Abstract
The 2005–2006 outbreak of Fusarium keratitis associated with soft hydrophilic contact lens wear was both unprecedented and unexpected. More than 250 cases have been reported worldwide that have primarily been associated with Bausch & Lomb ReNu with MoistureLoc and, more recently, with Bausch and Lomb ReNu MultiPlus multipurpose contact lens disinfecting solutions. This article documents the outbreak, presenting the time line and the historical and scientific basis for its occurrence. Underlying causes are explored including likely mechanisms of contamination and subsequent corneal infection. Thorough exploration of this unique occurrence affords the opportunity to examine contact lens and lens care actions and interactions and to develop greater understanding of possible associated risks and ways to minimize them.
Keywords: alexidine, Bausch & Lomb, contact lens, fusarium keratitis, fusarium solani, microbial keratitis, ReNu with MoistureLoc, ReNu MultiPlus, PHMB
Introduction
Hydrophilic soft contact lenses were invented in 1961 by Czechoslovakian chemist Professor Otto Wichterle and first commercially introduced in the United States by Bausch & Lomb in 1971. In the more than three decades since their introduction, hydrophilic soft contact lenses have proven a comparatively safe and effective form of vision correction.
The majority of complications associated with hydrophilic lenses are minor and self-limiting, however contact lens related microbial keratitis (CL-MK) stands out as a potentially sight threatening and sometimes life altering event. The relative risk of infectious keratitis has been extensively investigated; however, the underling risk factors much less so. Length of continuous lens wear, particularly overnight wear, has been identified as a key factor in lens-related microbial keratitis. Several studies suggest that relative risk increases from approximately 4 in 10,000 for daily wear to 20 in 10,000 for extended wear (Poggio et al 1989; Schein et al 1989; Cheng et al 1999).
Other factors have been recognized but generally are assumed to play contributory or relatively minor roles in the genesis of CL-MK (Weissman and Mondino 2002). They include hypoxia, non-compliance with lens care regimens, blepharitis, diabetes mellitus, epithelial trauma, steroid use, tobacco use, and therapeutic lens use. Environmental factors such travel to warm climates have also been implicated.
The role contact lens materials play in the genesis of CL-MK has been explored, albeit indirectly. Post-approval surveillance and other studies regarding silicone hydrogel-based contact lenses have shown relatively little difference in the incidence of presumed microbial keratitis compared to conventional hydrogel lenses (Schein et al 2005). The roles of potentially important contributory factors such as lens care products, their constituents, lens designs, and interactions among these elements have been largely ignored both clinically and scientifically. Based on current knowledge, CL-MK appears to be both multifactorial and complex.
The overwhelming majority of CL-MK cases have been attributed to infection by Gram-negative bacteria, primarily Pseudomonas and Serratia spp (Cheng et al 1999; Schaefera et al 2001; Robertson et al 2007). Pseudomonas is one of the few pathogens known to be capable of penetrating an intact corneal epithelium (Fleiszig et al 1998). CL-MK has been associated with other pathogens including acanthamoeba spp. and, rarely fungi. The incidence of reported fungal keratitis has been low compared to bacterial keratitis with the exception of occurrences in tropical climates and in surface or immune compromised individuals, especially those wearing extended wear or therapeutic bandage contact lenses.
In South Florida, which has historically been the epicenter of fungal keratitis in the US, a review of records from January 1982 and January 1992 found trauma to be the most common risk factor (44%) (Rosa et al 1994). Five cases were linked to extended wear contact lenses (4%) and one to a therapeutic bandage contact lens (<1%).
In a retrospective review of culture-positive fungal keratitis at the Singapore National Eye Center and Singapore General Hospital from January 1991 to December 1995, twenty-nine cases of culture-positive fungal keratitis were reported. More than half of the patients had a history of ocular trauma while a quarter had prior topical corticosteroid therapy. Only two patients were contact-lens wearers (Wong et al 1997).
Reports describing a dramatic rise in the incidence of contact lens-related fungal keratitis later found to be associated with Bausch and Lomb ReNu with MoistureLoc multipurpose solution began in 2005 and continued into 2006. Initial reports came from Hong Kong and Singapore. By February 2006, reports appeared in the Asian press. On March 8, 2006, the United States Centers for Disease Control (CDC) received a report of three patients with contact lens-associated Fusarium keratitis from David S. Chu, MD, a New Jersey ophthalmologist and cornea specialist (US CDC 2006a). The outbreak had come to the United States and was soon to become a global mini-epidemic. This paper documents the Fusarium outbreak with particular focus on the time line, epidemiology, mechanisms underlying disinfectant failure and the relevant pathophysiology of fungal infection in contact lens wearers.
Origins of the outbreak: Time line
Although ReNu® with MoistureLoc’s® proclivity for fungal growth was first discovered accidentally in January of 2005 (Epstein 2007) its clinical impact remained unrecognized for more than a year. The first signs of an outbreak were discovered in Hong Kong and then in Singapore. Ophthalmologists at the Hong Kong Hospital Authority initially voiced concern in July 2005 during a periodic review of emerging cases regarding “an unusually high yield of Fusarium from patients’ clinical specimens sent for culture” (Hong Kong Hospital Authority 2006). As a result, Hong Kong’s health department issued an alert in August 2005 based on reports of four patients in public hospitals with CL-MK in July and August. The public was warned to clean contact lenses carefully and to use appropriate hygiene. These cases were reported to Bausch & Lomb and apparently were investigated; however, a link between these emerging cases and B&L lens care products was not found. Several months later investigators in Singapore uncovered the relationship.
On January 27, 2006, Donald Tan, MD, the deputy director of Singapore’s National Eye Center (SNEC) sent a letter to the Singapore Ministry of Health (MOH) describing thirteen Fusarium cases in contact lens wearers all of whom used Bausch & Lomb’s ReNu with MoistureLoc multipurpose solution. As a result, the Singapore Ministry of Health issued a public alert regarding the “Increasing Incidence of Contact Lens Related Fungal Corneal Infections” on February 17, 2006. The alert detailed nineteen patients that had been treated at SNEC for culture positive fungal keratitis due to Fusarium since May 2005 and an additional three cases reported by Changi General Hospital.
As the numbers of infected patients in Singapore mounted, the MOH advised that “as a precautionary measure for contact lens users to discontinue using Bausch and Lomb’s ReNu multipurpose contact lens solution for the time being” on February 20, 2006 (Update 2). On February 21 (Update 3), the Singapore MOH announced that Bausch & Lomb was voluntarily suspending sales of ReNu multipurpose solutions. They further announced “In view of the potentially serious adverse visual consequences of fungal corneal infection, the Ministry of Health strongly advises all contact lens users as a precautionary measure to discontinue the use of B & L ReNu multipurpose contact lens solution for the time being, until the causes behind this recent increase in infections can be more clearly ascertained. B&L will advise consumers on what to do with existing stocks of their product.”
