Abstract
Aging, Alzheimer disease, and hypertension, major determinants of cognitive dysfunction, are associated with profound alterations in the structure and function of cerebral blood vessels. These vascular alterations may impair the delivery of energy substrates and nutrients to the active brain, and impede the clearance of potentially toxic metabolic byproducts. Reactive oxygen species derived form the enzyme NADPH oxidase are key pathogenic effectors of the cerebrovascular dysregulation. The resulting alterations in the homeostasis of the cerebral microenvironment may lead to cellular dysfunction and death and to cognitive impairment. The prominent role that cerebrovascular oxidative stress plays in conditions associated with cognitive impairment suggests new therapeutic opportunities to counteract and, possibly, reverse the devastating effects of cerebrovascular dysfunction on the brain.
Keywords: dementia, VCI
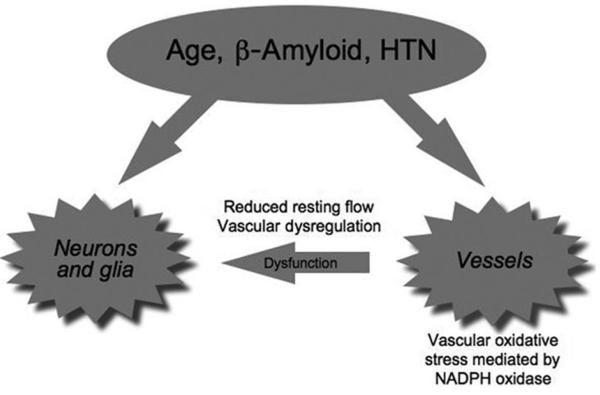
Aging, hypertension, and Alzheimer diseases (AD) are major determinants of cognitive impairment. Although normal aging may affect only selected aspects of cognitive function, advanced age is a powerful risk factor for the cognitive decline caused by hypertension and AD.1 Furthermore, midlife hypertension is a risk factor for developing AD later in life.2 Therefore, the effects of hypertension, aging, and AD on cognition are intertwined and highly interactive. The brain has no energy reserves, and its structural and functional integrity depends on a continuous and well regulated blood supply matched to its energetic needs.3 The cerebral circulation is endowed with regulatory mechanisms that assure an adequate delivery of blood to the brain. Disruption of these mechanisms alters the balance between delivery of energy substrates and clearance of metabolic waste and leads to brain dysfunction. Recent studies have revealed that aging, hypertension, and AD trigger common signaling pathways that lead to deleterious effects on the regulation of the cerebral circulation. These findings reinforce the notion that cerebrovascular dysfunction plays a key role in the cognitive impairment associated with these conditions (Figure 1). This article will briefly review the cerebrovascular effects of aging, hypertension, and AD, and will examine their common mechanisms and their potential role in the associated cognitive dysfunction.
Figure 1.
Aging, hypertension (HTN), and β-amyloid produce cerebrovascular alterations that act in concert with other mechanisms to produce neuronal dysfunction.
Major Factors Regulating the Cerebral Circulation
The cerebral circulation is equipped with control mechanisms that assure that the brain receives an adequate amount of blood at all times. Thus, if the energy demands of the brain rise because of increased neural activity, cerebral blood flow (CBF) also increases, a phenomenon termed functional hyperemia.4 The increase in flow is thought to enhance the delivery of nutrients and the clearance of metabolic byproducts and heat produced by brain activity, but it may also influence neural activity via effects on astrocytes.3,5 Another control mechanism is cerebrovascular autoregulation, defined as the ability of cerebral blood vessels to maintain stable CBF despite changes in arterial pressure within a certain range.6 Thus, changes in arterial blood pressure are coupled with corresponding changes in vascular resistance that maintain CBF nearly constant between 60 and 150 mm Hg (mean arterial pressure). Cerebral endothelial cells participate in cerebrovascular control by releasing vasoactive agents, such as the vasodilator nitric oxide or the vasoconstrictor endothelin.7 Cerebral blood vessels are extremely sensitive to changes in respiratory gases in the circulating blood. Thus, hypoxia and hypercapnia increase CBF markedly, a response aimed at maintaining adequate cerebral oxygenation in conditions of respiratory compromise.
