Abstract
Background:
To compare nepafenac 0.1% with placebo and ketorolac 0.5% for prevention and treatment of ocular pain and inflammation after cataract surgery.
Methods:
In a multi-center, randomized, placebo- and active-controlled, double-masked clinical trial, 227 patients with cataract were randomized to receive nepafenac 0.1%, ketorolac 0.5%, or placebo TID beginning 1 day pre-operatively and continuing for 21 days postoperatively. At each postoperative visit, cure rates and clinical success rates (≤5 aqueous cells and no flare) were calculated, and investigators evaluated patients’ pain. On Day 7, patients judged ocular comfort after study drug instillation.
Results:
Nepafenac 0.1% produced significantly more cures compared to placebo at Day 14 (76.3% vs 59.2%, p = 0.0241), more clinical successes from Day 7 onward (p < 0.05), and more pain-free patients from Day 3 onward (p < 0.05). Nepafenac 0.1% was superior to ketorolac 0.5% in terms of clinical success at Day 14 (p = 0.0319) and in percentage of pain-free patients at Day 3 (p = 0.0366). Nepafenac 0.1% also demonstrated less discomfort upon instillation than ketorolac 0.5% (p = 0.0158).
Conclusion:
The anti-inflammatory efficacy of nepafenac 0.1% is better than that of placebo; it is also more comfortable and at least equal to ketorolac 0.5% in the prevention and treatment of postoperative ocular pain and inflammation.
Keywords: NSAIDs, cataract, inflammation, nepafenac, ketorolac
Introduction
Ocular inflammation, which is common after cataract surgery, may cause patients to have postoperative pain and photophobia. Complications resulting from inflammation include increased intraocular pressure, posterior capsular opacification, cystoid macular edema, and decreased visual acuity. Topical steroid therapy effectively treats inflammation, but can increase intraocular pressure, inhibit wound healing, increase the likelihood of infection, or worsen an existing one (Heier et al 2000; Simone and Whitacre 2001). Cataract surgeons have therefore been interested in decreasing dependence on steroid use alone, seeking alternative or complementary treatments for postoperative inflammation that are equally effective but have fewer complications than steroid therapy (O’Brien 2005). Combination therapy of steroids with nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to produce a synergistic effect on postoperative inflammation (Flach 1994; Heier et al 2000).
Topical NSAIDs reduce inflammation by inhibiting prostaglandin synthesis and have been shown to be clinically effective in controlling inflammation after cataract surgery (Flach et al 1988; Flach et al 1998; Heier et al 1999; Simone and Whitacre 2001; Flach 2002; Bodaghi et al 2005). Several clinical studies have shown that NSAIDs are as effective as steroids in the treatment of postoperative pain and inflammation (Flach et al 1989; Brennan et al 1993; Simone et al 1999; Reddy et al 2000). The NSAIDs ketorolac 0.5% (Allergan Inc, Acular®, Irvine, CA, USA), diclofenac 0.1% (Novartis Ophthalmics, Voltaren Ophthalmic®, Duluth, GA, USA), and bromfenac 0.09% (ISTA Pharmaceuticals Inc, Xibrom™, Irvine, CA, USA) are all available in some markets for the treatment of postoperative inflammation following cataract surgery.
Pre-operative initiation of NSAID therapy may reduce postoperative inflammation more than when treatment is initiated after surgery (Roberts 1996; Solomon et al 1997; Flach 2002). Pre-operative regimens may initiate treatment anywhere from 3 days to immediately prior to surgery; regardless, pre-operative treatment with NSAIDs followed by postoperative combination therapy with NSAIDs and steroids has become common practice for the prevention and treatment of ocular inflammation following cataract surgery (Flach 2002).