On April 12, 2006 in an Update on Contact Lens Related Fungal Infections, the Singapore MOH reported the following: “A comprehensive case-control study (comparing contact lens users with infection and contact lens users without corneal infection was undertaken in Feb–Mar 2006 to investigate risk factors for the spike in fungal corneal infection. The study found a strong association between corneal infection and the use of ReNu solution. This association remained strong even after taking into consideration sociodemographic, lens, hygiene and environmental factors. The findings are also consistent with recent observations made in the US and Hong Kong.”
Based largely on the quickly circulating report of New Jersey cornea specialist Dr. David Chu, the City of New York Department of Health and Mental hygiene issued 2006 Alert #6 on March 14, 2006, warning both public and professional communities about the association of Fusarium keratitis and soft contact lens wear.
After meeting with representatives of Bausch & Lomb, on March 30, 2006 officers of the American Optometric Association (AOA) and the AOA Contact Lens & Cornea Section met on March 31, 2006. An urgent response to the Fusarium outbreak was approved and on April 3, 2006, the AOA issued a press release and a clinical advisory regarding the Fusarium outbreak in the United States. Optometrists in the United States were advised to be vigilant and consider a fungal etiology when encountering CL-MK. The press quickly disseminated this information and on April 5, 2006, Good Morning America, a national morning television program televised a segment on the Fusarium outbreak.
On March 31, 2006, Bausch & Lomb issued their first press release discussing the Fusarium outbreak and their management of the situation. They described interaction with the US CDC and collaboration of the Johns Hopkins Wilmer Eye Institute and the CDC to implement a surveillance program at leading corneal treatment centers in the US. Top experts in corneal infectious disease as well as microbiologists specializing in fungi were retained to collect and culture clinical isolates of Fusarium from patient samples and identify the genotypes of retained specimens in an attempt to determine if they represented uncommon variants. Bausch & Lomb also announced their collaboration with health authorities in Singapore and Hong Kong in completing the aforementioned case control study. The remainder of the press release focused on poor patient compliance as a root cause of the outbreak and the results of extensive testing that confirmed that ReNu with MoistureLoc was sterile and effective. Bausch & Lomb again reminded contact lens wearers of the importance of proper lens care in an April 7, 2006 press release.
The public response from US government agencies followed soon after. On April 10, 2006, the US Centers for Disease Control and Prevention (CDC) and US Food and Drug Administration (FDA) issued public health alerts about the rise in reports in the US of CL-related Fusarium keratitis.
The CDC reported 109 cases of suspected fungal keratitis in seventeen different states. Of particular note was the geographic distribution, with many of the cases occurring in areas where fungal keratitis was rarely if ever clinically encountered. This included states in the Northeast and North Dakota. Complete data was available for thirty of the case patients as of April 10. Twenty-eight of the thirty case patients wore soft contact lenses, and twenty-six of those twenty-eight used different types of Bausch & Lomb ReNu brand contact lens solution in the month prior to the onset of infection. Eight patients required corneal transplantation (US CDC 2006a).
In a terse response to the initial CDC report Bausch and Lomb announced on April 10, 2006, that it was temporarily suspending U.S. shipments of ReNu® with MoistureLoc® lens care solutions produced in its Greenville, SC, manufacturing facility. This limited response to the worsening crisis was not well received by the press or the financial community with several critical articles and commentaries quickly appearing in the media. Subsequently, in a press release issued April 13, 2006, Bausch & Lomb requested that retailers remove US-manufactured ReNu with MoistureLoc from shelves and recommended that consumers switch to another solution pending further investigation. The company also placed ads in USA Today and regional newspapers on April 14 and 16 with Bausch & Lomb Chairman and CEO Ron Zarrella explaining the situation and providing guidance on alternatives.
In a May 5, 2006 update, the US CDC reported 102 confirmed cases, twelve possible cases and eighty-one cases still under investigation from thirty-one US states and territories (US CDC 2006b).
Of the fifty eight confirmed cases for which CDC had complete data:
56 wore contact lenses
32 reported using any B&L ReNu with MoistureLoc
15 reported using any B&L ReNu MultiPlus
7 reported using any unspecified B&L ReNu
3 reported using any AMO product
3 reported using any Alcon product
Some cases reported using more than one type of solution, therefore the solution categories are not mutually exclusive.
In a response to the CDC, dated May 3, 2006 (which was initially released on May 2), Bausch & Lomb offered the following: “These preliminary data require further examination and should be placed in proper context. Studies suggest that Fusarium keratitis occurs among contact lens wearers at a low rate. It would be expected that the distribution of lens care products associated with these cases would be roughly proportional to the products’ relative market share. In the small sample of cases CDC has analyzed to date, the 27-percent representation of ReNu® MultiPlus® solution is well below its approximate 40-percent market share. The 57-percent share of cases preliminarily reported for the company’s MoistureLoc formula is significantly and disproportionately higher than its U.S. market share of less than 10 percent, which represents approximately 2.3 million users. This disproportionate representation of the MoistureLoc formula in the CDC case reports is the reason Bausch & Lomb voluntarily withdrew MoistureLoc from the market while the investigation to determine the cause of these unusual infections continues.” It should be noted that no explanation was offered for the disproportionately low incidence of cases associated with other products, particularly Alcon Laboratory’s OptiFree products that were linked to only three cases (5%) despite having a market share comparable to ReNu MultiPlus, which was associated with 27% of cases.
A May 12, 2006, CDC press release detailed reports of 122 confirmed cases, fifteen possible cases and sixty cases still under investigation from thirty-three U.S. states and territories. Seventy-five reports included insufficient evidence to classify them as cases or carry other non-Fusarium diagnoses. States or territories with at least one confirmed or possible case included: Arkansas, Arizona, California, Connecticut, Florida, Georgia, Iowa, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Maryland, Michigan, Missouri, North Carolina, North Dakota, New Jersey, New York, Ohio, Oklahoma, Pennsylvania, Puerto Rico, Tennessee, Texas and Vermont. States where cases were currently under investigation included: Indiana, Minnesota, Mississippi, Nevada, Oregon, Rhode Island, and Virginia (US CDC 2006c).