Aging
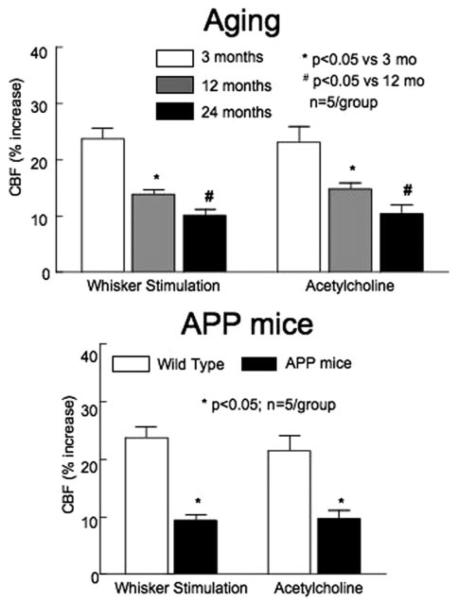
Cerebral blood vessels undergo profound changes with aging. In the cerebral cortex and hippocampus cerebral capillaries are reduced in number and have thickened and fibrotic basement membranes.8 Pericytes, which replace smooth muscle cells in capillaries, undergo a degenerative process, whereas endothelial cells appear elongated and exhibit a reduced number of mitochondria.8 White matter vessels (diameter: 100 μm) of aged individuals appear more tortuous, a phenomenon not observed in gray matter vessels.9 Aging is associated with a reduction in resting CBF and a dysfunction of the mechanisms regulating the cerebral circulation.8,9 The cerebrovascular relaxation induced by the endothelium-dependent vasodilator acetylcholine is impaired in older animals, and the increases in CBF induced by hypercapnia, hypoxia, or hypotension are attenuated (see10 for references). Recent data indicate that aging also alters the increase in CBF produced by neural activity. Thus the increase in CBF produced by stimulation of the whiskers in mice (functional hyperemia) is attenuated in an age dependent manner (Figure 2). These alterations reduce cerebral perfusion, deplete cerebrovascular reserves, and increase the susceptibility of the brain to vascular insufficiency and ischemic injury.8,11
Figure 2.
The increase in cortical CBF produced by whisker stimulation or by topical superfusion with acetylcholine is attenuated with aged C57Bl/6 mice, or in Tg2576 mice overexpressing the amyloid precursor protein (APP), a model of AD.
Hypertension
Chronic hypertension is associated with profound changes in the structure of cerebral blood vessels. Hypertension promotes atherosclerosis in cerebral arteries12 and induces lipohyalinosis, a pathological process characterized by fibrinoid necrosis of the vascular wall.13 Lipohyalinosis affects penetrating arteries and arterioles supplying the white matter (usually <300 μm in diameter), and can lead to small white matter strokes (lacunes) or brain hemorrhage.13 Hypertension induces adaptive changes in systemic and cerebral arteries known as hypertrophic and eutrophic remodeling. In hypertrophic remodeling smooth muscle cells grow inward, encroaching into the lumen of the artery.14 In eutrophic remodeling, smooth muscle cells undergo a rearrangement that leads to a reduction of the vessel lumen without significant changes in wall thickness.14 Chronic hypertension also alters the function of cerebral blood vessels. Hypertension reduces resting CBF regionally, and impairs cerebrovascular reactivity.14 Thus, functional hyperemia is attenuated in animal models of hypertension and in hypertensive patients as well.14 Hypertension attenuates endothelium-dependent response of cerebral arteries, whereas endothelium-independent responses are spared.14 Hypertension shifts the cerebrovascular autoregulation curve to the right, so that a higher pressure is needed to maintain adequate CBF,14 predisposing to hypoperfusion during hypothension.