Nepafenac 0.1% (Alcon Laboratories Inc, Nevanac®, Fort Worth, TX, USA) is a new ophthalmic NSAID and is the only one with a prodrug structure, making it a neutral molecule. This property, unlike the acidic nature of the other topical NSAIDs, allows nepafenac to rapidly penetrate the cornea, after which it is converted by intraocular hydrolases to its more active moiety amfenac (Ke et al 2000). Nepafenac is unique, in that its bioconversion to amfenac is targeted to the iris/ciliary body and, to an even greater extent, the retina/choroid, suggesting nepafenac may have prolonged activity in the vascularized tissues of the eye (Ke et al 2000). In support of this hypothesis, a rabbit model of paracentesis-induced ocular inflammation demonstrated that nepafenac exhibited a more complete and longer lasting inhibition of iris/ciliary body prostaglandin synthesis than diclofenac (Gamache et al 2000). The same was true in the posterior segment of the eye, where nepafenac demonstrated superior inhibition of retina/choroid prostaglandin synthesis compared to diclofenac. Taken together, this preclinical evidence suggests that nepafenac would compare favorably to conventional NSAIDs in the prevention and treatment of ocular inflammation associated with cataract surgery.
This study was designed based on regulatory guidance to compare the effectiveness of nepafenac 0.1% to both placebo and a well-studied NSAID (ketorolac 0.5%) for the prevention and treatment of pain and inflammation following cataract surgery (Flach et al 1988; Flach et al 1998; Heier et al 1999; Simone et al 1999; Heier et al 2000). The primary objective of this study was to compare the effect of nepafenac 0.1% and placebo on anterior segment inflammation at Day 14. All other comparisons of nepafenac 0.1% to ketorolac 0.5% and placebo for the prevention and treatment of postoperative pain and inflammation were performed at all time points. The dosing of the study drugs (three times daily) was congruent with the approved labeling of ketorolac 0.5% in Europe and was the most effective dosing regimen of nepafenac 0.1% demonstrated in a prior clinical study (Stewart 2005).
Materials and methods
Test, active, and control articles
Nepafenac Ophthalmic Suspension, 0.1% (nepafenac 0.1%) and nepafenac vehicle (placebo) were supplied by Alcon Laboratories, Inc. Ketorolac Tromethamine Ophthalmic Solution, 0.5% (ketorolac 0.5%) was supplied by Allergan, Inc. Nepafenac 0.1%, ketorolac 0.5% and placebo were sterile-transferred into identical bottles by Alcon Laboratories, Inc.
Study design
This was a multi-center, randomized, placebo- and active-controlled, double-masked clinical trial conducted at 15 ophthalmology clinics in France, Hungary, Italy, Portugal, Spain, and the UK. The study was approved by the appropriate Institutional Review Boards; patients underwent a process of informed consent. Patients were randomly assigned to one of three treatment groups: nepafenac 0.1%, ketorolac 0.5%, or placebo. Patients instilled 1 drop 3 times daily beginning 1 day before surgery, continuing on the day of surgery, and for 21 days thereafter. Additional medication was not dispensed unless the investigator declared a patient to be a treatment failure, at which time the patient was discontinued from the study. Treatment failure was defined as a patient presenting at any postoperative visit with more than 15 cells, very dense flare, or investigator-assessed ocular pain score of grade 4 or 5 on a 6-point ordered categorical scale from 0 (none) to 5 (severe). Patients were considered cured if the sum of their aqueous cells and flare ratings was 0 (ie, absence of cells and flare) at the current and all subsequent study visits. Clinical success was defined as an aqueous cells rating of 0 (none) or 1 (1–5 cells) and an aqueous flare rating of 0 (none) at the current and all subsequent study visits.
Postoperative examinations were conducted on Days 1, 3, 7, 14, 21, and 28; patients were required to have received a dose of their test article within 1–4 hours of each visit, except at Day 28 (the test article was stopped at Day 21). At the Day 7 visit, patients received dosing at the study site, after which the patients rated ocular discomfort on a 5-point scale from 0 (none) to 4 (severe).
Inclusion and exclusion criteria
Eligible patients were those who were 18 years or older, in need of cataract extraction by phacoemulsification and implantation of a posterior chamber intraocular lens (IOL), and who met all informed consent requirements. Exclusion criteria included the use of topical ocular, inhaled or systemic steroids within 14 days prior to surgery or topical ocular, inhaled or systemic NSAIDs within 7 days of surgery (except for up to 100 mg of aspirin), any cells or flare, investigator-assessed ocular pain at the pre-operative baseline visit, any corneal abnormality that prevented reliable Goldmann applanation tonometry, prior cataract surgery in the fellow eye within 60 days of screening visit or planned cataract surgery in the fellow eye prior to the Day 28 visit, planned multiple procedures during cataract/IOL implantation surgery (except for relaxing keratotomy for the correction of astigmatism), known or suspected hypersensitivity to NSAIDs or to any component of the study drugs, and a history of chronic or recurrent inflammatory eye disease in the study eye.
