Abstract
Purpose:
To report the short term anatomical and visual acuity response after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy.
Methods:
Ten eyes having proliferative diabetic retinopathy were treated with at least one intravitreal injection of bevacizumab, 1.25 mg in 0.05 mL. Patients underwent complete ophthalmologic evaluation at baseline and follow up visits including Snellen’s best corrected visual acuity (BCVA), fundus biomicroscopy and fluorescein angiography. Vitreous hemorrhage precluding laser treatment was present in 6 eyes.
Results:
Follow up ranged between 3 and 6 months (mean 4.2 ± 1.23 months). Improvement of vision was observed after one week with BCVA improved to 0.47 ± 0.26 compared with baseline BCVA of 0.365 ± 0.26 (P < 0.05). At last follow up, BCVA was 0.65 ± 0.33. Seven eyes had better visual acuity. Vitreous hemorrhage cleared completely in 4 eyes while 2 eyes had residual vitreous hemorrhage. Regression of retinal neovascularization was complete in 7 eyes, incomplete in 3 eyes. Regression was observed as early as 2 weeks after first injection. Reperfusion after regression was observed in 2 eyes. Patients received a mean of 1.7 ± 0.67 injections per eye. No complications were noted in all eyes. Pancryopexy was done in 1 eye and additional laser was done in 2 eyes.
Conclusion:
Intravitreal bevacizumab was safe and effective in treatment of proliferative diabetic retinopathy. It can induce effective regression of retinal neovascularization and rapid clearance of vitreous hemorrhage. It can be used as an adjunctive therapy with laser photocoagulation and to enhance absorption of vitreous hemorrhage with subsequent deferral from vitrectomy.
Summary:
Ten eyes having proliferative diabetic retinopathy were treated with intravitreal bevacizumab. Following injection, evident regression of retinal neovascularization and rapid clearance of vitreous hemorrhage as well as vision improvement was observed.
Keywords: intravitreal bevacizumab, proliferative diabetic retinopathy, retinal neovascularization, vascular endothelial growth factor, vitreous hemorrhage
Introduction
Diabetic retinopathy remains the leading cause of new blindness in young adults. Without treatment, 50% of those with proliferative disease will be blind within 5 years (Chew 1999). Hyperglycemia induces retinal hypoxia that up regulates a range of vasoactive factors which may lead to macular edema and/or angiogenesis and hence potentially sight threatening retinopathy (Malik et al 2005). A generally accepted explanation of the fibro-vascular proliferations found in proliferative diabetic retinopathy (PDR) is that widespread retinal ischemia leads to release of vasoproliferative factors such as vascular endothelial growth factor (VEGF) which diffuses through out the vitreous as well as the anterior segment inducing neovascularization (Wilkinson-Berka 2004). Vitreous levels of VEGF were elevated by approximately 3-fold in patients with advanced proliferative retinopathy (Malik et al 2005). Intraocular injection of VEGF causes micro vascular abnormalities and retinal ischemia (Tolentino et al 1996, 2002) Pan retinal laser photocoagulation (PRP) represents the main stay in the treatment of proliferative diabetic disease (DRSRG 1976, 1981). However, the laser therapy might be hindered by vitreous hemorrhage and or early cataract. Vitreous hemorrhage may be encountered in eyes having maximum laser therapy.
Intravitreal bevacizumab (Avastin, Genentech Inc., San Francisco, CA), a full length humanized monoclonal antibody to VEGF which was approved by the Food and Drug Administration for the treatment of colorectal cancer (Manzano et al 2006). It can inhibit both types of VEGF receptors: VEGFR-1 and VEGFR-2 (Manzano et al 2006). It has been recently shown to enhance the clearance of vitreous hemorrhage and induce involution of retinal neovascularization with no reported complications (Avery 2006; Davidorf et al 2006; Spaide and Fisher 2006). It has been also tried in the treatment of macular edema due to central retinal vein occlusion (Iturralde et al 2006).
In this series of cases, intravitreal bevacizumab was offered aiming at enhancing the absorption of vitreous hemorrhage with possible deferral from vitrectomy and possible laser addition and inducing regression of retinal neovascularization persistent after having maximum PRP with the aim of reducing the morbidity of PDR. This report describes the short term results that a series of eyes with PDR had to intravitreal bevacizumab.
Methods
This work was done in Magrabi Eye Hospital in Muscat, Sultanate of Oman, after approval from the ethical committee and a written informed consent from the patients. The study included 10 eyes with proliferative diabetic retinopathy which were treated with at least one intravitreal injection of bevacizumab (Avastin, Genentech); 1.25 mg in 0.05 mL. Indications for Avastin injection were:
Diabetic vitreous hemorrhage precluding laser treatment persistent for at least 3 months (6 eyes).
