Abstract
Papilledema is considered a neuro-ophthalmic emergency because of its capacity to induce irreversible end-organ damage and the often grave nature of its precipitating factor. Even more concern is warranted when papilledema presents in a pediatric setting. After excluded the contributions of intracranial masses, congenital malformations, ischemic insults and acute infections, the investigation must focus on determining the contributions of other uncharacteristic causes of pediatric pseudotumor cerebri. Pediatric pseudotumor cerebri is a rare clinical entity which shares few commonalities to the adult condition in regards to its predicating factors or symptoms. Without adequate medical history questioning, funduscopic evaluation and ancillary testing, the possibility of an erroneous diagnosis is plausible. This case report aims to disclose the toxic role levothyroxine sodium tablets (Synthroid®, Abbott Laboratories, Abbott Park, IL, USA) played in inducing pseudotumor cerebri in a pediatric patient being treated for congenital hypothyroidism.
Keywords: pseudotumor cerebri, papilledema, levothyroxine, Synthroid®, hypothyroidism
Background
Pseudotumor cerebri (PTC) is a condition that classically presents with optic disc elevation and edema resulting from increased intracranial pressure in absence of hydrocephalus, space occupying lesion, or any other intracranial disease. Studies show that obese women in their childbearing years are most frequently affected with an incidence rate more than a hundred times that of the general population (90/100,000 women vs 0.9–1.7/100,000 people) (Campos and Olitsky 1995). The symptom complex of a patient with PTC is variable but commonly includes nausea, emesis, amaurosis fugax, diplopia, tinnitus, retrobulbar pain, and postural headaches (Campos and Olitsky 1995). Although there are few cases of pediatric PTC in literature, current reports suggest that pediatric PTC symptoms are typically less severe as compared to adults and no sexual predilection or correlation with obesity has been shown to exist. Symptoms appear to parallel adult-like symptoms the older the child is at time of presentation (Raghaven et al 1997). Emotional irritability and somnolence are two key symptoms that appear to more commonly afflict infants and younger children who suffer from pediatric PTC (Raghaven et al 1997).
In both the adult or pediatric presentations, preservation of vision and visual field are principle concerns that must be addressed immediately. It has been shown that 85% of the patients presenting with signs and symptoms of PTC possess a visual field abnormality (Cincirpini et al 1999). In pediatric PTC cases, the degree of visual field loss is between 13%–38% with 46% of these cases demonstrating an enlarged blind spot (Cincirpini et al 1999). Although the cause of pediatric PTC is often a mystery, the contributions of several secondary factors have surfaced including various endocrine disorders and iatrogenic causes (Wilson and Baker 2005) (Table 1). The following report illustrates the toxic contribution of oral levothyroxine therapy (Synthroid®, Abbott Laboratories, Abbott Park, IL, USA) employed for the treatment congenital hypothyroidism in precipitating a case of pediatric PTC.
Table 1.
Reported secondary causes of pseudotumor cerebri (Wilson and Baker 2002)
|
|
Congenital hypothyroidism affects approximately 1 in every 4000 infants in the United States and is caused by an infant’s inability to produce an adequate level of thyroid hormone (Bourgeois and Varma 2005). Principal causes of this congenital deficiency include a malfunctioning thyroid gland, an iodine deficiency, or defective thyroid metabolism. Because of the thyroid hormone’s critical role in promoting neuronal myelination for normal central nervous system development during the early postpartum period, supplemental therapy must be initiated early in a child’s life (Bourgeois and Varma 2005).
Levothyroxine sodium (Synthroid®, Abbott Laboratories, Abbott Park, IL, USA) is used as a replacement therapy in the setting of diminished or absent thyroid function due to primary thyroid gland dysfunction or secondary etiologies such as autoimmune destruction, trauma, surgery, radio-ablation, drug-toxicity or infections. Synthroid® is a synthetic replacement for thyroxine (T4), the principal hormone that is synthesized and released by the thyroid gland. As a therapeutic agent, levothyroxine sodium has a narrow therapeutic range. If the fractions of levothyroxine sodium administered are sub-optimal, the desired reversal of the hypothyroid-state is not attained. Unfortunately, the main complication reported to arise in pediatric administration of levothyroxine sodium is usually the result of over-administration of the drug or an allergic reaction to the synthetic formulation (Bourgeois and Varma 2005). Adults with clinically-confirmed thyroid dysfunction often require 1.6 μg/kg of levothyroxine per day, whereas pediatric patients often require higher fractions, up to 4 μg/kg/day. Careful titration of the administered dosages of levothyroxine sodium is performed every 6 weeks after initiating therapy till the desire level of free T4 and TSH are attained. Regular assays are collected thereafter to avoid over- or under-medicating patients with thyroid dysfunction.