Post-operative examinations
At the Day 1, 3, 7, 14, 21, and 28 visits, the following examinations were performed: best-corrected logMAR visual acuity, intraocular pressure measurement, investigator assessment of ocular pain, and slit-lamp evaluation of the anterior segment (aqueous cells and flare). Additionally, at the Day 7 visit, patients assessed ocular discomfort following instillation of the study drug. A dilated fundus examination was conducted at the Day 28 visit.
Safety analysis
All patients receiving study medication were included in the safety analysis. Safety evaluation included adverse event reports (solicited and voluntary) and ocular parameters (best-corrected logMAR visual acuity, intraocular pressure measurement, slit-lamp evaluation, and dilated fundus examination).
Statistics
All statistical analyses were conducted on the intent-to-treat data set. The primary efficacy variable was the percentage of patients who achieved a cure at Day 14, defined as aqueous cells score = 0 and aqueous flare score = 0. A chi-square test of independence was conducted to assess the superiority of nepafenac 0.1% relative to placebo at Day 14. Eye drop comfort evaluation at the Day 7 visit was conducted for nepafenac 0.1% compared to ketorolac 0.5% as well as placebo using two-sample t-tests.
Additional analyses were conducted for nepafenac 0.1% compared to placebo and ketorolac 0.5% for percentage of patients with clinical success (aqueous cells score = 0 or 1 and aqueous flare score = 0) and for investigator assessment of ocular pain. Chi-square tests of independence were conducted to assess the superiority of nepafenac 0.1% relative to ketorolac 0.5% and placebo at each time point.
Results
Of 360 screened patients, 227 patients were enrolled in this study (77 nepafenac, 73 ketorolac, and 77 placebo). All 227 patients received at least one dose of study medication and were therefore included in the safety analysis. Patients who received study medication, completed cataract surgery, and had at least one follow-up visit were included in the intent-to-treat (ITT) population, which accounted for 225 of the 227 patients enrolled in the study.
Patient demographics are shown in Table 1. The mean age of the total population was 72 years, with ages ranging from 42 to 90. Forty percent of patients were male and over 96% were Caucasian. The groups were statistically equivalent with regard to age, gender, race and difficulty of cataract surgery.
Table 1.
Patient demographics (intent-to-treat population)
| Nepafenac (n=76) n (%) | Ketorolac (n=73) n (%) | Placebo (n=76) n (%) | |
|---|---|---|---|
| Age (mean years) | 71.7 | 72.9 | 71.6 |
| Sex | |||
| Male | 34 (44.7) | 28 (38.4) | 28 (36.8) |
| Female | 42 (55.3) | 45 (61.6) | 48 (63.2) |
| Race | |||
| Caucasian | 74 (97.4) | 70 (95.9) | 73 (96.1) |
| Black | 0 | 2 (2.7) | 1 (1.3) |
| Asian | 1 (1.3) | 0 | 2 (2.6) |
| Hispanic | 1 (1.3) | 1 (1.4) | 0 |
Note: No statistically significant differences were observed among groups.
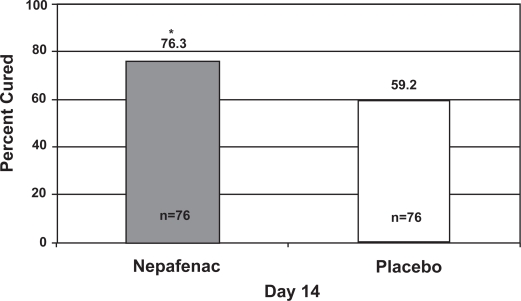
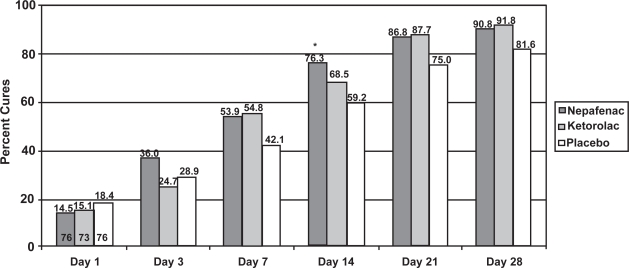
Figure 1 illustrates the cure rates of nepafenac 0.1% and placebo at Day 14, the primary endpoint of this study. Significantly more patients treated with nepafenac 0.1% were cured compared to those treated with placebo (76.3% vs 59.2%, p = 0.0241). Figure 2 shows the cure rates of all 3 treatment groups across all postoperative visits. Although the nepafenac group had a higher cure rate than the placebo group from Day 3 onward, only the Day 14 visit reached statistical significance (p = 0.0241). No significant difference in cure rates between the nepafenac and ketorolac groups was observed at any time point.
