Abstract
Purpose:
To assess visual and anatomical outcomes following the surgical removal of subfoveal choroidal neovascular membranes (CNVMs) in older patients without clinical evidence of diffuse disease of the retinal pigment epithelium (RPE).
Methods:
We retrospectively reviewed records of consecutive patients aged 50 years or older who underwent surgical removal of subfoveal CNVMs. Patients with clinical evidence for age-related macular degeneration (AMD) (>5 small drusen), angioid streaks, and myopic degeneration were excluded.
Results:
Twenty-two patients (8 women) ranged in age from 50 to 85 years (median, 67 years). All membranes were 100% classic, with a mean size of 2.5 MPS disc areas (range, 1 to 6.5). Best postoperative visual acuity (VA), measured a median 12.5 weeks after surgery, improved (>3 Snellen lines) in 10 eyes (45%) and worsened in 0 eyes, with 8 eyes (36%) achieving 20/50 or better. Over a mean follow-up of 37 months (range, 6 to 114 months), CNVM recurrence was seen in 13 eyes (59%), causing loss of VA from best postoperative levels in 5 eyes (23%). On final follow-up, 4 eyes (18%) retained acuity of 20/50 or better, 12 eyes (55%) had disciform scarring, and 5 eyes (23%) had geographic atrophy. Improvement in best postoperative VA occurred in a higher percentage of eyes with focal (58%) compared with idiopathic (30%) disease, but this trend was not statistically significant (p = 0.23, Fisher’s exact test).
Conclusions:
Surgical removal of subfoveal CNVMs may result in substantial visual improvement in older patients without other clinical evidence for AMD, particularly in eyes with focal diseases of the RPE-Bruch’s membrane complex.
Keywords: choroidal neovascularization, idiopathic choroidal neovascular membrane, presumed ocular histoplasmosis syndrome, submacular surgery
Introduction
Choroidal neovascularization remains a major cause of central vision loss in adults. In the 1980s, laser photocoagulation was the only proven treatment for choroidal neovascular membranes (CNVMs). However, laser photocoagulation of subfoveal CNVMs damages the overlying neurosensory retina, resulting in immediate loss of visual acuity (MPSG 1991). Although immediate loss of visual acuity associated with verteporfin photodynamic therapy (PDT) is uncommon, this treatment is generally disappointing given that few treated eyes experience substantial improvement in visual acuity (TARMDPTSG 1999, 2001; Rosenfeld et al 2004). More recently, nonselective anti-vascular endothelial growth factor (VEGF) therapy with intravitreal ranibizumab and bevacizumab has been demonstrated to result in substantial visual improvement in a significant subset of treated eyes with exudative age-related macular degeneration (AMD) (Rosenfeld et al 2005; Avery et al 2006; Brown et al 2006; Spaide et al 2006). Given these results, anti-VEGF therapy has rapidly become the standard of care for subfoveal CNVM in AMD and other conditions.
In the early 1990s, submacular surgery was pioneered as a treatment with the potential to markedly improve visual acuity in eyes with subfoveal CNVMs (Thomas and Kaplan 1991). Surgical results in various series have confirmed that significant improvements in visual acuity can be attained with submacular surgery in eyes with presumed ocular histoplasmosis syndrome (POHS) and other focal disorders of the retinal pigment epithelium-Bruch’s membrane complex (Berger and Kaplan 1992; Thomas et al 1992, 1994; Berger et al 1997; Holekamp et al 1997; Sears et al 1999; Uemura and Thomas 2000). However, the Submacular Surgery Trials showed that surgical removal of CNVMs did not result in visual benefit compared with laser photocoagulation or natural history in eyes with subfoveal membranes complicating AMD (SSTPSI 2000; SSTRG 2004a). Other series have similarly shown that significant visual improvement following submacular surgery is rare in eyes with AMD (Berger and Kaplan 1992; Thomas et al 1992, 1994; Ormerod et al 1994; Roth et al 1997).
The Submacular Surgery Group H trial evaluated patients with subfoveal CNVMs which were idiopathic or related to POHS. This study did not find a large benefit to surgery compared to observation over a 24-month period for the overall study group. However, patients with preoperative visual acuity worse than 20/100 had the largest benefit. In addition, subgroup analysis found that surgery appeared to be more beneficial for patients older than age 45 years (SSTRG 2004b).