Although not life-threatening, negligence of the signs of hypothyroidism (fatigue, dry skin, cold intolerance, and weight gain) in a pediatric patient may have serious repercussions later in life by engendering memory impairment, mental retardation, cardiac dysfunction and myxedema coma. Equally significant is the fact that congenital or iatrogenically-induced states of hyperthyroidism can predispose a patient to early-onset osteoporosis and cardiac complications (atrial fibrillation, supraventricular arrhythmias). In light of the significant clinical consequences associated with improper thyroid-replacement therapy, the pediatric dosing schedule of levothyroxine sodium is kept at the minimum dosage which yields normal, stable thyroid function tests without noting tachycardia, palpitations, tremors, agitation, hyperactivity, weight loss, fever or diaphoresis in the child (Bourgeois and Varma 2005). A standardized dosing regimen has been published to assist in the reduction of deleterious toxic sequelae (Bourgeois and Varma 2005) (Table 2). The neuro-ophthalmic complication of levothyroxine-induced PTC has been reported in literature to arise with the initiation or during the course of therapy in a pediatric setting. The medical community at large must be alert to the signs and symptoms of this neuro-ophthalmic emergency that may be induced in response to toxic levels of levothyroxine sodium and be cognoscente of the fact it may arise in isolation of other more common indicators of iatrogenic-hyperthyroidism. Furthermore, clinicians should consider levothyroxine-induced PTC as a situation that warrants re-investigation or adjustment of the current levels of levothyroxine sodium replacement therapy.
Table 2.
Recommended initial dosing schedule of levothyroxine for pediatric patients (Bourgeois and Varma 2005)
| Age | Recommended dosage level |
|---|---|
| <6 months | 8–10 μg/kg/d PO or 25–50 μg/d PO |
| 6–12 months | 6–8 μg/kg/d PO or 50–75 μg/d PO |
| 1–5 years | 5–6 μg/kg/d PO or 75–100 μg/d PO |
| 6–12 years | 4–5 μg/kg/d PO or 100–150 μg/d PO |
| >12 years | 2–3 μg/kg/d PO or 150 μg/d PO |
| IV/IM = 50%–75% of PO dose | |
Case report
An 11-year-old Caucasian female presented for a routine eye exam complaining of chronic headaches for the last 12 months. She reported supra-nuchal, radiating pain that was rated as an “8” on a 10 point scale. These headaches were exacerbated during postural changes such as while tying her shoes. Nausea, vomiting, visual aura, amaurosis fugax, phonophobia, diplopia, tinnitus and an altered mental status were not reported. Medical history was remarkable for juvenile diabetes mellitus and congenital hypothyroidism. The patient’s mother noted that these diagnoses were assigned in infancy. Current medical therapy included insulin NPH 100 units/ml s.c. bid (Novolin N®, Nov Nordisk Pharmaceuticals, Princeton, NJ, USA), and levothyroxine 100 μg p.o. qd (Synthroid®, Abbott Laboratories, Abbott Park, IL, USA).
Visual acuities were recorded as 20/30 OD and 20/40 OS without correction. Pupils were equal, round with brisk, symmetrical responses to light; no afferent pupillary defect was noted. Versions were full, smooth, and accurate in all gazes. Color vision was normal with Ishihara pseudo-isochromatic plate testing. Cover test uncovered an 8 prism diopter esophoria at near and orthophoria at distance. Manifest refraction yielded a prescription of OD: −1.00 −0.25 × 105, OS: −0.50 −0.75 × 105 with a +1.00 add to attain 20/20 OU at all distances and eliminate her near phoria.