Figure 1.
Percentage of patients cured (no aqueous cells or flare) at Day 14 with nepafenac 0.1% or placebo.
*p = 0.0241, chi-square test.
Note: The number at the bottom of each bar represents the number of evaluable patients in each arm.
Figure 2.
Cure rate of nepafenac 0.1%, ketorolac 0.5%, and placebo at each visit.
*p = 0.0241, chi-square test.
Note: The numbers at the bottom of the Day 1 bars represent the number of evaluable patients in each arm. The number of evaluable patients did not vary across time points.
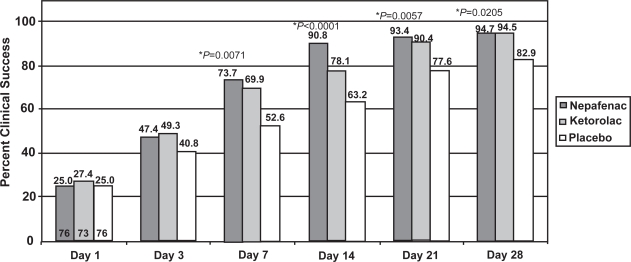
As shown in Figure 3, significantly more patients achieved clinical success with nepafenac 0.1% than with placebo beginning on Day 7 and continuing through Day 28 (p < 0.05). The clinical success rate of nepafenac 0.1% was superior to that of ketorolac 0.5% at Day 14 (p = 0.0319). At other time points, the clinical successes of nepafenac 0.1% and ketorolac 0.5% were not statistically different.
Figure 3.
Clinical success rate of nepafenac 0.1%, ketorolac 0.5%, and placebo at each visit.
*nepafenac 0.1% vs placebo, chi-square test. †nepafenac 0.1% vs ketorolac 0.5%, chi-square test.
Note: The numbers at the bottom of the Day 1 bars represent the number of evaluable patients in each arm. The number of evaluable patients did not vary across time points.
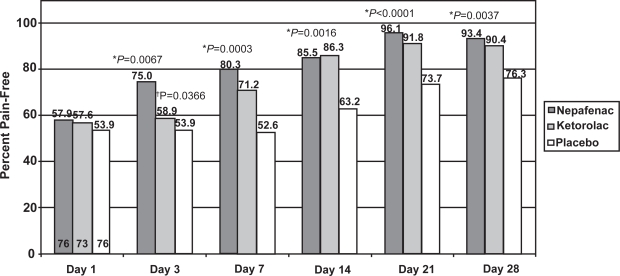
The percentage of patients with no ocular pain was significantly higher in the nepafenac 0.1% group compared to the placebo group at each time point starting with Day 3 (Figure 4, p < 0.05). Furthermore, more patients treated with nepafenac 0.1% were pain-free compared to those treated with ketorolac 0.5% at Day 3 (p = 0.0366). At other time points, the percent of patients with no ocular pain was not statistically different between the nepafenac 0.1% and ketorolac 0.5% groups.
Figure 4.
Pain-free rate of nepafenac 0.1%, ketorolac 0.5%, and placebo at each visit.
*nepafenac 0.1% vs placebo, chi-square test. †nepafenac 0.1% vs ketorolac 0.5%, chi-square test.
Note: The numbers at the bottom of the Day 1 bars represent the number of evaluable patients in each arm. The number of evaluable patients did not vary across time points.