Although anti-VEGF therapy appears very promising for CNVM in all clinical contexts, its use has been studied extensively only in patients with AMD. Furthermore, a substantial percentage of patients treated with effective anti-VEGF agents do not respond with significant improvement in visual acuity. Based on the surprising suggestion from the Submacular Surgery Group H study that increased age may be a positive prognostic factor for submacular surgery in non-AMD eyes, we were interested in evaluating our experience with such patients. The purpose of this study was to assess long-term visual and anatomical outcomes following the surgical removal of CNVMs in older patients without separate clinical evidence of AMD, angioid streaks, or myopic degeneration.
Patients and methods
We retrospectively reviewed records of 31 consecutive patients aged 50 years or older who underwent surgical removal of subfoveal CNVMs by one surgeon (MWJ) between June 1994 and July 2001. Patients with evidence of diffuse retinal pigment epithelium (RPE) disease, including AMD, myopic degeneration, and angioid streaks, were excluded. In this study, the presence in either eye of greater than 5 small drusen or geographic atrophy of the RPE was considered diagnostic of AMD. Of the 31 eyes, 3 eyes with AMD, 2 eyes with myopic degeneration, 1 eye with angioid streaks, and 1 eye with coexisting diabetic macular edema were excluded. Additionally, 2 eyes were excluded because postoperative follow-up was less than 6 months. The study group consisted of 22 eyes of 22 patients. Diagnoses included idiopathic CNVMs (10 eyes), presumed ocular histoplasmosis (7 eyes), juxtapapillary CNVMs (2 eyes), birdshot chorioretinopathy (1 eye), idiopathic central serous chorioretinopathy (1 eye), and multifocal choroiditis (1 eye). No eyes had features suggestive of polypoidal choroidal vasculopathy.
Comprehensive ophthalmic examination including visual acuity, anterior segment biomicroscopy, intraocular pressure, and fundus examination, was performed preoperatively and postoperatively. Visual acuity was obtained in a nonstandardized fashion using Snellen charts with manifest refraction or with spectacle correction and pinhole. Visual acuities worse than 20/400 were classified retrospectively as either 5/200 or 2/200. Fundus photography and fluorescein angiography were performed on all patients. Review of medical records and photographic studies was performed after approval from the Institutional Review Board (IRB) of our institution.
Detailed informed consent was obtained prior to surgical intervention. Pars plana vitrectomy was performed with peeling of the posterior cortical vitreous. A small retinotomy was made with a retinal perforator. Balanced Salt Solution (BSS, Alcon Laboratories, Fort Worth, TX) was used to elevate the retina from the surface of the CNVM. Subretinal forceps were placed through the retinotomy and used to gently separate and remove the neovascular complex. Neither tissue plasminogen activator nor endolaser photocoagulation were employed. Elevation of the infusion pressure was used to achieve hemostasis. A partial (approximately 50%) fluid-air exchange was performed and patients were instructed to maintain face-down positioning for the remainder of the day. Repeat surgical removal was generally offered to patients who had substantial visual recovery following the initial surgery and subsequently developed a recurrent subfoveal CNVM with vision loss. During repeat surgery, previous sclerotomy sites were avoided and the location of the access retinotomy was chosen with no consideration of the intial retinotomy site (which was typically invisible).
Primary outcome measures were best postoperative visual acuity, final visual acuity, and anatomic status of the macula at the final follow-up examination. Other outcomes evaluated included recurrence of neovascularization, time between surgery and recurrence, and the occurrence of surgical complications.
Patients with focal diseases of the RPE-Bruch’s membrane complex were compared with patients with idiopathic disease. The Student’s t-test was used to compare the age of the two groups. The Fisher’s exact test was used to compare the percentage of patients achieving best postoperative visual acuity of 20/50 or better as well as 20/100 or better, and the percentage of patients gaining 3 or more lines of visual acuity. The log-rank test was used to compare the rate of recurrence and the time to recurrence between the two groups.
