrillin, fibulin and osteonectin) are associated with pelvic organ prolapse in women and compromised ECM in mice.31,32,33,36
Our findings of decreased HOXA13 expression in the vagina in women with POP and the data demonstrating that HOXA13 regulates many different structural components of the ECM, strongly suggest that HOXA13 is a candidate gene for further investigation in determining the molecular mechanisms involved in the development of POP. This data is also consistent with our previous findings that HOXA11, another HOXA gene involved in the development of the uterus and uterosacral ligaments, is dramatically decreased in women with POP. Like HOXA13, expression of HOXA11 was also independent of estrogen, age, parity and BMI.14 These findings combined suggest that HOX genes may be key regulators in the maintenance of the ECM of the uterine support structures and vagina.
A possible explanation for variable clinical presentation of POP is the existence of several pathways regulating the urogenital ECM. We have demonstrated that there is a genetic predisposition for developing POP independent of menopause and leuprolide administration which is associated with hypoestrogenism. Current in vitro and animal studies have shown that estrogen has positive effects on the ECM 15–17, 20 and others have shown that hypoestrogenism is associated with deterioration of the ECM and development of POP.5–7,19 The Women’s Health Initiative demonstrated that conjugated equine estrogen alone and conjugated equine estrogen with medroxyprogestesrone increased the risk of urinary incontinence among continent women and worsened the characteristics of urinary incontinence in symptomatic women after 1 year of treatment.37 Currently, there are no longitudinal studies evaluating the effect of hormone therapy on the quality of the ECM and its relationship with incontinence. It is possible that HT may benefit hypoestrogenic women presenting with POP and women with atrophic symptoms. However, identification of genetic tendencies toward developing POP is necessary as HT may not benefit this group of patients. The existence of two distinct pathways regulating the ECM in the vagina may explain the discrepant data on the effect of HT on urogenital symptoms.
Conclusion
Vaginal HOXA13 expression is diminished in women with POP compared to women with normal support. Expression of HOXA13 is not affected by menopause or exposure to leuprolide acetate, suggesting that estrogen and HOXA13 work through separate pathways in ECM metabolism of the vagina. Understanding genetic predispositions to developing POP may aid in identifying younger patients at risk for this disease who would benefit from preventative strategies and not from estrogen therapy.
Acknowledgments
Disclosure of funding: This work was supported by the NICHD Women’s Reproductive Health Research Career Development Program (grant 5K12HD047018).
References
- 1.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson EC, Victor FT, Tibblin G, Svardsudd K. Signs of genital prolapse in a Swedish population of women 20–59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 3.Drutz H, Alarab M. Pelvic organ prolapse: demographics and future growth prospects. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:S6–S9. doi: 10.1007/s00192-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 4.Olsen AL, Smith VJ, Berstrom JO, Colling JC, Clark A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 5.Swift SE, Pound T, Dias JK. Case-control study of etiologic factors in the development of severe pelvic organ prolapse. Int Urogynecol J. 2001;(12):187–192. doi: 10.1007/s001920170062. [DOI] [PubMed] [Google Scholar]
- 6.Kim CM, Jeon MJ, Chung DJ, Kim SK, Kim JW, Bai SW. Risk factors for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;98(3):248–51. doi: 10.1016/j.ijgo.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse:risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109(6):1396–1403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- 8.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187:56–63. doi: 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SR, Avery NXC, Rarlton JF, et al. Changes in the metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–1661. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 10.Liapis A, Bakas P, Pafiti A, et al. Changes in collagen type III in female patients with genuine strss incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol. 2001:9776–79. doi: 10.1016/s0301-2115(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 11.Makinen J, Kahari VM, Soderstrom KO, et al. Collagen synthesis in the vaginal connective tissue of patients with and without uterine prolapse. Eur J Obstet Gynecol Reprod Biol. 1987;24:319–325. doi: 10.1016/0028-2243(87)90157-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:80–87. doi: 10.1007/s001920200020. [DOI] [PubMed] [Google Scholar]