With the evidence continuing to mount linking ReNu with MoistureLoc to the Fungal keratitis outbreak, Bausch & Lomb, in a press conference and subsequent press release dated May 15, 2006, announced a global voluntary recall of MoistureLoc. Ron Zarrella, B&L CEO was quoted, “After an extensive investigation involving thousands of tests, millions of dollars and collaboration with government agencies, health authorities and independent experts, there is no evidence of product contamination, tampering, counterfeiting or sterility failure. That leads us to conclude that some aspect of the MoistureLoc formula may be increasing the relative risk of Fusarium infection in unusual circumstances.”
In a final update, Fusarium Keratitis—United States, 2005–2006 published in the May 26, 2006 MMWR, the CDC reported 130 confirmed cases and announced its final determination (US CDC 2006d).
Among 125 contact lens wearers, 118 were able to identify which contact lens solution(s) they had used during the month before onset of infection. Seventy-five (64%) reported using Bausch & Lomb’s ReNu with MoistureLoc alone, fourteen (12%) reported using MoistureLoc in combination with another product, eight (7%) reported using an unspecified Bausch and Lomb solution, and twenty-one (18%) reported using only products other than MoistureLoc, from various manufacturers. Corneal transplantation was required in 31% of cases. The results of the CDC case-control investigation indicated increased risk for Fusarium keratitis associated with use of Bausch & Lomb’s ReNu with MoistureLoc.
The CDC data were subsequently updated and published in the Journal of the American Medical Association. As of June 30, 2006, 164 confirmed cases were identified in thirty-three states and one US territory. Corneal transplantation was required or planned in fifty-five patients (34%). Fusarium was not recovered from the factory, warehouse, solution filtrate, or unopened solution bottles; production of implicated lots was not clustered in time. Among thirty-nine isolates tested, at least ten different Fusarium species were identified, comprising nineteen unique multilocus genotypes (Chang et al 2006).
Summary reports from Singapore and the Untied States have been published. In Singapore from March 1, 2005, to May 31, 2006, sixty-six patients (sixty-eight affected eyes) were diagnosed with contact lens related Fusarium keratitis; the estimated annual national incidence was 2.35 cases per 10,000 contact lens wearers with more than 98% wearing soft, disposable lenses. Sixty-two patients (93.9%) reported using ReNu solution with forty-two patients (63.6%) specifically reporting use of ReNu with MoistureLoc. Most patients (81.8%) reported poor contact lens hygiene practices (Khor et al 2006).
Results of a subsequent case-control study evaluating risk factors for contact lens-related Fusarium keratitis in contact lens users in Singapore from March 2005 through May 2006 has been published. It included 61 patients with Fusarium keratitis and 188 population-based and 179 hospital-based control subjects. Patients with Fusarium keratitis were found to have been more likely to use Bausch & Lomb ReNu contact lens solutions than were either population-based or hospital-based control subjects. After controlling for age, sex, contact lens hygiene, and other factors, the use of ReNu with MoistureLoc significantly increased the risk of Fusarium keratitis. The risk associated with ReNu with MoistureLoc was 5 times higher compared to the still significant risk of using ReNu MultiPlus. The authors concluded that use of ReNu contact lens solutions significantly increased the risk of contact lens-related Fusarium keratitis in Singapore and called for further investigations into the role of ReNu MultiPlus in the development of Fusarium keratitis in other populations (Saw et al 2007).
In the United States, several groups reported on the outbreak. Bascom Palmer Eye Institute in Miami, Florida, reported treating ten cases of soft contact lens-associated keratomycosis between 1969 and 1992. Three cases of fungal keratitis associated with contact lenses were seen between 1969 and 1977, two between 1977 and 1982 and five between 1982 and 1992. In contrast, between January 2004 and April 2006, thirty-four cases at Bascom Palmer were attributed to Fusarium infection (Alfonso, Cantu-Dibildox, et al 2006). Fungal pathogens in CL-MK increased from 26.7% (eight of thirty) to 50% (eighteen of thirty-six) of isolates in 2005. Fusarium species (66 of 122, 54.1%) were the most frequent fungal pathogens (Alfonso, Miller, et al 2006). Fungal keratitis is rare in non-tropical or sub-tropical areas. Despite this well recognized climate-associated pattern, Fusarium CL-MK was reported in states including Pennsylvania, Ohio, and California even during the cold winter months (Bernal et al 2006; Cohen 2006; Jeng et al 2006).
Origins of the problem
A variety of possible etiologies were proposed and disseminated to professional groups and the press by Bausch & Lomb soon after the Fusarium outbreak was identified in the US. During a March 30, 2006 briefing on the status of the Fusarium investigation presented to the AOA and AOA Contact Lens & Cornea Section leadership by B&L Director of Medical Marketing Gary Orsborn, MS, OD, the following possible etiologies were suggested:
Poor patient hygiene and non-compliance with manufacturer or practitioner recommendations
Contamination of the manufacturing plant or some aspect of the manufacturing process
Contamination of bottles after leaving the factory either during transport or storage
The recent Asian tsunami and hurricane Katrina causing shifts in the ecosystem resulting in new, more virulent strains of Fusarium and possibly other fungi
Significant diversity of genotypes found in clinical isolates
Fusarium contamination of non-preserved fluoroquinolones drops used to treat CL-MK resulting in super-infection
Of these, poor patient compliance was highlighted as the most likely etiologic factor. Initial data from the Singapore Eye Research Institute found that more than 80% of infected patients reported poor hygienic practices. (Khor et al 2006). However, comparison to historical data suggests that patient non-compliance did not worsen significantly during the Fusarium outbreak, certainly not sufficiently to explain such a large scale occurrence. Claydon and Efron reported contact lens non-compliance in the use of recommended care and maintenance regimens in 40 to 91% of contact lens patients with many who were confused or ignorant about their behavior (Claydon and Efron 1994). Other researchers reported similar findings (Sokol et al 1990).
Non-compliance as a primary factor was made even more unlikely by genetic analysis of clinical isolates, which revealed a diverse array of Fusarium solani genotypes. The broad genetic diversity and the fact that a preponderance of phylogenetic species are viewed as commonly found and associated with sinks and drains suggest the source of inoculation in these cases of keratitis was most likely from strains resident in the water systems of the patients’ homes (Levy et al 2006). Simple logic would suggest that non-hygienic patients who failed to wash their hands prior to handling lenses would be less likely to encounter and transfer a fungal inoculum to the lens, case or eye than a compliant patient exposed to sinks and drains during hand washing.