Selected case reports
Case 4
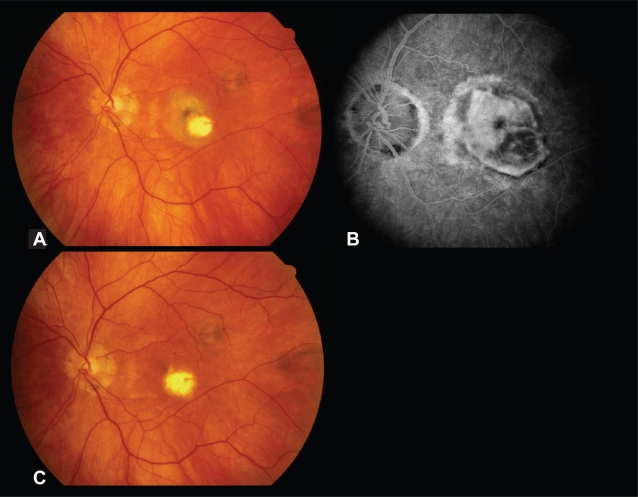
A 59-year-old man with presumed ocular histoplasmosis syndrome had a history of extrafoveal CNVM in the left eye treated twice with laser photocoagulation. Following the second treatment, he developed increased exudation and extension of the membrane into the foveal center (Figure 1A, B), with decline in visual acuity to 20/200. He underwent pars plana vitrectomy with membrane removal and partial fluid-air exchange. The membrane readily separated from the RPE, but was firmly adherent to the laser scar. Following complete removal, the subfoveal RPE appeared to be intact. On final follow-up 36 months postoperatively, the visual acuity in the left eye was 20/20, and clinical examination revealed no signs of recurrent CNVM (Figure 1C).
Figure 1.
Case 4. Preoperative fundus photograph (A) and fluorescein angiogram (B) of the left eye demonstrating recurrent subfoveal choroidal neovascularization following laser photocoagulation of an extrafoveal CNVM. (C) Three years postoperatively, the visual acuity is 20/20.
Case 7
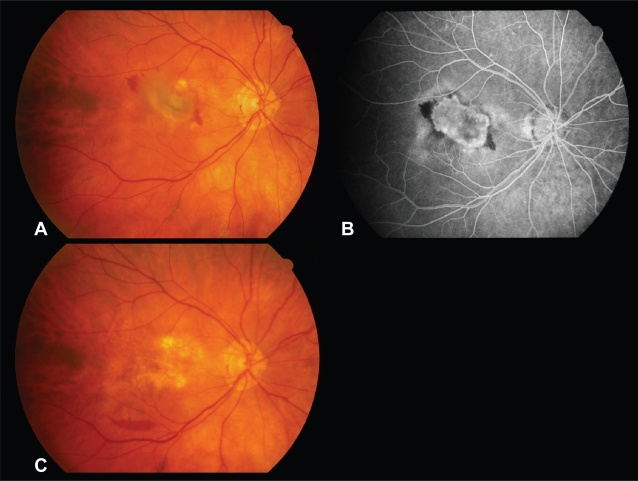
A 74-year-old woman with history of presumed ocular histoplasmosis syndrome developed a large CNVM emanating from an atrophic choroidal scar superior to the fovea in the right eye (Figure 2A, B). Visual acuity declined to 2/200. She underwent uncomplicated pars plana vitrectomy with CNVM removal. One week postoperatively, she had an area of RPE loss in the macular center (Figure 2C). Best postoperative visual acuity was 20/400 and final visual acuity 6.5 months after surgery was 5/200.
Figure 2.
Case 7. Preoperative fundus photograph (A) and fluorescein angiogram (B) of the right eye demonstrating a subfoveal CNVM arising from an atrophic choroidal scar due to POHS. (C) Fundus photograph of the right eye 1 week postoperatively shows absence of the retinal pigment epithelium (RPE) in the macular center.