Alzheimer Disease
AD has long been recognized to be associated with structural and functional alterations of cerebral blood vessels.9 Thus, especially in the temporal-parietal cortex, cerebral microvessels become irregular and fragmented, vascular basement membranes become thicker, and cerebral capillaries appear atrophic and reduced in number.8 Cerebral endothelial cells are swollen and exhibit irregular nuclei,8 whereas cerebral myocytes are hypercontractile.15 In advanced cases, amyloid deposition occurs in cerebral vessels (amyloid angiopathy), resulting in smooth muscle cell degeneration and weakening of the vascular wall.16 CBF and glucose utilization are reduced in the temporal-parietal cortex of patients with AD.17 Flow-dependent vasodilatation in the brachial artery is attenuated, an effect correlated with the severity of the dementia.18 However, the pathogenic significance of these structural and functional alterations in sporadic AD has remained elusive because it has been unclear whether the microcirculatory vasculopathy is secondary to neuronal dysfunction or to the regionally-selective neurodegeneration characteristic of the disease. Recent studies using mouse models of AD have revealed that Aβ, the peptide linked to the pathological manifestations of AD, has profound effects on the regulation of cerebral blood vessels. Transgenic mice overexpressing the amyloid precursor protein (APP) have reduced CBF and attenuated cerebrovascular reactivity to functional hyperemia and endothelium-dependent vasodilators (Figure 2).4 Furthermore, autoregulation is markedly impaired.4 These cerebrovascular alterations are observed in the absence of amyloid plaques or amyloid angiopathy, and occur in young APP mice when they are still behaviorally normal. Acute application of Aβ1–40 to the cerebral cortex of normal mice reproduces the cerebrovascular effects observed in APP mice,4 linking the Aβ peptide to the mechanisms of the vascular dysfunction. These experimental observations have provided evidence that cerebrovascular dysfunction may be an early pathogenic event in AD and have been supported by clinical studies demonstrating that changes in cerebral perfusion precede the onset of the dementia in AD patients.17 Furthermore, a growing number of epidemiological and pathological studies have emphasized the role of vascular risk factors in AD.9 These clinical, epidemiological, and experimental observations, collectively, support the contention that cerebrovascular abnormalities play a pathogenic role in the early stages of AD.4,9
NADPH Oxidase: A Final Common Pathway to Vascular Oxidative Stress in Aging, Hypertension, and AD
Reactive oxygen species (ROS) have long been implicated in the pathogenesis of hypertension, AD, and aging.19 In particular, oxidative stress in cerebral blood vessels has been found to play a major role in the cerebrovascular dysfunction associated with these conditions.4,14 Although there are several sources of vascular ROS,20 the enzyme NADPH oxidase has emerged as a major source of vascular oxidative stress. NADPH oxidase is a multiunit enzyme discovered in neutrophils that is also present in vascular cells, particularly in cerebral blood vessels.21,22 The enzyme comprises membrane bound (p22phox and Nox) and cytoplasmic subunits (p47phox and p67phox) and requires the small GTPase Rac for its activation.21 Nox, a subunit present in 5 homologues (Nox1 through 5), is the catalytic site of the enzyme.21 NADPH oxidase-derived superoxide has been implicated in the cerebrovascular dysfunction associated with the hypertension induced by angiotensin II (AngII), aging, and Aβ.4,10,14 Mice lacking Nox2 do not exhibit cerebrovascular oxidative stress