Failure to induce complete regression of retinal neovascularization after performing complete PRP with no apparent untreated retinal areas (4 eyes).
Cases with significant tractional retinal detachment were not included in the study.
Patients were exposed to complete ophthalmologic evaluation at base line and follow up visits including Snellen’s best corrected visual acuity, fundus biomicroscopy and fluorescein angiography. After informed consent, 1.25 mg in 0.05 mL bevacizumab was injected intravitreally under complete sterile preparation in the operating theater in the usual sterile fashion (Aiello et al 2004) using topical anesthesia. Prophylactic topical antibiotic (gatifloxacine) was given for 2 days post-injection. Patients were followed up after one week, one month, and then monthly thereafter. Reinjection was considered after at least one month of the first injection on a case by case basis. Laser treatment or cryopexy for anterior retina was given after clearance of the hemorrhage on individual basis. The main outcome measurements were visual acuity improvement, clearance of vitreous hemorrhage, regression of neovascularization on fluorescein angiography, and complications related to injection or to the drug.
Statistical analysis
Values were expressed as mean ± standard deviation. Paired samples t test was used to compare visual acuity changes. P < 0.05 was considered statistically significant.
Results
Follow up period ranged between 3 and 6 months with mean follow up of 4.2 ± 1.23 months.
Baseline characteristics
A total of 10 eyes from 8 patients were reviewed. The patients included 5 males and 3 females with an average age 45.3 ± 13 years (range between 23 and 59 years). Four patients had type 1 diabetes and 4 had type 2 diabetes. All eyes had previous PRP; which was complete in 7 eyes and insufficient in 3 eyes. Vitreous hemorrhage precluding laser treatment was present in 6 eyes while in the other 4 eyes, maximum laser therapy was not able to induce regression of retinal neovascularization. One eye had clinically significant macular edema. Two eyes were pseudophakic. Mean baseline best corrected visual acuity (BCVA) was 0.365 ± 0.26.
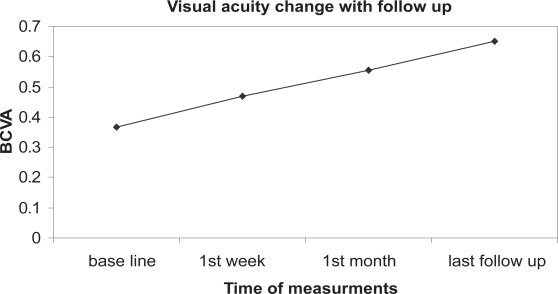
Improvement of vision (Figure 1) was observed after one week with mean BCVA improved to 0.47 ± 0.26, a difference from baseline that was significant (P < 0.05). Of the 10 eyes, six eyes improved 2 lines or more, 1 eye acquired 1 line while 3 eyes kept the same baseline vision. Only in 1 eye, visual improvement was ascribed to improvement of macular edema. The visual improvement was maintained till the end of follow up. After one month, mean BCVA was 0.555 ± 0.32. At last follow up, Mean BCVA was 0.65 ± 0.33. The improvement of vision was statistically significant at all post injection evaluation points in comparison to the baseline (P < 0.05).
Figure 1.
shows improvement of vision following bevacizumab injection which is maintained up to the end of follow up.
Out of 6 eyes having vitreous hemorrhage, 4 eyes had complete clearance of hemorrhage between 4 and 8 weeks, while 2 eyes had incomplete clearance with residual vitreous hemorrhage (Figures 2 and 3). Significant reduction of leakage was observed in angiography as early as 2 weeks after injection. Regression of retinal neovascularization was complete in 7 eyes (70%) within 4 to 8 weeks using 1 to 2 injections (Figures 2 and 3). Three eyes had significant, however, incomplete regression of neovascularization. Reperfusion after regression was observed in 2 eyes, 3 and 4 months from the first injection (Figures 3 and 4). Re-bleeding occurred in one eye in association with reperfusion. Reperfusion regressed 2 weeks after re-injection in both cases. Six eyes (60%) required more than one injection. Frequency of injections per eye ranged between 1 to 3 injections with a mean value of 1.7 ± 0.67. Reasons for repeated injection were reperfusion in one case, reperfusion plus re-vitreous hemorrhage in one case and incomplete regression of vitreous hemorrhage and/or neovascularization in 4 cases. A total of 17 bevacizumab injections were performed in the whole work at the end of follow up period. No ocular or systemic adverse events were noted during the follow up period including endophthalmitis, clinically evident inflammation, rise of intraocular pressure, rhegmatogenuos retinal detachment and systemic thrombo-embolic events. No progression to tractional detachment was observed. Pancryopexy to the anterior retina was done in one eye (previously had maximum PRP) and additional laser was done in 2 eyes. Additional procedures were all performed after 3 months following the first injection.