Case 14
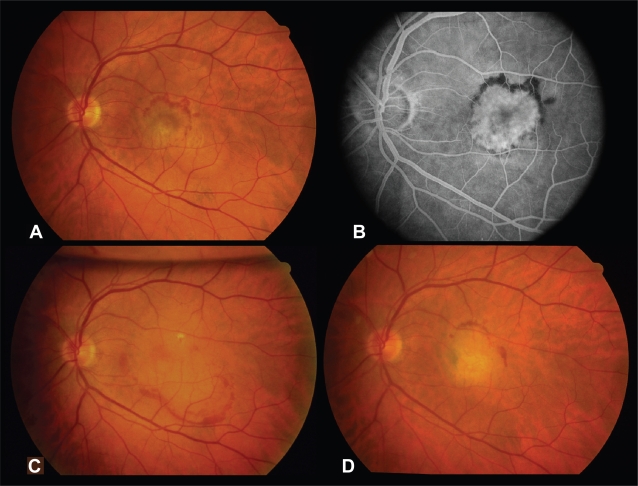
A 65-year-old man with an idiopathic subfoveal CNVM (Figure 3A, B) in the left eye was referred for surgery after he had been followed for 7 months with growth of the CNVM, increased exudation, and decline of best-corrected visual acuity to 20/200. He underwent uncomplicated pars plana vitrectomy with membrane removal. Three days postoperatively, the macular RPE appeared to remain intact (Figure 3C). Visual acuity improved to 20/25 one month following surgery. He developed a recurrent subfoveal CNVM 7 weeks postoperatively, for which he sought external beam irradiation elsewhere (Figure 3D). On final follow-up 114 months postoperatively, there was disciform scarring in the left macula with visual acuity of 20/200.
Figure 3.
Case 14. Preoperative fundus photograph (A) and fluorescein angiogram (B) of the left eye demonstrating an idiopathic subfoveal CNVM. (C) Fundus photograph of the left eye demonstrating intact retinal pigment epithelium 3 days after submacular surgery. (D) Fundus photograph 56 months postoperatively demonstrating disciform scarring after the patient underwent external beam irradiation for a recurrent CNVM.
Results
Twenty-two patients (8 women, 14 men) ranged in age from 50 to 85 years (median, 67 years). Follow-up ranged from 6 to 90 months (mean, 35 months). Seventeen patients (77%) had follow-up greater than 1 year (Table). Six eyes (27%) had a history of laser photocoagulation for a juxtafoveal or extrafoveal CNVM prior to surgery and one (5%) had received previous verteporfin PDT. All CNVMs were subfoveal and 100% classic, with a mean size of 2.5 MPS disc areas (range, 1 to 6.5). Five eyes (23%) were pseudophakic and 17 (77%) eyes were phakic preoperatively.
Table.
Demographic and clinical data
| No | Sex/Age/Eye | Diagnosis | Previous therapy | CNVM Size (MPS DA) | Preoperative VA | Best postoperative VA | Final VA | Follow-up (Mos) | Recurrence (time to recurrence, wks) | Final macular status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/50/OS | POHS | 2 | 20/200 | 20/25 | 20/100 | 45 | Yes (10) | CNVM | |
| 2 | F/56/OS | POHS | 6.5 | 5/200 | 20/200 | 20/200 | 13 | No | RPE
Mottling |
|
| 3 | F/56/OS | POHS | 1.5 | 20/200 | 20/30 | 20/30 | 17 | Yes (1) | RPE
Mottling |
|
| 4 | M/59/OS | POHS | Laser | 3.5 | 20/200 | 20/20 | 20/20 | 36 | No | RPE
Mottling |
| 5 | M/60/OD | POHS | 2.5 | 20/80 | 20/100 | 20/100 | 6 | Yes (4) | Disciform | |
| 6 | F/61/OS | POHS | Laser | 2 | 20/100 | 20/100 | 20/100 | 10.5 | No | Atrophy |
| 7 | F/74/OS | POHS | 4 | 2/200 | 20/400 | 5/200 | 6.5 | No | Atrophy | |
| 8 | F/56/OD | Birdshot | 4 | 20/300 | 20/30 | 20/50 | 90 | Yes (48) | Disciform | |
| 9 | F/61/OD | CSR | 1.5 | 20/300 | 20/200 | 5/200 | 60 | Yes (8) | Disciform | |
| 10 | M/67/OD | MFC | Laser | 1 | 20/200 | 20/40 | 20/400 | 31 | No | Atrophy |
| 11 | M/67/OS | Juxtapapillary | 3 | 20/200 | 20/60 | 20/60 | 17 | No | RPE