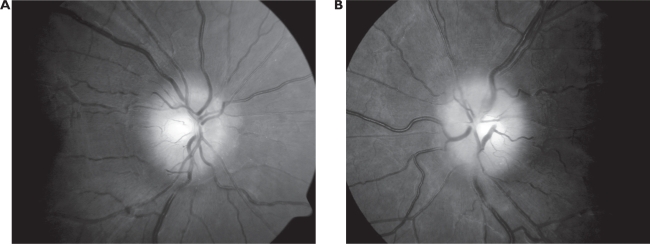
Anterior segment was grossly unremarkable. Goldmann applanation uncovered intraocular pressures of 16 mmHg OD, 16 mmHg OS. Blood pressure readings were recorded as 128/84 mmHg. Funduscopic evaluation revealed bilaterally edematous, elevated and non-hemorrhagic optic nerves (Figure 1). Cup-to-disc ratio was approximated at 0.30 H/V OD, 0.30 H/V OS. Spontaneous venous pulsations were absent OU. The lenticular and vitreal media were clear. Peripheral eye grounds were clear, flat and intact 360° OU.
Figure 1.
Funduscopic images of non-hemorrhagic, elevated, and edematous optic nerves noted in the right (1-A) and left (1-B) eyes of our patient. No spontaneous venous pulsations were detected at initial examination.
Automated Humphrey® visual field testing (SITA-Standard, 30-2, size III stimulus) results were deemed unreliable likely owing to the patient’s age; nevertheless mild bilateral blindspot enlargement was discernible.
Immediate referral to a pediatric neuro-ophthalmologist was initiated with a request for T-1 weighted MRI imaging of the patient’s head/orbit and lumbar puncture studies including cerebrospinal fluid (CSF) cultures and opening pressures. No space-occupying lesion, congenital aterio-venous malformations or hydrocephalus was detected during neuroimaging studies. Cerebral spinal fluid cultures revealed the absence of infection or pleocytosis. Opening pressures were not recovered by attending physician at the time of the lumbar puncture.
After consulting with the patient’s endocrinologist, the patient’s neuro-ophthalmologist initiated acetazolamide 250 mg bid p.o. (Diamox®, Lederle Laboratories, Pearl River, NY, USA) in place of modulating her current levothyroxine-therapy. Fortunately, the patient was afforded partial relief from her headache pain over the course of the next several weeks. Repeated automated perimetry testing has demonstrated stability in the pattern of bilateral blind-spot enlargement. Six months after her initial presentation, funduscopic evaluation demonstrates an improved degree of optic nerve head elevation, but a persistent pattern of blindspot enlargement on visual field testing.
Patient was counseled as to the benefit of a second opinion from another pediatric neuro-ophthalmologist and endocrinologist to ensure that visual morbidity would be minimized. Patient denied additional intervention and was eventually lost to follow-up.
Discussion
Pediatric pseudotumor cerebri (PTC) is a rarely reported phenomenon in neuro-ophthalmology literature. Nevertheless, awareness and quick intervention are essential to prevent unnecessary vision loss from this reversible clinical entity. Diagnosing pediatric PTC is challenging since children and young adults exhibit a clinical presentation that is unlike that reported in adults manifesting this condition. In considering this diagnosis, the astute clinician must employ a step-wise approach that is in keeping with the criteria listed in the modified Dandy Criteria to ensure absolute certainty in their diagnosis (Smith 1985; Langford 2002) (Table 3). In-depth probing of a patient’s medical, social and family history may help to uncover the contributions of a secondary factor in manifesting PTC (Wilson and Baker 2005) (Table 1). When no identifiable cause can be found, it is appropriate to label this clinical entity as idiopathic intra-cranial hypertension.
Table 3.
Modified dandy criteria for diagnosis of pseudotumor cerebri (Smith 1985; Langford 2002)
|
This case report illustrates how medical history questioning provided the basis for determining the cause of the PTC to be a toxic level of levothyroxine. A review of current literature suggests that there are at least half a dozen reports outlining the role levothyroxine played in inducing secondary PTC in a child or young adult (Van Doop et al 1983; McVie 1984; Huseman and Torkelson 1985; Rohn 1985; Campos and Olitsky 1995; Rovet and Ehrlich 1995; Raghaven et al 1997; Williams 1997; Langford 2002; Selva et al 2002). Raghavan et al (1997) described a 5 month old infant who developed PTC shortly after initiation of levothyroxine (50 μg/kg) therapy. Campos and Olitsky (1995) described a case of a 7½ year old patient who developed severe, widespread headaches after 1 week of commencing levothyroxine therapy (75 μg p.o. qd) for hypothyroidism. This child also reported concomitant symptoms of eye pain, tinnitus, nausea and vomiting. Only after titrating the levels of levothyroxine being administered to the patient (50 μg/day), did the patient’s symptoms resolve over the next few months despite persistent papilledema on a subsequent funduscopic examination. Neuroimaging studies and lumbar puncture confirmed a diagnosis of pseudotumor cerebri owing to toxic levothyroxine levels (Campos and Olitsky 1995). McVie (1984) described a 13 year old girl whose severe headaches and concurrent papilledema which began after only 3 days of starting levothyroxine therapy resolved once levothyroxine therapy was discontinued and subsequently re-initiated at a level of 50 μg/kg.