Possible contamination of the manufacturing facility was considered. Extensive investigation by both the US FDA and B&L found no point source of contamination. Likewise, no problems were uncovered with the packaging or transport of the product. The genetic diversity of isolates weighed heavily against this theory throughout the investigation. No contamination was found in any bottles, and all tested product was found stable and effective. This additional information confirmed that the outbreak was due to disinfection failure occurring during product use by patients.
The issue of climatic change was considered by several people including Bausch and Lomb’s CEO, Ronald Zarrella. He was quoted in a Washington Times article published April 13, 2006 “There’s been theories all the way from, ‘Has the tsunami ... hurricanes and the effect of environmental factors created mold levels that are unprecedented?”
More recently, Iyer, and coworkers from the University of Florida at Gainesville published a retrospective review of fungal keratitis at their institution from 1999 through June 2006 (Iyer et al 2006). Their results suggest that, at least at their institution, contact lenses represent a major risk factor for fungal keratitis; furthermore, the incidence of contact lens–related fungal keratitis was increasing even before the Fusarium outbreak in 2005 and 2006.
Fungal contamination of non-BAK preserved antibiotic bottles has been anecdotally described in scientific posters and the ophthalmic press, but the clinical relevance has never been established (Mack 2006; Ashford 2006). Other studies suggest that microbial contamination of the contents of an antibiotic bottle is unlikely.
Cultures of bottles used by individual patients and in a multidose office or hospital setting have demonstrated no clinically significant contamination (Mason et al 2005). Fungi are saprophytes that derive nutrition from organic matter. The environment within an antibiotic bottle does not support fungal growth. Additional evidence that fluoroquinolones did not foster the Fusarium outbreak is the reported efficacy of commercial non-preserved moxifloxacin 0.5% (Vigamox, Alcon Labs, Ft. Worth, TX) against culture positive Fusarium cases (Munir et al 2006).
While climatic change may be influencing shifts in the prevalence and severity of mycotic disease, the contribution of climatic shift in the Fusarium keratitis outbreak is likely small. Other factors, specifically solution formulation issues and inadequate testing, are more likely key contributors to this epidemic (US FDA 2006).
Current disinfectant standards for contact lens care products
In 1997, the US Food and Drug Administration adopted an updated standard for contact lens care product disinfection. A 172-page guidance document containing specific direction for still current “stand-alone” disinfection approval was disseminated (US FDA 1997). Subsequently, the International Organization for Standardization (ISO) developed and promulgated a newer standard, which was designed to harmonize numerous differing country-specific standards (Rosenthal, Sutton, et al 2002). The ISO 14729 procedure was introduced in 2001 (ISO 2001).
The current ISO 14729 standard consists of two parts. A “Stand Alone” test which evaluates the disinfecting solution’s innate antimicrobial activity. Upon passing this test, the product is exempted from additional testing using the “Regimen Test”. The Stand Alone test assumes that in conjunction with rubbing and rinsing steps, the disinfectant will be capable of passing a more stringent regimen criteria. The Regimen Test evaluates the antimicrobial efficacy of the entire regimen as described in the package insert (e.g., rubbing, rinsing, and disinfecting).
The US FDA had come under harsh criticism during the Fusarium outbreak for maintaining insufficiently robust standards and failing to revise them despite significant advances in contact lens materials and lens care products. However, current ISO standards were actually exceeded at the time of the outbreak.
Evidence had been accumulating for several years regarding the interaction between disinfecting solutions and lens materials. Loss of disinfecting efficacy after lenses were soaked in biguanide disinfecting products for various periods of time has been demonstrated in several studies (Rosenthal, Henry, et al 2002; Dannelley et al 2004). These reports were largely ignored. Current FDA (ISO) stand-alone testing protocols are in-vitro tests and do not incorporate contact lenses.
Etiology of the fusarium outbreak
Postulating a mechanism that explains how a multipurpose contact lens disinfection solution could become a key element in a world wide outbreak of fungal keratitis is a daunting task. All modern contact lens solutions, including ReNu with MoistureLoc must pass relatively stringent testing that challenges effectiveness against a variety of potential pathogens – including Fusarium solani.
In January 2005, newly introduced Bausch and Lomb ReNu with MoistureLoc was subjected to a small pilot study exploring its compatibility with silicone hydrogel and conventional hydrogel lens materials (Epstein 2007). New unworn lenses were placed directly in conventional soft lens flat-pack cases containing B&L ReNu with Moisture-Loc. When the lenses were checked approximately a week later, several were covered with what appeared to be fungus. This was an unanticipated finding. In the northeast United States, fungal contamination of contact lenses is rare and usually is observed as small isolated focal fungal colonies invading the matrix of the lens. However, these lenses were extensively coated by fungal growth. Consultation with several knowledgeable colleagues suggested that this was of no clinical significance and the lenses were placed in storage and forgotten about. The experiment was recalled and the lenses recovered and cultured in May 2006. Several organisms were found including Fusarium solani. The experiment was repeated and several generations of lenses with significant fungal growth were produced using the original solution as an inoculum (Epstein 2007). This observation has been tested in several labs and reproduced. Publication is pending.
Previous research has shown how biocides can absorb into contact lens materials resulting in the solution losing disinfectant efficacy (ISO 2001; Rosenthal, Sutton, et al 2002). Recently, the contact lens uptake characteristics and fungicidal activity of alexidine, a small molecular weight monomeric biguanide in ReNu with MoistureLoc, has been compared to polyhexamethylene biguanide (PHMB) in Bausch & Lomb ReNu MultiPlus and AMO Complete MoisturePLUS and POLYQUAD (polyquaternium-1) and ALDOX (myristamidopropyl dimethylamine) used in OPTI-FREE RepleniSH (Rosenthal et al 2006).
Approximately 30% to 60% of the PHMB and alexidine were depleted from the solution within six hours. Diminished antimicrobial activity corresponded to decreases in disinfectant concentration throughout the course of the evaluation. The polyquaternium-1 based solution maintained biocide concentration and consequently retained nearly 100% of its fungicidal activity.
Of note, the diminishing alexidine concentration of ReNu with MoistureLoc was reflected in reduced antimicrobial efficacy. ReNu with MoistureLoc showed an average 37% less fungicidal activity and an average of 54% less alexidine after a 6-hour soak period and showed an average 84% less activity and an average 88% less Alexidine biocide present in the 7-day old used solution. ReNu MultiPlus showed an average 71% less fungicidal activity with an average 19% less PHMB after six hours and 94% less fungicidal activity with an average drop of 50% biocide after seven-days.