Mottling |
|
| 12 | F/85/OD | Juxtapapillary | Laser | 1.5 | 20/200 | 20/300 | 20/400 | 26.5 | Yes (26) | Disciform |
| 13 | M/60/OD | Idiopathic | 1 | 20/200 | 20/300 | 2/200 | 25 | Yes (2.5) | Disciform | |
| 14 | M/65/OS | Idiopathic | 2.5 | 20/200 | 20/25 | 20/200 | 114 | Yes (7) | Disciform | |
| 15 | M/70/OD | Idiopathic | 1 | 20/100 | 20/200 | 20/400 | 76 | Yes (14) | Disciform | |
| 16 | M/73/OD | Idiopathic | Laser | 1.5 | 20/200 | 20/30 | 20/30 | 54 | No | RPE
Mottling |
| 17 | M/74/OS | Idiopathic | PDT (1) | 3 | 5/200 | 20/400 | 5/200 | 24 | No | Atrophy |
| 18 | M/75/OD | Idiopathic | 4 | 20/300 | 20/25 | 20/400 | 28.5 | Yes (36) | Disciform | |
| 19 | M/76/OS | Idiopathic | 2 | 5/200 | 20/400 | 20/400 | 7.5 | Yes (1.5) | CNVM | |
| 20 | F/78/OD | Idiopathic | 3 | 2/200 | 20/400 | 20/400 | 69 | No | Atrophy | |
| 21 | M/83/OD | Idiopathic | 1.5 | 2/200 | 5/200 | 2/200 | 6.5 | Yes (12) | Disciform | |
| 22 | M/83/OD | Idiopathic | Laser | 3 | 20/300 | 20/100 | 20/200 | 56.5 | Yes (16) | Disciform |
Abbreviations: VA, visual acuity; POHS, presumed ocular histoplasmosis; CSR, central serous retinopathy; MFC, multifocal choroiditis; MPS DA, size of standard optic disc (1.77 mm2) adopted by the Macular Photocoagulation Study Group; PDT, photodynamic therapy; CNVM, choroidal neovascular membrane; RPE, retinal pigment epithelium.
Preoperative visual acuity ranged from 20/80 to 2/200 (median, 20/200). Best postoperative visual acuity was measured a median 12.5 weeks after surgery (range, 1 day to 69 months) and ranged from 20/20 to 5/200 (median, 20/100). When compared with preoperative visual acuity, the best postoperative visual acuity improved by 3 or more Snellen lines in 10 eyes (45%) and worsened in 0 eyes, with 8 eyes (36%) achieving 20/50 or better.
Over a mean follow-up of 37 months, CNVM recurrence was seen in 13 eyes (59%), causing loss of visual acuity (3 or more lines) from best postoperative levels in 5 eyes (23%). Mean time to recurrence was 14 weeks after surgery, ranging from 1 to 48 weeks. Management of recurrences consisted of observation in 7 eyes, laser photocoagulation in 3 eyes, repeat surgical removal in 2 eyes, and radiation teletherapy in 1 eye. Of note was Patient #1 who had 2 recurrences, each treated with repeat pars plana vitrectomy and removal of the CNVM. Visual acuity returned to 20/25 following the third surgery. A subsequent recurrence in the same eye was treated with photodynamic therapy, with final visual acuity of 20/100.
Operative complications included peripheral retinal breaks in 5 eyes (23%), limited suprachoroidal hemorrhage in 1 eye (5%), intraoperative foveal break in 1 eye (5%), and postoperative vitreous hemorrhage in 1 eye (5%). There were no cases of retinal detachment or endophthalmitis in this series. Of the 17 phakic eyes in this study, 15 (88%) had progression of nuclear sclerosis and 4 (24%) underwent cataract surgery during the follow-up period.