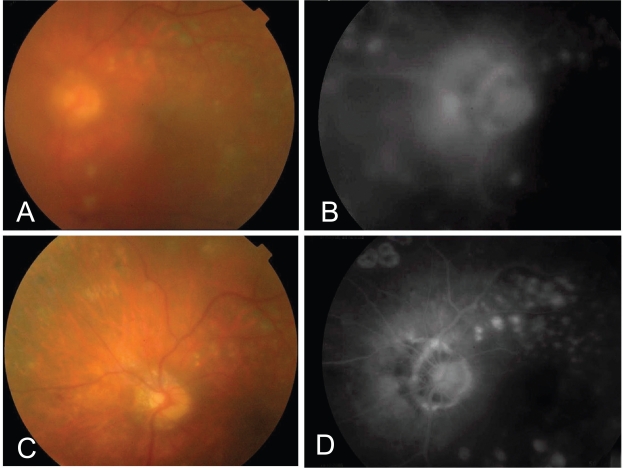
Figure 2.
This 53 years old patient presented with dense vitreous hemorrhage obscuring fundus details 4 months after having intensive laser therapy. One month after first bevacizumab injection, (A) Fundus details could be partially seen due to starting absorption of the blood and (B) the angiogram showed NVD. C, One month after second bevacizumab injection, the blood was completely absorbed and the angiogram (D) showed complete regression of the NVD.
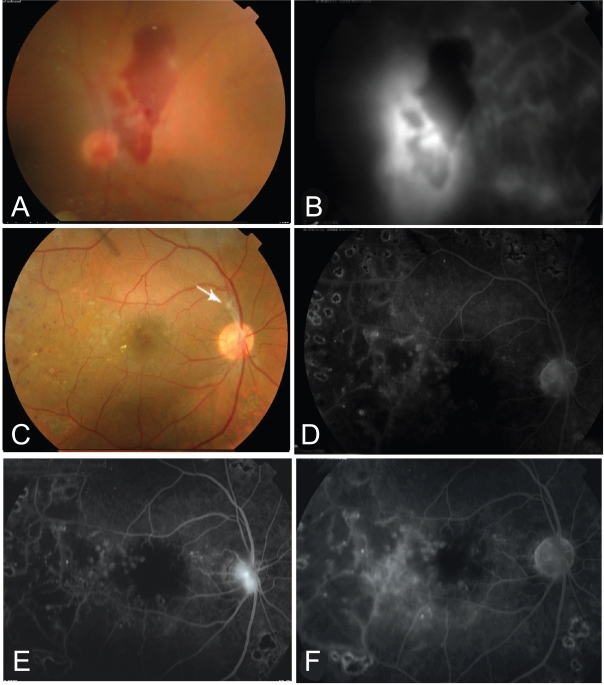
Figure 3.
A 40 years old patient with PDR and vitreous hemorrhage (A). B, The fluorescein angiogram showed evident neovascularization at the disc (NVD) with evident nasal ischemia. C, One month following bevacizumab injection there was near complete absorption of vitreous hemorrhage with a fibrous tuft arising from the optic disk (arrow). The macula looks dry with marks of previous grid. The angiogram (D) showed near complete regression of the neovascularization. E, three months following single injection, the angiogram showed evidence of reperfusion of NVD. F, 2 weeks following re-injection, the angiogram showed again complete regression of the NVD.
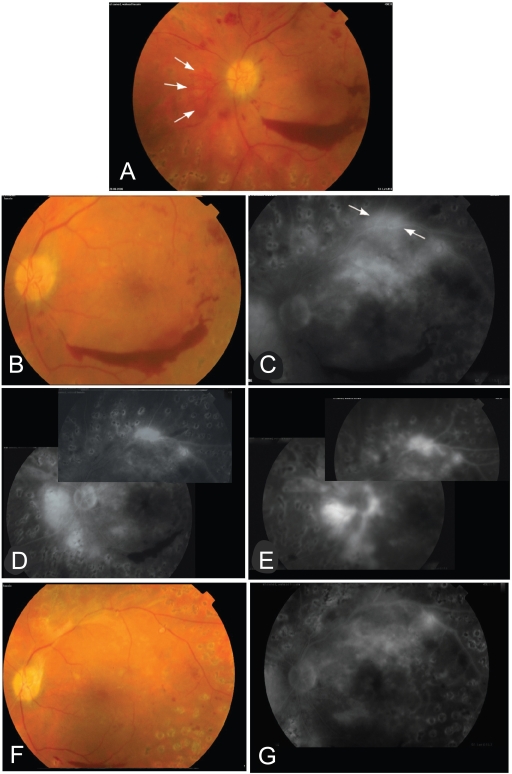
Figure 4.