Although standardized dosing regimens have been proposed, no clear consensus exists for determining a safe initial dosing schedule for pediatric patients suffering from congenital hypothyroidism to prevent systemic complication from the drug itself. (Table 2) The current reported cases infer that the toxicities arising from levothyroxine therapy arise from improper initial dosing selection or dosages that over-shoot the therapeutic window. This in turn suggests that the toxic effects are independent of cumulative dosing of this synthetic hormone. Selva et al (2002) looked at initial dosing schedules of patients with congenital hypothyroidism and suggested titration of the initial dosage is essential to limit levothyroxine’s harmful effects on neurologic development. They concluded that initial dosing of ∼12–15 μg/kg raised thyroxine levels to target levels within 3 days, normalized thyroid function tests within 2 weeks of initiating therapy and presented few deleterious complications in the majority of patients. Because of the variability in patient pharmacokinetic responses to any medication, it is advisable that all children be monitored closely during the initiation phase of levothyroxine therapy to limit the occurrence of neurological side effects.
In literature, few authors propose a definitive treatment paradigm or clinically superior therapy to resolve pediatric cases of PTC. Two studies suggest that initial modulation of the level of thyroid replacement hormone is often sufficient to promote the resolution of the signs and symptoms of PTC (Van Dop et al 1983; McVie 1984). Other reports infer that no change to the patient’s thyroid replacement regimen is needed, since the signs and symptoms of PTC have the capacity to self-resolve (Prendes and McLean 1978). However, if prompt resolution of PTC is warranted, medical intervention using oral carbonic anhydrase inhibitors or immunosuppressive therapy have shown to be effective at reducing CSF production and reversing the signs of papilledema in other clinical scenarios (Baker et al 1985). Surgical intervention should only be considered if medical therapy fails to achieve an appropriate endpoint, headaches persist, or progressive vision loss ensues. Optic nerve sheath fenestration (ONSF) is a neuro-ophthalmic procedure designed to create an alternative outflow channel for the CSF which encircles the intra-orbital portion of the optic nerve. It is a procedure that is limited almost exclusively to the treatment of PTC and has been shown to manifest significant benefits along with an adequate safety profiles in children suffering from pediatric PTC (Lee et al 1998; Thuente and Buckley 2005). Lee et al (1998) reported on the effects of optic nerve sheath fenestration (ONSF) in 12 children who were previously unresponsive to typical pharmacological intervention. They noted that 66% of patients’ showed improvements in visual acuity, while 17% showed stability and the remaining 17% experienced a worsening in their level of acuity. A similar breakdown was noted with regards to the patient’s peripheral vision, with 2/3 of pediatric PTC patients’ experiencing an improvement or stability in their side-vision; while 1/3 suffered from continued deterioration following ONSF. In a follow-up investigation by Thuente and Buckley (2005), the outcome of ONSF in 17 eyes (12 children) that were previously unresponsive to medical therapy was once again examined. Seven of the twelve children only required unilateral ONSF to attain an improvement in the degree of papilledema in the contralateral eye. For those who manifested more advanced papilledema at presentation, bilateral ONSF was performed within 3–4 months of this initial surgical intervention. Similarly to Lee and colleagues (1998) initial investigation, the level of visual acuity and degree of visual field attenuation was more likely to be improved or stabilized following ONSF; these findings were noted to most often arise in conjunction with a regression in the degree of papilledema. As such, the authors surmised that ONSF is a safe and effective adjunctive therapy for pediatric cases of medically-unresponsive PTC.
Conclusion
Pre-cautionary measures should always be taken with pediatric patients receiving levothyroxine therapy for congenital hypothyroidism. Despite its widespread use and relatively benign safety profile, levothyroxine maintains a capacity to induce visually devastating sequelae in young patients. We advise considering levothyroxine as a potential cause of secondary PTC especially if the signs and symptoms arise shortly after commencing therapy or arise in a pediatric setting. Despite the lack of neuro-ophthalmic literature citing the contributions of presumed levothyroxine-induced pediatric PTC, awareness of this rare clinical occurrence is vital to ensure prompt diagnosis and appropriate management.