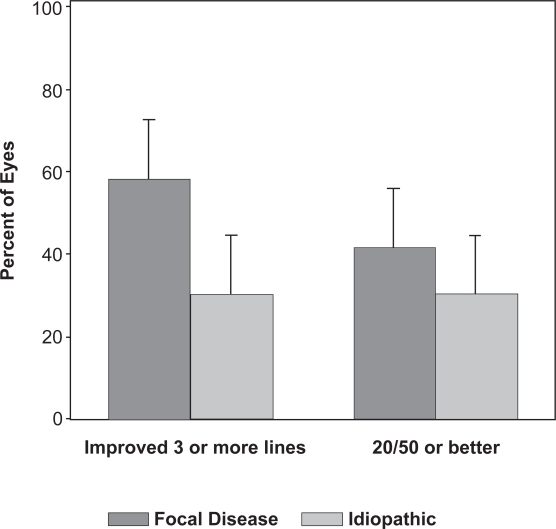
Patients with focal disease were significantly younger than patients with idiopathic disease (mean age 63 versus 74 years, p = 0.007, Student’s t-test). Improvement in best postoperative visual acuity of 3 or more lines occurred in a higher percentage of eyes with focal (7/12, 58%) compared with idiopathic (3/10, 30%) disease, but this difference was not statistically significant, presumably due to small sample size (p = 0.23, Fisher’s exact test) (Figure 4). Likewise, an insignificantly higher percentage of eyes with focal (5/12, 42%) compared with idiopathic disease (3/10, 30%) achieved a best postoperative visual acuity of 20/50 or better. There was no significant difference in CNVM recurrence rate between the focal (50%) and idiopathic (70%) groups (p = 0.75, log-rank test).
Figure 4.
Best postoperative visual acuity in eyes with focal diseases of the RPE compared to eyes with idiopathic CNVM (p = 0.23). Error bars represent 1 standard error.
Discussion
Our results demonstrate that surgical removal of subfoveal CNVMs may result in significant visual improvement in older patients without clinical evidence of diffuse RPE disease such as AMD. In our series, best postoperative visual acuity improved substantially in 45% of eyes, with 36% achieving reading visual acuity. Although our study was not controlled, the outcomes compare favorably to previously reported series in younger patients (Berger and Kaplan 1992; Thomas et al 1992, 1994; Berger et al 1997; Holekamp et al 1997; Sears et al 1999; Uemura and Thomas 2000). This observation suggests that taken alone, senescence of RPE cells and Bruch’s membrane does not preclude the recovery of photoreceptor function after the surgical removal of a subfoveal CNVM. Previous reports suggest that patients with AMD do not experience substantial visual improvement following surgical removal of CNVMs, with 0%–9% achieving reading visual acuity at any postoperative time point (Thomas et al 1992, 1994; Ormerod et al 1994; Roth et al 1997). A pilot study for the Submacular Surgery Trials reported results in patients with post-laser recurrent CNVMs secondary to AMD. Of the 34 surgically treated eyes, only one achieved best postoperative visual acuity of 20/40 or better. At the 24-month follow-up, none of the patients maintained reading visual acuity (SSTPSI 2000). Similarly, of the 220 surgically treated eyes in the Submacular Surgery Group N trial, only one achieved a best postoperative visual acuity of 20/40 or better (SSTRG 2004a).
In contrast to patients with AMD, significant improvements in visual acuity can be attained with submacular surgery in POHS and other focal disorders of the RPE-Bruch’s membrane complex (Berger and Kaplan 1992; Thomas et al 1992, 1994; Berger et al 1997). Various factors may contribute to the difference in outcomes between AMD and focal disorders of the RPE, including underlying disease process, growth pattern of the neovascular complex, and patient age. CNVMs in focal diseases arise from focal dehiscences in an otherwise healthy RPE-Bruch’s membrane complex. In contrast, neovascular AMD develops in the setting of widespread dysfunction of the RPE-Bruch’s membrane complex. Histological studies (Gass 1994) have shown that CNVMs in these two disease categories differ with respect to their location relative to the RPE. In AMD, CNVMs are typically located beneath and inseparable from the RPE layer, which results in extensive removal of RPE during surgical extraction of the CNVM. Loss of RPE leads to postoperative atrophy of the choriocapillaris and photoreceptors, severely compromising visual outcomes. In POHS and other focal inflammatory conditions, CNVMs are located anterior to and separable from the RPE and may therefore be removed without significant alteration of the underlying RPE layer.
Animal models have been used to examine the consequences of RPE removal. Valentino and co-authors (Valentino et al 1995) and Del Priore and co-authors (1995) studied RPE repopulation after submacular surgery. These investigators found that the treated (denuded) area can be repopulated by RPE cell migration and proliferation as early as 1 week after surgery. Regrowth of the RPE was only observed when Bruch’s membrane was intact. Del Priore and co-authors (1996) used mitomycin-C for pharmacologic inhibition of RPE healing followed by RPE debridement in porcine eyes. They found that absence of the RPE led to atrophy of the choriocapillaris by 1 week after surgery. These findings may be important in explaining the poor surgical outcomes in eyes with AMD compared with eyes with focal RPE diseases. In eyes with AMD, larger amounts of the RPE may be removed during submacular surgery, senescent and diseased RPE may have less ability to regenerate, and the RPE may be unable to repopulate diseased Bruch’s membrane. In addition, choroidal hypoperfusion may further impair the physiologic mechanisms required for visual recovery (Thach et al 1996).