- 13.Takano CC, Girao MJ, Sartori MG, et al. Analysis of collagen in parametrium and vaginal apex of women with pelvic organ prolapse. Int J Urogynecology Pelvic Floor Dysfunct. 2002;13:342–345. doi: 10.1007/s001920200076. [DOI] [PubMed] [Google Scholar]
- 14.Connell KA, Guess MK, Chen H, Andikyan V, Bercik R, Taylor HS. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J Clin Invest. 2008;118(3):1050–1055. doi: 10.1172/JCI34193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson SJ, Nelson LD, Sharpe DT, Thornton MJ. 17 β-estradiol regulates the secretion of TGF-β by cultured human dermal fibroblasts. Biomater Sci Polymed. 2008;19(8):1097–1109. doi: 10.1163/156856208784909354. [DOI] [PubMed] [Google Scholar]
- 16.Zbucka M, Miltyk W, Bielawski T, Surazynski A, Wolczynski Palka J. Mechanism of collagen biosynthesis up-regulation in cultured leiomyoma cells. Folio Histochem Cytobiol. 2007;45(1):S181–5. [PubMed] [Google Scholar]
- 17.Tizk DEE, Hassan HA, Al-Marzouqi AH, Ramadan GA, Al-Kedrah SS, Daoud SA, Fahim MA. Combined estrogen and ghrelin administration restores number of blood vessels and collagen type I/III ratio in the urethral and anal canal submucosa of old ovariectomized rats. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:547–552. doi: 10.1007/s00192-007-0462-1. [DOI] [PubMed] [Google Scholar]
- 18.Markiewicz M, Asano Y, Znoyko S, Gong Y, Watson DK, Trojanowska M. Distinct effects of gonadectomy in male and female mice on collagen fibrillogenesis in the skin. J Dermatological Sci. 2007;47:217–226. doi: 10.1016/j.jdermsci.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moalli PA, Talarco LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol. 2004;190:620–7. doi: 10.1016/j.ajog.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Moalli PA, Debes KM, Meyn LA, Howden NS, Abramowitch SD. Am J Obstet Gynecol. 2008. April 4, Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. (epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the hoxa cluster genes. Biol Reprod. 1997;57:1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 22.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84:1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- 24.Utsch B, McCabe CD, Galbraith K, Gonzalez R, Born M, Dötsch J, Ludwig M, Reutter H, Innis JW. Molecular Characterization of HOXA13 Polyalanine Expansion Proteins in Hand–Foot–Genital Syndrome. J Med Genet. 2007;143A:3161–3168. doi: 10.1002/ajmg.a.31967. [DOI] [PubMed] [Google Scholar]
- 25.Frisén L, Lagerstedt K, Tapper-Persson M, Kockum I, Nordenskjöld A. A novel duplication in the HOXA13 gene in a family with atypical hand-foot-genital syndrome. J Med Genet. 2003 Apr;40(4):e49. doi: 10.1136/jmg.40.4.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, Beemer FA, Hennekam RC, Scambler PJ. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000 Jul;67(1):197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997 Feb;15(2):179–80. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- 28.Vitiello D, Pinard R, Taylor HS. Gene expression profiling reveals putative HOXA10 downstream targets in the periimplantation mouse uterus. Reprod Sci. 2008 May;15(5):529–35. doi: 10.1177/1933719108316911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams TM, Williams ME, Kuick R, Misek D, McDonagh K, Hanash S, Innis JW. Candidate downstream regulated genes of HOX group 13 transcription factors with and without monomeric DNA binding capability. Dev Biol. 2005 Mar 15;279(2):462–80. doi: 10.1016/j.ydbio.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 31.Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice:pregnancy- induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170(2):578–89. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006 Aug 8;8(19):1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 33.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers Danlos syndrome. Am J Obstet Gynecol. 2000 May;182(5):1021–3. doi: 10.1067/mob.2000.105410. [DOI] [PubMed] [Google Scholar]
- 34.Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40(2):199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001 May;107(9):1049–54. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker TH, Baneyx G, Cardó-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem. 2005 Oct 28;280(43):36483–93. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- 37.Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, Aragaki A, Naughton MJ, Wallace RB, McNeeley SG. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005 Feb 23;293(8):935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]