and are protected from the alterations in endothelium-dependent relaxation and functional hyperemia induced by AngII, Aβ, or aging.4,10,14 Similarly, a peptide inhibiting the assembly of NADPH oxidase or a pharmacological inhibitor of this enzyme blocks the ROS production and cerebrovascular dysfunction induced by AngII, aging, and Aβ.4,10,14 In mice overexpressing APP, deletion of Nox2 improves cerebrovascular reactivity and cognitive function, implicating NAPDH oxidase–derived ROS in the mechanisms of the cerebrovascular dysfunction and cognitive deficits.23
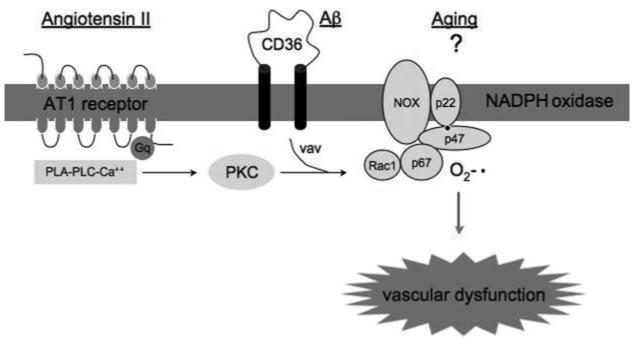
The specific molecular cascades leading to NADPH activation in hypertension, aging, and Aβ have not been elucidated in full, but a great deal is known about the mechanisms of NADPH oxidase activation by AngII and Aβ (Figure 3). In vascular cells, activation of AngII AT1 receptors leads to activation of protein kinase C, which, in turn, phosphorylates p47phox leading to the assembly of the enzyme and ROS production.24 In microglia, Aβ leads to phosphorylation of the guanine nucleotide exchange factor Vav through interaction with the scavenger receptor CD36 and the associated tyrosine kinases Syk and Lyn.25 The downstream target of Vav, the small GTPase Rac1, is GTP-loaded and leads to NADPH oxidase activation.25
Figure 3.
AngII, Aβ, and aging induce vascular oxidative stress by activating NADPH oxidase. See text for details.
How Does Cerebrovascular Dysfunction Lead to Cognitive Deficits?
Aging, AD, and hypertension are often associated with focal (lacunes) or diffuse (laukoaraiosis) white matter abnormalities, which can have a profound impact on the severity of dementia.9 The periventricular white matter and the white matter of the basal ganglia and centrum semiovale are particularly susceptible to damage by reduced cerebral perfusion. These regions are located at the border between different arterial territories and are prone to hypoperfusion.9,26 Moreover, in AD, aging, and hypertension, these brain regions exhibit marked capillary loss and an increase in microvascular tortuosity, changes that increase resistance to flow and reduce tissue perfusion.9 Therefore, subcortical white matter regions are highly vulnerable to injurious stimuli.
These structural and functional cerebrovascular alterations are likely to have major impact also in brain regions primarily involved in cognition, such as the neocortex and the hippocampus. Reduced blood flow at rest and during activation may impair the delivery of energy substrates and nutrients to active brain cells, creating a mismatch between the energy supply and demand. Furthermore, the clearance of byproducts of brain activity, which is flow dependent, is also likely to be reduced. For example, Aβ is released during synaptic activity27 and is cleared via vascular transport mechanisms involving the lipoprotein receptor.15 Therefore, the deficit of cerebrovascular regulation in AD brains may impair the normal clearance of Aβ and facilitate accumulation in brain and blood vessels.
Alterations in the blood–brain barrier (BBB), well described in aging, hypertension, and AD, will disrupt the exchange between blood and brain.15 These BBB alterations, in concert with the disturbance in cerebral perfusion, compromise the homeostasis of the cerebral microenvironment and increase the vulnerability of the brain to injury. Finally, neuronal protein synthesis, a process essential for memory formation and synaptic plasticity, is suppressed by hypoperfusion.28 Therefore, reduced protein synthesis may also contribute to the cognitive dysfunction by reducing the formation and consolidation of new memories and by altering synaptic plasticity.