References
- Baker RS, Carte D, Hendrick EB, et al. Visual loss in pseudotumor cerebri of childhood. Arch Ophthalmol. 1985;103:1681–6. doi: 10.1001/archopht.1985.01050110075029. [DOI] [PubMed] [Google Scholar]
- Bourgeois M, Varma S.2004. Congenital hypothyrodism (edited November 30, 2004) [online]. Accessed 9 September 2005. URL: http://www.emedicine.com
- Campos SP, Olitsky S. Idiopathic intracranial hypertension after L-thyroxine therapy for acquired primary hypothyroidism. Clin Pediatrics. 1995;34:334–7. doi: 10.1177/000992289503400608. [DOI] [PubMed] [Google Scholar]
- Cincirpini G, Donahue S, Borchet M. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J of Ophthalmology. 1999;127:178–82. doi: 10.1016/s0002-9394(98)00386-9. [DOI] [PubMed] [Google Scholar]
- Huseman CA, Torkelson RD. Pseudotumor cerebri following treatment of hypothalamic and primary hypothyroidism. Am J Dis Child. 1985;138:927–31. doi: 10.1001/archpedi.1984.02140480029010. [DOI] [PubMed] [Google Scholar]
- Langford C. Pseudotumor cerebri in a pre-pubescent child-case report. Optometry. 2002;73:700–3. [PubMed] [Google Scholar]
- Lee A, Partrinely J, Edmond J. Optic nerve sheath decompression in pediatric pseudotumor cerebri. Ophthalmic Surg Lasers. 1998;29:514–17. [PubMed] [Google Scholar]
- McVie R. Abnormal TSH regulation, pseudotumor cerebri, and empty sellae after replacement therapy in juvenile hypothyroidism. J Pediatrics. 1984;105:768–70. doi: 10.1016/s0022-3476(84)80302-9. [DOI] [PubMed] [Google Scholar]
- Prendes JL, McLean W. Pseudotumor cerebri during treatment for hypothyroidism. South Med J. 1978;71:977. doi: 10.1097/00007611-197808000-00033. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Dimartino-Nardi J, Saenger P, et al. Pseudotumor cerebri in an infant after L-thyroxine therapy for transient neonatal hypothyroidism. J Pediatrics. 1997;130:478–80. doi: 10.1016/s0022-3476(97)70215-4. [DOI] [PubMed] [Google Scholar]
- Rohn R. Pseudotumor cerebri following treatment of hypothyroidism. Am J Dis Child. 1985;139:752. doi: 10.1001/archpedi.1985.02140100014015. [DOI] [PubMed] [Google Scholar]
- Rovet JF, Ehrlich RM. Long-term effects of L-thyroxine therapy for congenital hypothyroidism. J Pediatrics. 1995;126:380–6. doi: 10.1016/s0022-3476(95)70452-3. [DOI] [PubMed] [Google Scholar]
- Selva KA, Mandeal SH, Rien L, et al. Initial treatment dose of L-thyroxine in congenital hypothyroidism. J Pediatr. 2002;141:786–92. doi: 10.1067/mpd.2002.128887. [DOI] [PubMed] [Google Scholar]
- Smith JL. Whence pseudotumor cerebri? J Clin Neuro-ophthalmol. 1985;5:55–6. [PubMed] [Google Scholar]
- Thuente D, Buckley E. Pediatric optic nerve sheath decompression. Am Academy of Ophthalmol. 2005;112:724–7. doi: 10.1016/j.ophtha.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Van Dop C, Conte FA, Koch TK, et al. Pseudotumor cerebri associated with initiation of levothyroxine therapy for juvenile hypothyroidism. N Engl J Med. 1983;308:1076–80. doi: 10.1056/NEJM198305053081807. [DOI] [PubMed] [Google Scholar]
- Williams JB. Adverse effects of thyroid hormones. Drugs Aging. 1997;11:460–9. doi: 10.2165/00002512-199711060-00005. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Baker MJ.2002. Pseudotumor cerebri: pediatric perspective (edited January 4, 2002) [online]. Accessed 3 November 2005. URL: http://www.emedicine.com