Loss of disinfection efficacy during the disinfection process appears the most likely initiator of sustained Fusarium contamination of the solution, lens and case. As biocide is lost through contact lens material absorption, disinfectant efficacy is reduced and the kill curve rapidly diminishes. Fusarium solani is a relatively hearty organism and may survive longer than most other pathogens that may be inadvertently inoculated by the patient during lens handling. Despite the activity of alexidine against Fusarium, clearly some organisms survive the initial high concentration of the biocide and persist while disinfectant levels fall to levels that are more hospitable to the organism. Fusarium strains have shown several properties that aid in their survival and may account for their selection (Zhang et al 2006). This is likely exacerbated by non-compliant reuse of the solution with “topping off” since the lens has been shown to absorb disinfectant (Rosenthal et al 2006). However, the mere survival of Fusarium organisms does not account for the level of infection observed.
The FDA/ISO disinfection standard represents a time kill curve. Product passage requires sufficient kill within a stipulated time. The contribution of possible organism growth was not considered when the standards were promulgated. However, advances in lens care design as well as competitive forces prompted Bausch & Lomb and other companies to formulate products with additives that boost moisture retention to improve patient comfort. The MoistureLoc formula contains polyquaternium-10, an organic compound commonly used in hair-care products. Polyquaternium-10 is a weakly cationic cellulose derivative. Its charge facilitates adherence to the lens surface for sustained wettablity. Its cellulose content serves as a nutritive media that may facilitate fungal growth especially as disinfectant levels fall to sub-lethal levels. This accounts at least in part for the observed pattern of fungal growth on lenses. The persistence of Fusarium during the decaying disinfection cycle supports the concept that ReNu with MoistureLoc may function as a selective culture media.
Research performed by B&L has shown how the accumulated polymer in MoistureLoc can serve to isolate and protect fungus. Polymer coating the lens surface, as it was designed to do, would serve to shield fungal organisms from high levels of disinfectant likely present at the surface of the lens. It would thus facilitate growth (Levy et al 1990). As disinfectant levels decline, the growth curve would overtake and exceed the kill curve of the disinfectant. This interaction has never been observed with contact lens care products as most predicate products lacked nutritive properties and disinfectant levels were assumed to remain static; however, these principals derive from current pharmacokinetic-pharmacodynamic models of in vivo antibiotic function (Mueller et al 2004). Furthermore, they offer one of the few logical explanations for the intensity and geographic diversity of this epidemic.
The relation between corneal staining (barrier disruption) and infection
Humans have been exposed to microbial pathogens since the origin of the species. As a result, a variety of effective defenses to bacterial, viral, fungal protozoan, and parasitic infection have evolved. This is especially true of the ocular surface, which is habitually in direct contact with the environment. Defenses against microbial infection include: rapid epithelial healing, tight junctions, and brisk cell turn over. These defenses are so effective that only five bacteria are capable of invading an intact cornea: Neisseria gonorrhoeae, Corynebacterium diphtheriae, Listeria, Haemophilus aegyptius, and invasive strains of Pseudomonas aeruginosa (Fleiszig et all 1998).
Physical epithelial barriers comprise the first line of defense against infection. Penetration of these innate natural host barriers is therefore a key prerequisite for microbial infection. This is especially true of fungi. Although host defenses are complex and interwoven, intact epithelium serves as a primary defense against infection. This concept is so fundamental to infectious disease that it is unquestioned. The importance of the epithelium in protection against infection is obvious when the barrier is breached, as in wounds and burns, where infection is an obvious major cause of mortality and morbidity (Janeway et al 2001). Disruption of physiologic epithelial barriers increases the risk of infection.
As discussed previously, fungal keratitis is relatively rare, especially outside of tropical and subtropical areas. Because fungi do not typically invade intact cornea, trauma, usually caused by plants or vegetable matter is considered the most common cause of fungal keratitis (Liesegang and Forster 1980). Ocular and systemic disease and prior corticosteroid treatment are additional risk factors (Thomas 2003). In developing an animal model of Fusarium keratitis, Forster and Rebell found it necessary to scarify the cornea and pretreat with subconjunctival corticosteroids to obtain sustained culture-positive ulcers in high percentage of eyes (Forster and Rebell 1975).
A review of the clinical and microbiology records of the New York Eye and Infirmary identified sixty-one cases of fungal keratitis in fifty-seven patients between January 1, 1987 and June 1, 2003, a sixteen-year period. Of 5083 positive corneal cultures, sixty-one eyes in fifty-seven patients were positive for fungus representing 1.2% of cases. Human immunodeficiency virus (HIV) seropositivity, chronic ocular surface disease, and trauma were the most commonly associated risk factors (Ritterband et al 2006).
Contact lens associated fungal keratitis is rarely encountered, even in subtropical climates. In a 10 year review of records from January 1982 to January 1992 at South Florida’s Bascom Palmer Eye Institute only 4% of fungal keratitis cases were associated with contact lens wear (Rosa et al 1994). Fungal infection in cosmetic contact lens wearers is rare, again representing only 4% of overall fungal keratitis cases in another large series (Wilhelmus et al 1988). Other estimates are as low as 1% of all cases of CL-MK (Rosenthal et al 2006).
Fluorescein staining (staining) of the ocular surface reveals epithelial trauma and barrier disruption. Staining is typically observed after instilling sodium fluorescein solution or an alternative vital dye and examining the eye with a biomicroscope using special lighting and, in some cases, filtering techniques. Low grade corneal staining has been reported to occur spontaneously in successful contact lens wearers and non-contact lens wearers (Schwallie et al 1997; Jalbert et al 1999). Corneal staining may be asymptomatic even in severe cases. As a result, poor correlation exists between observed staining and patient symptoms (Jones et al 2002; Garofalo et al 2005; Epstein 2006). Regardless of cause or extent, staining represents a breakdown of normally protective barriers and logically increases the risk of infection and inflammation (Carnt et al 2007).
Over the past few years, numerous reports describing corneal staining in contact lens wearers using different combinations of multipurpose solutions (MPS) and contact lens materials have been published, especially with the biguanide disinfectants such as Bausch and Lomb ReNu MultiPlus (Epstein 2002; Jones et al 2002; Pritchard et al 2003; Lebow and Schachet 2003; Andrasko et al 2006). Bausch and Lomb ReNu with MoistureLoc has also been associated with significant corneal staining in several investigations (Townsend et al 2005; Lebow and Schachet 2006).