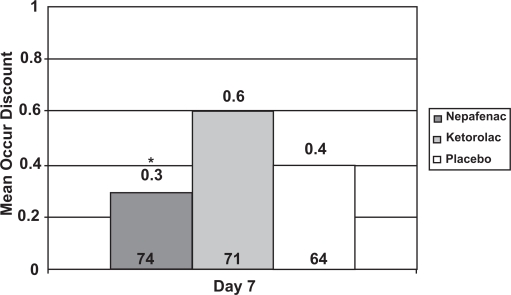
At the Day 7 visit only, patients evaluated the ocular discomfort of the study medication. Mean ocular discomfort was significantly lower for nepafenac 0.1% compared to ketorolac 0.5% (p = 0.0158) but not to placebo (Figure 5).
Figure 5.
Mean ocular discomfort of eye drops at Day 7.
*p = 0.0158 nepafenac 0.1% vs ketorolac 0.5%, t-test, on a 0 to 5 scale.
Note: The number at the bottom of each bar represents the number of evaluable patients in that arm. Sixteen patients had missing ocular discomfort scores, for a total of 209 evaluable patients.
All 227 patients who were enrolled in the study received at least one dose of study drug and were therefore included in the safety analysis. Two serious adverse events (hospitalizations due to diabetes mellitus and a branch retinal vein occlusion) were reported, both of which were unrelated to the use of study medication. Adverse events in the overall safety population were predominantly mild or moderate in intensity, and generally did not interrupt patient continuation in the study. The most frequent treatment-related adverse event with nepafenac 0.1% was eyelid margin crusting (2.6%); no eyelid margin crusting was reported with ketorolac 0.5% or placebo. For patients treated with ketorolac 0.5% or placebo, ocular hyperemia was the most frequent adverse event (2.7% and 5.2%, respectively), whereas the nepafenac group did not experience ocular hyperemia. No clinically relevant differences were observed between the treatment groups in the overall safety population. No safety concerns were identified for intraocular pressure, visual acuity, ocular signs (corneal edema, bulbar conjunctival injection, and chemosis), or dilated fundus parameters based upon an analysis of changes from baseline.
Discussion
Nepafenac 0.1% is the newest topical NSAID available for the treatment of ocular pain and inflammation associated with cataract surgery. It has unique properties, including rapid corneal permeability and targeted intraocular activation due to its prodrug structure.
As expected from previous studies (Stewart 2005; Lane et al 2007), nepafenac 0.1% met its primary objective in the current study by demonstrating superiority over placebo in the percentage of patients with clinical cures at Day 14. In addition, nepafenac 0.1% produced more clinical successes than placebo at every time point beginning with Day 7. This confirms that nepafenac 0.1% is effective in the prevention and treatment of ocular inflammation. Nepafenac 0.1% was also superior to placebo in the management of pain, as shown by the fact that significantly more patients treated with nepafenac 0.1% were pain-free at every time point after Day 1. By Day 14, over 90% of patients treated with nepafenac 0.1% reported no pain, suggesting that this drug is highly effective in the treatment of pain related to cataract surgery. In fact, nepafenac has shown analgesic efficacy in PRK-related ocular pain (Colin and Paquette 2006).
Most interesting, however, is the comparison between nepafenac 0.1% and ketorolac 0.5%. Previous studies have established the effectiveness of ketorolac 0.5% for the treatment of both pain and inflammation following cataract surgery (Flach et al 1988; Flach et al 1989; Heier et al 1999; Simone et al 1999; Solomon et al 2001). Therefore, ketorolac 0.5% was used as a benchmark against which the efficacy of nepafenac 0.1% was measured. The current study was not powered to detect the superiority of nepafenac 0.1% relative to ketorolac 0.5%. Despite this fact, nepafenac 0.1% reached statistical superiority compared to ketorolac 0.5% in both clinical success rate at Day 14 and pain assessment at Day 3. This illustrates the robust nature of the data and indicates that nepafenac 0.1% is an effective and safe drug in treating ocular inflammation.