Conclusions
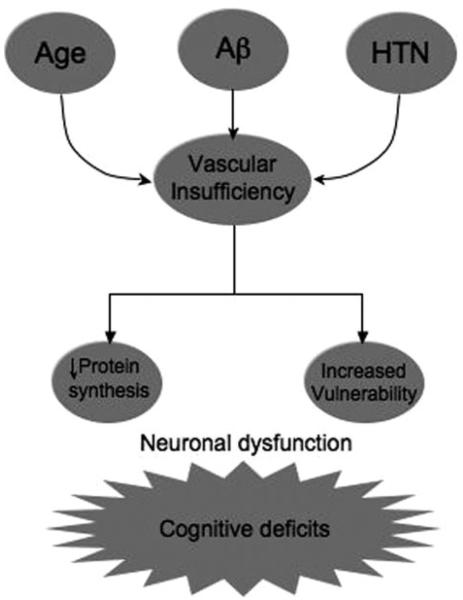
The evidence presented above indicates that cerebrovascular dysfunction is a common pathological factor in hypertension, aging, and AD (Figure 4). The cerebrovascular and BBB alterations may impair brain function by altering the homeostasis of the brain cell microenvironment and by increasing the vulnerability of susceptible regions to ischemichypoxic brain damage. The inhibition of protein synthesis induced by hypoperfusion may alter brain plasticity and memory formation. These processes act in concert to produce cognitive deficits (Figure 4). The finding that the cerebrovascular alterations are mediated by oxidative stress raises the possibility that antioxidants could be used to counteract the deleterious effect of ROS on cerebral blood vessels. However, the failure of trials using antioxidants in cardiovascular diseases29 suggests that more effective approaches are needed to protect the blood vessels from oxidative damage. Treatments using inhibitor of vascular isoforms of NADPH oxidase may offer a new strategy to curtail cerebrovascular oxidative stress. In addition, preventive measures to counteract risk factors for vascular diseases would be most desirable not only for cerebrovascular health, but also for prevention of AD.
Figure 4.
Potential mechanisms of cognitive dysfunction by vascular insufficiency. Relatively small reductions in CBF inhibit protein synthesis, which is essential for normal cognitive function. Furthermore, reduced CBF may increase the vulnerability of the brain to ischemic injury, particularly in borderzone regions of the hemispheric white matter.
Sources of Funding
This work was supported by grants from the National Institutes of Health (NS38252, HL18974). C.I. is the recipient of a Javits Award from NIH/NINDS.
Footnotes
Disclosures None.
References
- 1.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 2.Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C. Neurovascular regulation in the normal brain and in alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 5.Moore CI, Cao R. The hemo-neural hypothesis: On the role of blood flow in information processing. J Neurophysiol. 2008;99:2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:162–192. [PubMed] [Google Scholar]
- 7.Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 8.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 9.Kalaria RN. Linking cerebrovascular defense mechanisms in brain ageing and Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.10.020. In press. [DOI] [PubMed] [Google Scholar]
- 10.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 11.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 12.Droste DW, Ritter MA, Dittrich R, Heidenreich S, Wichter T, Freund M, Ringelstein EB. Arterial hypertension and ischaemic stroke. Acta Neurol Scand. 2003;107:241–251. doi: 10.1034/j.1600-0404.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblum WI. Fibrinoid necrosis of small brain arteries and arterioles and miliary aneurysms as causes of hypertensive hemorrhage: A critical reappraisal. Acta Neuropathol. doi: 10.1007/s00401-008-0416-9. 2008. [DOI] [PubMed] [Google Scholar]
- 14.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Auerbach ID, Sung SH, Wang Z, Vinters HV. Smooth muscle cells and the pathogenesis of cerebral microvascular disease (“Angiomyopathies”) Exp Mol Pathol. 2003;74:148–159. doi: 10.1016/s0014-4800(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KA, Albert MS. Perfusion abnormalities in prodromal ad. Neurobiol Aging. 2000;21:289–292. doi: 10.1016/s0197-4580(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 18.Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, Cankurtaran ES, Aytemir K, Kabakci G, Ariogul S. Assessment of endothelial function in alzheimer's disease: Is Alzheimer's disease a vascular disease? J Am Geriatr Soc. 2007;55:1613–1617. doi: 10.1111/j.1532-5415.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 19.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM. Reactive oxygen species: Influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth JD. Nox enzymes, ros, and chronic disease: An example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AA, Drummond GR, Schmidt HH, Sobey CG. Nadph oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 23.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overex-pressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008; 105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 29.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]