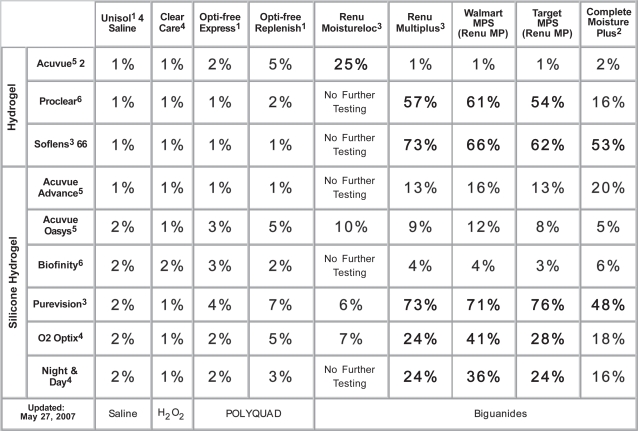
The research of Andrasko and associates has been of particular value in understanding contact lens material and MPS product interactions. This series of well-constructed double masked (ongoing) studies has already evaluated more than fifty different combinations of contact lenses and lens care products. Average corneal staining area is measured and reported. This data is charted on a color-coded grid for ease of interpretation (http://www.staininggrid.com – Figure 1). Results confirm that staining varies substantially depending upon the MPS and lens combination tested.
Figure 1.
Staining grid (Used by permission of Gary Andrasko, OD).
Biguanide-based MPS showed higher levels of corneal disruption when paired with silicone hydrogel lenses. In particular, the combination of B&L's Purevision (Balafilcon A) lens and ReNu MultiPlus (PHMB) MPS produced the highest levels of average corneal staining area – 73%. This finding is consistent with prior reports (Epstein 2002; Jones et al 2002; Lebow and Schachet 2003).
B&L ReNu with MoistureLoc in combination with the conventional hydrogel Vistakon Acuvue 2 had relatively high levels of corneal staining – 25%. Although the CDC did not specifically report on the relation of lens type and infection rate, personal communication with clinicians at Bascom Palmer Eye Institute in Miami, FL and the Singapore National Eye Institute in Singapore suggested the majority of contact lens-related Fusarium infections were in patients wearing Acuvue 2 or similar hydrophilic materials.
The risk of developing microbial keratitis depends upon several factors including: the extent of epithelial barrier disruption, the magnitude of the infectious inoculum (introduced or present) within the ocular environment, the virulence of the organism and finally, the effectiveness of the host inflammatory response (Lawin-Brussel et al 1993). The larger the surface area of epithelial compromise, the greater the probability that an organism (or multiple organisms) will gain entry into damaged areas of epithelium or corneal stroma and cause infection. Likewise, the larger the number of potentially infectious organisms present, the greater the probability of infection. Increased virulence of a pathogen will increase the chances of infection while effective humeral defenses will decrease the odds of infection.
The presence of a contact lens may play a role in infection by interfering with the normal ocular immune response. Since the cornea is avascular, polymorphonuclear leukocytes (PMN) must migrate from the conjunctival circulation to the site of infection. This acute inflammatory response was blocked by a contact lens in a rabbit model of Pseudomonas keratitis (Lawin-Brussel et al 1995). In that regard, a contact lens may function as a mechanical anti-inflammatory agent. The presence of a contact lens may have been a contributory factor in the ReNu with MoistureLoc outbreak.
Conclusion
The Fusarium outbreak was a virtually unprecedented occurrence in health care. Nearly 200 cases of Fusarium keratitis were reported to the US CDC, sixty-six cases were reported in Singapore and others occurred in Malaysia, China, India and several European countries. The full extent of the outbreak may never be known.
Some question if Bausch and Lomb waited too long before issuing a global recall of ReNu with MoistureLoc, worsening an already bad situation. Despite the convincing evidence of a link between the Fusarium outbreak and ReNu with MoistureLoc established in Singapore and mounting evidence in the US, B&L initially only stopped production and distribution, not sales or use of the product. Several days later, a limited recall was initiated in the US and patients and professionals were advised to discontinue using MoistureLoc. It wasn’t until weeks later that a global recall was issued. It appears that there was dissention within B&L during this period. Angela J. Panzarella, B&L vice president for global vision care, was quoted: “I wish we had made the decision the first day not to sell or use it.” (Feder 2006).
Although many of the facts of this outbreak will ultimately become clearer with time and additional investigation, there remains a great opportunity to learn from this experience.
In analyzing the specific factors that contributed to widespread Fusarium infection, comparison to normal trends of microbial keratitis is essential. Normal “background” CL-MK has been estimated to range from approximately 4 in 10,000 for daily wear to 20 in 10,000 for extended-wear (Schein et al 1989; Cheng et al 1999). At the height of the Fusarium epidemic in Singapore the estimated annual national incidence was 2.35 cases per 10,000 wearers (Khor et al 2006).
Considering that fungal keratitis normally manifests as 1 in every 5 cases of microbial keratitis in Singapore and contact lens related keratitis is generally a small fraction of that (approximately 6%), it is obvious that the numbers reported in Singapore are many times greater than expected (Wong et al 1997; Houang et al 2001). It is likely that analysis of annualized infection incidence during the Fusarium outbreak would yield similar numbers for the US and other areas (Alfonso, Cantu-Dibildox et al 2006).
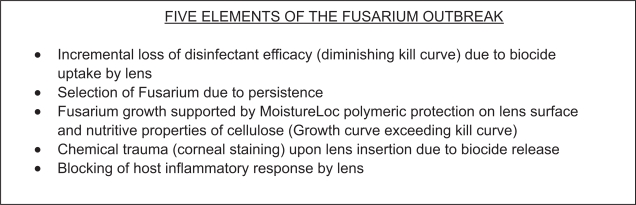
While some of the core elements of the infectious outbreak such as biocide uptake, loss of disinfection efficacy, and some of the effects of the polymeric additives have already been investigated and reported, some critically important pieces of this puzzle remain unconfirmed. Five key elements of Fusarium infection associated with ReNu with MoistureLoc are depicted in Figure 2. Corneal staining likely plays an important role in the genesis of infection and deserves further investigation.
Figure 2.
Key elements of Fusarium infection.
Of sixty-six patients infected in Singapore, sixty-two reported using B&L ReNu solutions. Of these, 63.6% reported using ReNu with MoistureLoc and the remaining 30.3% appear to have been using B&L ReNu MultiPlus. In the CDC data, 69% of patients used ReNu with MoistureLoc while 18% used ReNu MultiPlus. Even though this was below the expected usage rate based on market share, it still represents a disproportionate number compared to other brands.