This 43 years old patient presented with mild vitreous hemorrhage and preretinal hemorrhage 3 months after complete PRP. A, showed NVDs on the disk and a tuft of neovessels nasal to the disk (arrows). NVE on the upper arcade are not shown in the color photo. He had visual acuity of 0.6. One month after first bevacizumab injection, (B) showed better visualization of the retinal details and regression of NVDs on the disk and (C) the angiogram showed no NVD on the disk with residual leakage from NVE on the upper arcade (arrows) and NVD nasal to the disk. There is upper macular intraretinal leakage (edema).The central macula however was dry. He was given a second injection. Six weeks after 2nd injection, D, angiogram showed residual activity of NVE on the upper arcade and nasal to the disk but no vessels on the disk. After 4 months from 1st injection, re-vitreous hemorrhage was observed. E, angiogram showed reperfusion of NVDs on and nasal to the disk and NVE on the upper arcade. A 3rd injection was given. F&G,4 weeks after injection, angiogram showed disappearance of NVD and NVE. Final visual acuity was 0.9. Cryopexy to the anterior retina was done to stabilize the final outcome.
Discussion
Retinal neovascularization and macular edema are central features of diabetic retinopathy, a major cause of blindness in working age adults. The currently established treatment for diabetic retinopathy targets the vascular pathology by laser photocoagulation (Caldwell et al 2005). According to diabetic retinopathy study (DRSRG 1976, 1981), PRP reduced the risk of severe vision loss by more than 50%. This approach is associated with significant adverse effects due the destruction of neural tissue and is not always effective.
Characterization of the molecular and cellular processes involved in vascular growth and hyper permeability has led to the recognition that the angiogenic growth factor and vascular permeability factor VEGF plays a pivotal role in the retinal microvascular complications of diabetes. Thus, VEGF represents an important target for therapeutic intervention in diabetic retinopathy. Agents that directly inhibit the actions of VEGF and its receptors show considerable promise (Caldwell et al 2005). Pegaptanib (Macugen) has been reported to cause involution of retinal neovascularization (MDRSG 2006).
In the current series, bevacizumab intravitreal injection was followed by rapid improvement of vision as early as one week following injection. This reflects rapid clearance of vitreous hemorrhage. It reflects improvement of macular edema only in one eye not associated with vitreous hemorrhage. Vitreous hemorrhage cleared either completely or significantly resulted in improvement of vision which lasted up to the end of follow up. Clearance of media opacity precluding PRP has also made additional laser possible. Vitrectomy, the therapeutic modality in cases with persistent vitreous bleeding, could be avoided. Regression of retinal neovascularization was observed as early as 2 weeks. Avery (2006) reported regression in 1 case 1 week following intravitreal bevacizumab. Complete regression in this work was achieved in 70% of eyes. Avery and colleagues (2006) reported complete resolution of retinal neovascularization in 73% of cases. Spaide and Fisher (2006) reported on 2 cases with improvement of vision as early as 1 week after bevacizumab injection. Spaide and Fisher (2006) suggested a mechanism to explain this rapid clearance of vitreous hemorrhage. They suggested that persistent diabetic bleeding is due to repeated attacks of bleeding as the vitreous hemorrhage keeps its red coloration all the time, so involution of neo-vessels results in stoppage of new attacks of bleeding while the absorption process clears the preexisting blood. This significant absorption of blood within one week is relatively rapid. The author believes that bevacizumab might have an enhancing effect on the absorption of blood but the mechanism is not clear.