Ocular comfort of any topical medication is important, as this factor may have implications for patient compliance. In this study, the mean ocular discomfort score of nepafenac 0.1% was statistically lower than the mean score for ketorolac 0.5%, showing that patients judged nepafenac to be more comfortable upon instillation. At least two reasons could contribute to this difference. First, there may simply be molecular differences between nepafenac and ketorolac that result in the increased ocular comfort of nepafenac. Another possible explanation is that nepafenac 0.1% is formulated as a suspension whereas ketorolac 0.5% is a solution. Because of the discomfort associated with the instillation of ketorolac 0.5%, a less concentrated form (0.4%) has been developed to reduce patient complaints of stinging and burning. In clinical trials with ketorolac 0.5%, up to 40% of patients reported stinging and burning (Acular package insert 2002). However, in clinical trials with ketorolac 0.4%, a similar number of patients (20%–40%) complained of stinging and burning upon instillation (Acular LS package insert 2003). In the only head-to-head trial of these 2 agents in patients undergoing cataract surgery, ketorolac 0.4% significantly reduced stinging and burning compared to ketorolac 0.5% at Day 1 (20% vs 65%, p = 0.001), but not at Week 1 (50% vs 40%) or Month 1 (30% vs 40%; Sandoval et al 2006).
This study demonstrates that nepafenac 0.1% is superior to placebo for the prevention and treatment of ocular pain and inflammation resulting from cataract surgery. Nepafenac also shows at least equal efficacy (for both ocular pain and inflammation measures) compared to ketorolac 0.5%. Moreover, nepafenac 0.1% is more comfortable upon instillation than ketorolac 0.5%.
Acknowledgments
Members of the Internacional C-04-65 Study Group: A Brezin, Hopital Cochin, Paris; B Cochener, CHU Morvan, Brest; P Rozot, Clinique Monticelli, Marseille; L Vissac, Hôpital Purpan, Toulouse; FRANCE. A Bereczki, Petz Aladar Teaching Hospital, Györ; Z Öri, Vaszary Kolos Hospital, Esztergom; HUNGARY. M Nardi, Università degli Studi di Pisa, Pisa; R Ratiglia, Università degli Studi di Milano, Milan; C Sborgia, Università degli Studi di Bari, Bari; ITALY. C Lobo, Aibili, Coimbra; JP Silva, H San Jose, Lisbon; PORTUGAL. R Barraquer, Centro Oftalmología Barraquer, Barcelona; J Cano, H Municipal de Badalona; Barcelona; SPAIN. I Cunliffe, Birmingham Heartlands Hospital, Birmingham; R Packard, King Edward VII Hospital, Windsor, Berkshire; UK.
This study was funded by Alcon Laboratories, Inc., Fort Worth, TX, USA. Competing interests of the authors: E Zagato, S Potts, G Sullins, and R Notivol are Alcon Laboratories employees.
References
- ACULAR® [package insert] Palo Alto, CA: Allergan, Inc; 2002. [Google Scholar]
- ACULAR LS® [package insert] Palo Alto, CA: Allergan, Inc; 2003. [Google Scholar]
- Bodaghi B, Weber ME, Arnoux YV, et al. Comparison of the efficacy and safety of two formulations of diclofenac sodium 0.1% eyedrops in controlling postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2005;15:702–11. doi: 10.1177/112067210501500608. [DOI] [PubMed] [Google Scholar]
- Brennan KM, Brown RM, Roberts CW. A comparison of topical non-steroidal anti-inflammatory drugs to steroids for control of post cataract inflammation. J Am Soc Ophthalmic Reg Nurses. 1993;XVIII:8–11. [PubMed] [Google Scholar]
- Colin J, Paquette B. Comparison of the analgesic efficacy and safety of nepafenac ophthalmic suspension compared with diclofenac ophthalmic solution for ocular pain and photophobia after excimer laser surgery: a phase ii, randomized, double-masked trial. Clin Ther. 2006;28:527–36. doi: 10.1016/j.clinthera.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Flach AJ, Lavelle CJ, Olander KW, et al. The effect of ketorolac tromethamine solution 0.5% in reducing postoperative inflammation after cataract extraction and intraocular lens implantation. Ophthalmology. 1988;95:1279–84. doi: 10.1016/s0161-6420(88)33034-4. [DOI] [PubMed] [Google Scholar]