These data when viewed in the context of the high levels of corneal staining reported with biguanide based MPS like ReNu MultiPlus, the recent data of Rosenthal and coworkers regarding loss of disinfectant efficacy with ReNu MultiPlus and MoistureLoc and the report of Iyer et al regarding the ongoing increase in fungal CL-MK paints a disturbing picture; one that may be only the tip of the iceberg (Dannelley et al 2004; Iyer et al 2006; Mack 2006).
60 million individuals throughout the world wear contact lenses. While the risk of infection associated with contact lenses is generally perceived as small, perhaps a more appropriate perspective is that infection rates may in reality be needlessly high, especially if we can use this experience to identify specific risk factors and learn to modify them. During the Fusarium outbreak, many patients experienced intense pain, lost their sight or even their eyes. For these patients and for the millions of other contact lens wearers, it is critical that a thorough and comprehensive investigation be conducted. It is hoped that this paper will serve as a starting point and as a result contact lenses will continue to evolve into an ever safer and more effective form of vision correction.
References
- Alfonso EC, Cantu-Dibildox J, Munir WM, et al. Insurgence of Fusarium keratitis associated with contact lens wear. Arch Ophthalmol. 2006;124:941–7. doi: 10.1001/archopht.124.7.ecs60039. [DOI] [PubMed] [Google Scholar]
- Alfonso EC, Miller D, Cantu-Dibildox J, et al. Fungal keratitis associated with non-therapeutic soft contact lenses. Am J Ophthalmol. 2006;42:154–5. doi: 10.1016/j.ajo.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Andrasko GJ, Ryen KA, Garofalo RJ, et al. 2006. Compatibility of silicone hydrogel lenses with multipurpose solutions ARVO 2006 Poster 2392/B44, Association for Research in Vision and Ophthalmology, May 2, 2006, Ft. Lauderdale, FL.
- Ashford WC. Refining Patient and Dosage Management, Ophthalmology Management. 2006. Nov,
- Bernal MD, Acharya NR, Lietman TM, et al. Outbreak of Fusarium keratitis in soft contact lens wearers in San Francisco. Arch Ophthalmol. 2006;124:1051–3. doi: 10.1001/archopht.124.7.ecr60006. [DOI] [PubMed] [Google Scholar]
- Carnt N, Jalbert I, Stretton S, et al. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci. 2007;84:309–15. doi: 10.1097/OPX.0b013e318046551b. [DOI] [PubMed] [Google Scholar]
- Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–63. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contactlens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–5. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Claydon BE, Efron N. Non-compliance in contact lens wear. Ophthalmic Physiol Opt. 1994;14:356–64. [PubMed] [Google Scholar]
- Cohen EJ. Fusarium keratitis associated with soft contact lens wear. Arch Ophthalmol. 2006;124:1183–4. doi: 10.1001/archopht.124.8.1183. [DOI] [PubMed] [Google Scholar]
- Dannelley KH, Waworuntu RV. Effectiveness of contact lens disinfectants after lens storage. Eye Contact Lens. 2004;30:163–5. doi: 10.1097/01.icl.0000133562.84179.5d. [DOI] [PubMed] [Google Scholar]
- Epstein AB. SPK with daily wear of silicone hydrogel lenses and MPS. Contact Lens Spectrum. 2002;17:30. [Google Scholar]
- Epstein AB. Contact lens care products effect on corneal sensitivity and patient comfort. Eye Contact Lens. 2006;32:128–32. doi: 10.1097/01.icl.0000178850.55802.1c. [DOI] [PubMed] [Google Scholar]
- Epstein AB. Recent Fusarium outbreak. Eye Contact Lens. 2007;33:55–6. doi: 10.1097/ICL.0b013e31802e6236. [DOI] [PubMed] [Google Scholar]
- Feder BJ. WORLD: from asia to america, how Bausch’s crisis grew. The New York Times. 2006. May 18,
- Fleiszig SM, Lee EJ, Wu C, et al. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 1998;24:41–7. [PubMed] [Google Scholar]
- Forster RK, Rebell G. Animal model of Fusarium solani keratitis. Am J Ophthalmol. 1975;79:510–15. doi: 10.1016/0002-9394(75)90629-7. [DOI] [PubMed] [Google Scholar]
- Garofalo RJ, Dassanayake N, Carey C, et al. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens. 2005;31:166–74. doi: 10.1097/01.icl.0000152489.99455.db. [DOI] [PubMed] [Google Scholar]
- Hong Kong Hospital Authority May202006. Accessed 26 August 2007. URL: http://www.newsinferno.com/archives/1226
- Houang E, Lam D, Fan D, et al. Microbial keratitis in Hong Kong: relationship to climate, environment and contactlens disinfection. Trans R Soc Trop Med Hyg. 2001;95:361–7. doi: 10.1016/s0035-9203(01)90180-4. [DOI] [PubMed] [Google Scholar]
- ISO ISO 14729. Ophthalmic optics – contact lens care products – microbiological requirements and test methods for products and regimens for hygienic management of contact lenses 2001.
- Iyer SA, Tuli SS, Wagoner RC. Fungal keratitis: emerging trends and treatment outcomes. Eye and Contact Lens. 2006;32:267–71. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- Jalbert I, Sweeney DF, Holden BA. The characteristics of corneal staining in successful daily and extended disposable contact lens wearers. Clin Exp Optom. 1999;82:4–10. doi: 10.1111/j.1444-0938.1999.tb06778.x. [DOI] [PubMed] [Google Scholar]
- Janeway C, Traver P, Walport M, et al. Immunobiology: the immune system in health and disease. 6th edn. Oxford: Garland Sciences; 2001. [Google Scholar]
- Jeng BH, Langston RHS, Hall GS, et al. A cluster of soft contact lens-related Fusarium keratitis. Poster 450 presented at the American Academy of Ophthalmology Meeting; 10–13 November; Las Vegas, NV, USA. 2006. [Google Scholar]
- Jones L, MacDougall N, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002;79:753–61. doi: 10.1097/00006324-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Khor WB, Aung T, Saw SM, et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–73. doi: 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- Lawin-Brussel CA, Refojo MF, Leong FL, et al. Effect of Pseudomonas aeruginosa concentration in experimental contact lens-related microbial keratitis. Cornea. 1993;12:10–18. doi: 10.1097/00003226-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Lawin-Brussel CA, Refojo MF, Leong FL, et al. Scanning electron microscopy of the early host inflammatory response in experimental Pseudomonas keratitis and contact lens wear. Cornea. 1995;14:355–9. doi: 10.1097/00003226-199507000-00002. [DOI] [PubMed] [Google Scholar]
- Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multipurpose solutions and two brands of soft contact lenses. Eye Contact Lens. 2003;29:213–20. doi: 10.1097/01.icl.0000081601.75812.03. [DOI] [PubMed] [Google Scholar]
- Lebow KA, Schachet JL.2006. Differences in clinical performance of multipurpose solutions with a silicone hydrogel lens ARVO 2006. Poster 99/B387, Association for Research in Vision and Ophthalmology, 30 April, Ft. Lauderdale, FL, USA.