Reperfusion was observed in 2 cases in the current series 3 and 4 months after first injection. Avery and colleagues (2006) reported leakage recurrence as early as 2 weeks and as late as 11 weeks. Spaide and Fisher (2006) reported reperfusion in one case 3 months after injection which responded to re-injection. In the current series, reperfusion regressed 2 weeks after re-injection. Reperfusion means that the effect obtained by bevacizumab might be short term and explains the need for repeated injection and or additional procedures (laser or cryopexy) to stabilize bevacizumab induced regression. It has been reported that bevacizumab has half life time of 4.32 days in the vitreous cavity of phakic rabbits and expected to last in the vitreous cavity approximately for 22 days (Bakri et al 2006). A single dose was found to provide complete VEGF blockade for a minimum of 4 weeks with an intravitreal half life of unbound bevacizumab between 2 and 6 days (Beer et al 2006). Reperfusion is because bevacizumab blocks the VEGF already secreted in the vitreous cavity but further secretion from the ischemic retina will continue. It has been reported that a high VEGF level was maintained in the vitreous cavity even after vitrectomy for PDR (Itakura et al 2004). The author believes that several factors play a role in the persistence of bevacizumab induced regression which include the severity of retinal ischemia, the activity of PDR and the extent of previous PRP. This therapeutic effect, despite being short term, seems effective in changing the sequence of events in cases of diabetic vitreous hemorrhage leading to rapid functional improvement with subsequent deferral from vitrectomy. In this series, no progression of tractional element of the fibrovascular tissue nor progression of tractional detachment was observed. Spaide and Fisher (2006) reported localized tractional retinal detachment in one case which did not change one month after bevacizumab injection. However, Arevalo and colleagues (2006) reported on 1 eye out of 25 eyes treated with bevacizumab in which PDR progressed to tractional retinal detachment requiring vitrectomy. This means that there is a risk of progression of tractional detachment in some cases which requires close follow up after bevacizumab injection. Intravitreal bevacizumab showed positive biological effect in treatment of PDR with no safety concerns at least for the short term but long term safety and efficacy could not be predicted according to the results of this study being non randomized, uncontrolled and retrospective with relatively short term follow up. Further research is warranted based on the accumulating reports supporting similar findings.
Intravitreal bevacizumab was effective in treatment of proliferative diabetic retinopathy without safety concerns. It can induce effective regression of retinal neovascularization and rapid clearance of vitreous hemorrhage. It can be used as an adjunctive therapy with laser photocoagulation and to enhance absorption of vitreous hemorrhage with subsequent deferral from vitrectomy.
Footnotes
Disclosure
This work has been presented as paper presentation in the EGVRS (Egyptian VitreoRetinal Society) International Conference in Luxor, Egypt from February 28th to March 2nd 2006, and in the MEACO 2007 conference in Dubai, United Arab Emirates (UAE) from March 29th to April 1st, 2007. The author has no proprietary interest in this study. This work has been done in Magrabi Eye and Ear Hospital, Muscat, Sultanate of Oman.
References
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreal injections. Retina. 2004;24:13–19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Wu L, Sanchez JG, et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy. Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 213. [Google Scholar]
- Avery RL. Regression of retina and iris neovascularization after Intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–3. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Castellarine AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 139. [Google Scholar]
- Bakri SJ, Snyder M, Pulido JS, et al. Pharmacokinetics of Intravitreal bevacizumab (Avastin). Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 77. [Google Scholar]
- Beer PM, Wong S, Falk NS, et al. Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two human subjects. Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 77. [Google Scholar]
- Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–24. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- Chew EY. Major clinical trials of vitreoretinal diseases. In: Regillo CD, Brown GC, Flyn HW, editors. Vitreoretinal disease, the essentials. New York: Theme Medical Publishers Inc; 1999. pp. 667–77. [Google Scholar]
- Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- Itakura H, Kishi S, Kotajima N, et al. Persistent secretion of vascular endothelial growth factor into the vitreous cavity in proliferative diabetic retinopathy after vitrectomy. Ophthalmology. 2004;111:1880–84. doi: 10.1016/j.ophtha.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment for macular edema in central retinal vein occlusion: A short term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- [MDRSG] Macugen Diabetic Retinopathy Study Group Changes in retinal neovascularization following Pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113:23–8. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Malik RA, Li C, Aziz W, et al. Elevated plasma CD105 and vitreous VEGF levels in diabetic retinopathy. J Cell Mol Med. 2005;9:692–7. doi: 10.1111/j.1582-4934.2005.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano RPA, Peyman GA, Khan P, et al. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–61. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- [DRSRG] The Diabetic Retinopathy Study Research Group Preliminary reports on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–96. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- [DRSRG] The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: Clinical applications of diabetic retinopathy study (DRS) findings, DRS report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreal injection of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–8. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, McLeod DS, Toamoto M, et al. Pathological features of vascular endothelial growth factor-induced retinopathy in nonhuman primate. Am J Ophthalmol. 2002;133:373–85. doi: 10.1016/s0002-9394(01)01381-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: Vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Des. 2004;10:3331–48. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]