Experimental evidence suggests that age is an independent risk factor for severity of CNVM in a mouse model. Espinosa-Heidmann and co-authors (2002) found that older age was associated with CNVMs with larger surface area, greater vascularity and greater cellularity. However, the older age of our patients did not appear to have a significant adverse impact on visual and anatomic outcomes, as these outcomes were similar to those of other studies in patients with POHS and other focal RPE diseases (Berger and Kaplan 1992; Thomas et al 1992, 1994; Berger et al 1997; Holekamp et al 1997; Sears et al 1999; Uemura and Thomas 2000).
Other published series report a gain of 3 or more lines in 34%–40% of surgically treated eyes, with 16%–35% achieving reading visual acuity (Berger and Kaplan 1992; Thomas et al 1992, 1994; Berger et al 1997; Holekamp et al 1997). Similar results have also been reported in pediatric patients, with 25%–43% achieving reading visual acuity (Sears et al 1999; Uemura and Thomas 2000). In the Submacular Surgery Group H trial (SSTRG 2004b), 18% of surgically treated patients versus 13% of patients in the observation group had visual acuity better than 20/40 at 36 months. Interestingly, older age (greater than 45 years) was found to be a potential positive predictor of visual acuity outcome on subgroup analysis.
In our series, there was a strong trend toward better outcomes in eyes with focal disorders of the RPE-Bruch’s membrane complex, compared to those with idiopathic disease. Among patients with focal diseases, best postoperative visual acuity improved by 3 or more Snellen lines in 58% compared with 30% of eyes with idiopathic disease. In part because of the high recurrence rate (70%), final outcomes in eyes with idiopathic disease were disappointing, with only 10% maintaining reading vision on final follow-up. One explanation for this outcome is that idiopathic CNVMs may represent a sub-clinical form of AMD, where changes in the RPE-Bruch’s membrane complex are not yet clinically detectable. However, the initial postoperative recovery of reading vision in 30% of patients with idiopathic CNVMs suggests that submacular surgery may still have a role in this group of patients.
Subfoveal choroidal neovascularization typically has devastating effects on the central vision. Although anti-VEGF agents appear promising for the treatment of CNVMs, a large percentage of treated patients do not experience a significant improvement in vision. The possibility of postoperative recovery of reading vision in a significant percentage of eyes suggests that submacular surgery should still be considered in select patients. We believe that submacular surgery is a reasonable treatment alternative for patients with subfoveal CNVMs due to focal disorders whose visual acuity is 20/100 or worse despite treatment with anti-VEGF agents.
Most of the patients in our series were managed before PDT and anti-angiogenic therapies were available. In the future, treatment of subfoveal CNVMs associated with idiopathic and focal disease will likely involve a multifaceted approach, combining pharmacologic therapies with surgery in select cases. We are hopeful that the use of effective anti-angiogenic drugs for postoperative recurrent CNVMs will increase both the magnitude and stability of the visual improvement associated with submacular surgery.
Footnotes
Disclosure
Presented in part at the Macula Society meeting, Naples, Florida, March 1, 2003. None of the authors have proprietary interest in the subject of this report.