- Levy B, Heiler D, Norton S. Report on testing from an investigation of Fusarium keratitis in contact lens wearers. Eye and Contact Lens. 2006;32:256–61. doi: 10.1097/01.icl.0000245556.46738.14. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- Mack R. A case of recalcitrant Fusarium solani keratitis leading to therapeutic penetrating keratoplasty in a patient with Fusarium solani contamination of a bottle of moxifloxacin 0.5% ophthalmic solution. Presented at: The American Society of Cataract and Refractive Surgery Annual Meeting; 17–22 March; San Francisco, CA, USA. 2006. [Google Scholar]
- Mason BL, Alfonso EC, Miller D. In-use study of potential bacterial contamination of ophthalmic moxifloxacin. J Cataract Refract Surg. 2005;31:1773–6. doi: 10.1016/j.jcrs.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Mueller M, de la Peña A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother. 2004;48:369–77. doi: 10.1128/AAC.48.2.369-377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir WM, Rosenfeld SI, Udell IJ, et al. Clinical response of contact lens associated fungal keratitis on topical fluoroquinolone therapy. Presented at the Ocular Microbiology and Immunology Group, 2006 40th Annual Meeting; 10 Nov; Las Vegas, NV, USA. 2006. [Google Scholar]
- Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–83. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- Pritchard N, Young G, Coleman S, et al. Subjective and objective measures of corneal staining related to multipurpose care systems. Cont Lens Anterior Eye. 2003;26:3–9. doi: 10.1016/S1367-0484(02)00083-8. [DOI] [PubMed] [Google Scholar]
- Ritterband DC, Seedor JA, Shah MK, et al. Fungal keratitis at the New York Eye and Ear Infirmary. Cornea. 2006;25:264–7. doi: 10.1097/01.ico.0000177423.77648.8d. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Petroll WM, Jester JV, et al. Current concepts: contact lens related Pseudomonas keratitis. Cont Lens Anterior Eye. 2007;30:94–107. doi: 10.1016/j.clae.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101:1005–13. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal RA, Dassanayake NL, Schlitzer RL, et al. Biocide uptake in contact lenses and loss of fungicidal activity during storage of contact lenses. Eye and Contact Lens. 2006;32:262–6. doi: 10.1097/ICL.0b013e31802b413f. [DOI] [PubMed] [Google Scholar]
- Rosenthal RA, Henry CL, Buck SL, et al. Extreme testing of contact lens disinfecting products. Contact Lens Spectrum. 2002;17:40–5. [Google Scholar]
- Rosenthal RA, Sutton SV, Schlech BA. Review of standard for evaluating the effectiveness of contact lens disinfectants. PDA J Pharm Sci Technol. 2002;56:37–50. [PubMed] [Google Scholar]
- Saw SM, Ooi PL, Tan DT, et al. Risk factors for contact lens-related Fusarium keratitis: a case-control study in Singapore. Arch Ophthalmol. 2007;125:611–17. doi: 10.1001/archopht.125.5.611. [DOI] [PubMed] [Google Scholar]
- Schaefera F, Bruttinb O, Zografosa L, et al. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85:842–7. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989;321:773–8. doi: 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- Schein OD, McNally JJ, Katz J, et al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112:2172–9. doi: 10.1016/j.ophtha.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Schwallie JD, Mckenney CD, Long WD, Jr, et al. Corneal staining patterns in normal non-contact lens wearers. Optom Vis Sci. 1997;74:92–8. doi: 10.1097/00006324-199702000-00020. [DOI] [PubMed] [Google Scholar]
- Sokol JL, Mier MG, Bloom S, et al. A study of patient compliance in a contact lens-wearing population. CLAO J. 1990;16:209–13. [PubMed] [Google Scholar]
- Thomas PA. Fungal infections of the cornea. Eye. 2003;17:852–62. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- Townsend W, Katims S, Rosen J. Investigating a new-generation multipurpose solution. Contact Lens Spectrum. 2005 Dec [Google Scholar]
- [US CDC] US Centers for Disease Control and Prevention Fusarium keratitis – multiple states. MMWR Morb Mortal Wkly Rep. 2006a;55:400–1. [PubMed] [Google Scholar]
- [US CDC] US Centers for Disease Control and Prevention 2006b. Press release – 5 May, 2006, Atlanta, GA.
- [US CDC] US Centers for Disease Control and Prevention 2006c. Press release – 12 May, 2006, Atlanta, GA.
- [US CDC] Centers for Disease Control and Prevention Update: Fusarium keratitis – United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006d;55:563–4. [PubMed] [Google Scholar]
- [US FDA] Food and Drug Administration 1997Guidance for industry – premarket notification (510(k)) guidance document for contact lens care productsAccessed 5 June 2007. URL: http://www.fda.gov/cdrh/ode/contlens.pdf
- [US FDA] Food and Drug Administration 2006. Warning letter (07-ATL-01) October 31, 2006. Accessed 5 June 2007. URL: http://www.fda.gov/foi/warning_letters/g6107d.htm [Google Scholar]
- Weissman BA, Mondino BJ. Risk factors for contact lens associated microbial keratitis. Cont Lens Anterior Eye. 2002;25:3–9. doi: 10.1016/s1367-0484(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Wilhelmus KR, Robinson NM, Font RA, et al. Fungal keratitis in contact lens wearers. Am J Ophthalmol. 1988;106:708–14. doi: 10.1016/0002-9394(88)90705-2. [DOI] [PubMed] [Google Scholar]
- Wong TY, Fong KS, Tan DT. Clinical and microbial spectrum of fungal keratitis in Singapore: a 5-year retrospective study. International Ophthalmology. 1997;21:127–30. doi: 10.1023/a:1026462631716. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ahearn DG, Noble-Wang JA, et al. Growth and survival of Fusarium solani–F. oxysporum complex on stressed multipurpose contact lens care solution films on plastic surfaces in situ and in vitro. Cornea. 2006;25:1210–16. doi: 10.1097/ICO.0b013e31802dd3a4. [DOI] [PubMed] [Google Scholar]