- Flach AJ, Jaffe NS, Akers WA. The effect of ketorolac tromethamine in reducing postoperative inflammation: double-mask parallel comparison with dexamethasone. Ann Ophthalmol. 1989;21:407–11. [PubMed] [Google Scholar]
- Flach AJ. Nonsteroidal anti-inflammatory drugs. In: Tasman W, editor. Duane’s foundations of clinical ophthalmology. Philadelphia: Lippincott; 1994. pp. 1–32. [Google Scholar]
- Flach AL, Dolan BJ, Donahue ME, et al. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology. 1998;105:1775–9. doi: 10.1016/S0161-6420(98)99053-4. [DOI] [PubMed] [Google Scholar]
- Flach AJ. Topical nonsteroidal anti-inflammatory drugs in ophthalmology. Int Ophthalmol Clin. 2002;42:1–11. doi: 10.1097/00004397-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Gamache DA, Graff G, Brady MT, et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357–70. doi: 10.1023/a:1007049015148. [DOI] [PubMed] [Google Scholar]
- Heier J, Cheetham JK, DeGryse R, et al. Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle-controlled clinical trial. Am J Ophthalmol. 1999;127:253–9. doi: 10.1016/s0002-9394(98)00413-9. [DOI] [PubMed] [Google Scholar]
- Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107:2034–8. doi: 10.1016/s0161-6420(00)00365-1. [DOI] [PubMed] [Google Scholar]
- Ke TL, Graff G, Spellman JM, et al. Nepafenac, a unique nonsteroidal pro-drug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation. 2000;24:371–84. doi: 10.1023/a:1007001131987. [DOI] [PubMed] [Google Scholar]
- Lane SS, Modi SS, Lehmann RP, et al. Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg. 2007;33:53–8. doi: 10.1016/j.jcrs.2006.08.043. [DOI] [PubMed] [Google Scholar]
- O’Brien TP. Emerging guidelines for use of NSAID therapy to optimized cataract surgery patient care. Curr Med Res Opin. 2005;21:1131–7. doi: 10.1185/030079905X50651. [DOI] [PubMed] [Google Scholar]
- Reddy MS, Suneetha N, Thomas RK, et al. Topical diclofenac sodium for treatment of postoperative inflammation in cataract surgery. Indian J Ophthalmol. 2000;48:223–6. [PubMed] [Google Scholar]
- Roberts CW. Pretreatment with topical diclofenac sodium to decrease postoperative inflammation. Ophthalmology. 1996;103:636–9. doi: 10.1016/s0161-6420(96)30641-6. [DOI] [PubMed] [Google Scholar]
- Sandoval HP, De Castro LE, Vroman DT, et al. Evaluation of 0.4% ketorolac tromethamine ophthalmic solution versus 0.5% ketorolac tromethamine ophthalmic solution after phacoemulsification and intra-ocular lens implantation. J Ocul Pharmacol Ther. 2006;22:251–7. doi: 10.1089/jop.2006.22.251. [DOI] [PubMed] [Google Scholar]
- Simone JN, Pendelton RA, Jenkins JE. Comparison of the efficacy and safety of ketorolac tromethamine 0.5% and prednisolone acetate 1% after cataract surgery. J Cataract Refract Surg. 1999;25:699–704. doi: 10.1016/s0886-3350(99)00023-1. [DOI] [PubMed] [Google Scholar]
- Simone JN, Whitacre MM. Effects of anti-inflammatory drugs following cataract extraction. Curr Opin Ophthalmol. 2001;12:63–7. doi: 10.1097/00055735-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Solomon KD, Turkalj JW, Whiteside SB, et al. Topical 0.5% ketorolac vs 0.03% flurbiprofen for inhibition of miosis during cataract surgery. Arch Ophthalmol. 1997;115:1119–22. doi: 10.1001/archopht.1997.01100160289004. [DOI] [PubMed] [Google Scholar]
- Solomon KD, Cheetham JK, DeGryse R, et al. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology. 2001;108:331–7. doi: 10.1016/s0161-6420(00)00543-1. [DOI] [PubMed] [Google Scholar]
- Stewart WC. Pre-operative and postoperative clinical evaluation of nepafenac 0.1% ophthalmic suspension for postcataract inflammation; Presented at the 2005 ASCRS meeting; Washington, DC. Apr, 2005. [Google Scholar]