References
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Berger AS, Conway M, Del Priore LV, et al. Submacular surgery for subfoveal choroidal neovascular membranes in patients with presumed ocular histoplasmosis. Arch Ophthalmol. 1997;115:991–6. doi: 10.1001/archopht.1997.01100160161004. [DOI] [PubMed] [Google Scholar]
- Berger AS, Kaplan HJ. Clinical experience with the surgical removal of subfoveal neovascular membranes. Ophthalmology. 1992;99:969–76. doi: 10.1016/s0161-6420(92)31869-x. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Hornbeck R, Kaplan HJ, et al. Debridement of the pig retinal pigment epithelium in vivo. Arch Ophthalmol. 1995;113:939–44. doi: 10.1001/archopht.1995.01100070113034. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Kaplan HJ, Hornbeck R, et al. Retinal pigment epithelial debridement as a model for the pathogenesis and treatment of macular degeneration. Am J Ophthalmol. 1996;122:629–43. doi: 10.1016/s0002-9394(14)70481-7. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner I, Hernandez EP, et al. Age as an independent risk factor for severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1567–73. [PubMed] [Google Scholar]
- Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118:285–98. [PubMed] [Google Scholar]
- Holekamp NM, Thomas MA, Dickinson JD, et al. Surgical removal of subfoveal choroidal neovascularization in presumed ocular histoplasmosis. Ophthalmology. 1997;104:22–6. doi: 10.1016/s0161-6420(97)30367-4. [DOI] [PubMed] [Google Scholar]
- [MPSG] Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–31. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, Puklin JE, Frank RN. Long-term outcomes after the surgical removal of advanced subfoveal neovascular membranes in age-related macular degeneration. Ophthalmology. 1994;101:1201–10. doi: 10.1016/s0161-6420(94)31200-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Saperstein DA, Bressler NM, et al. Photodynamic therapy with verteporfin in ocular histoplasmosis: uncontrolled, open-label 2-year study. Ophthalmology. 2004;111:1725–33. doi: 10.1016/j.ophtha.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- Roth DB, Downie AA, Charles ST. Visual results after submacular surgery for neovascularization in age-related macular degeneration. Ophthalmic Surg Lasers. 1997;28:920–5. [PubMed] [Google Scholar]
- Sears J, Capone A, Aaberg T, et al. Surgical management of subfoveal neovascularization in children. Ophthalmology. 1999;106:920–24. doi: 10.1016/S0161-6420(99)00510-2. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- [SSTPSI] Submacular Surgery Trials Pilot Study Investigators Sub-macular Surgery Trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration: I. Ophthalmic outcomes: Sub-macular Surgery Trials Pilot Study report number 1. Am J Ophthalmol. 2000;130:387–407. doi: 10.1016/s0002-9394(00)00729-7. [DOI] [PubMed] [Google Scholar]
- [SSTRG] Submacular Surgery Trials Research Group Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: Ophthalmic findings: SST report no. 11. Ophthalmology. 2004a;111:1967–80. doi: 10.1016/j.ophtha.2004.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [SSTRG] Submacular Surgery Trials Research Group Surgical removal vs observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic, I. Ophthalmic findings from a randomized clinical trial: Submacular Surgery Trials (SST) Group H Trial: SST report no. 9. Arch Ophthalmol. 2004b;122:1597–611. doi: 10.1001/archopht.122.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach AB, Marx JL, Frambach DA, et al. Choroidal hypoperfusion after surgical excision of subfoveal neovascular membranes in age-related macular degeneration. International Ophthalmology. 1996;20:205–13. doi: 10.1007/BF00175261. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Dickenson JD, Melberg NS, et al. Visual results after surgical removal of subfoveal choroidal neovascular membranes. Ophthalmology. 1994;101:1384–96. doi: 10.1016/s0161-6420(94)31172-9. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Grand MG, Williams DF, et al. Surgical management of subfoveal choroidal neovascularization. Ophthalmology. 1992;99:952–66. doi: 10.1016/s0161-6420(92)31888-3. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Kaplan HJ. Surgical removal of subfoveal neovascularization in the presumed ocular histoplasmosis syndrome. Am J Ophthalmol. 1991;111:1–7. doi: 10.1016/s0002-9394(14)76888-6. [DOI] [PubMed] [Google Scholar]
- [TARMDPTSG] Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age–related macular degeneration with verteporfin. One-year results of 2 randomized clinical trials-TAP report 1. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- [TARMDPTSG] Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- Uemura A, Thomas MA. Visual outcome after surgical removal of choroidal neovascularization in pediatric patients. Arch Ophthalmol. 2000;118:1373–78. doi: 10.1001/archopht.118.10.1373. [DOI] [PubMed] [Google Scholar]
- Valentino TL, Kaplan HJ, Del Priore LV, et al. Retinal pigment epithelial repopulation in monkeys after submacular surgery. Arch Ophthalmol. 1995;113:932–38. doi: 10.1001/archopht.1995.01100070106033. [DOI] [PubMed] [Google Scholar]