Abstract
Aim:
Evaluation of the diagnostic contribution of color duplex sonography of the temporal, carotid and vertebral arteries and doppler sonography of the periorbital arteries in patients with and without giant cell arteries (GCA) particularly to distinguish between arteritic and nonarteritic neuro-ophthalmological vascular complications (NOC).
Methods:
In a prospective study ultrasonographic findings in 85 GCA patients without NOC and 47 GCA patients with NOC were compared to those of 33 non GCA patients with NOC. Concentric hypoechogenic mural thickening (a so called halo) was considered as a GCA typical ultrasonographic finding. Absent or retrograde signals not corresponding to carotid occlusive disease were classified as a GCA typical doppler sonographic finding of the periorbital arteries.
Results:
GCA patients with NOC had significantly higher rates of abnormal ultrasonographic findings of the temporal (81% vs 62%) and periorbital arteries (32% vs 5%) than GCA patients without NOC. In patients with other diagnoses and NOC halos were found in 9%, whereas halos and stenosis and GCA typical findings of the periorbital arteries were absent.
Conclusion:
Typical ultrasonographic findings of the craniocervical arteries help to distinguish between arteritic and nonarteritic NOC. In patients with GCA typical ultrasonographic findings in at least 2 different arteries biopsy taking seems not obligatory.
Keywords: ultrasonography, giant cell arteritis, neuroophthalmological complications
Introduction
Giant cell arteritis (GCA) is a systemic vasculitis with a particular affinity to the superficial temporal artery (STA) and the extraocular parts of the central retinal, posterior ciliary and ophthalmic artery. Less common is the involvement of other branches of external carotid artery, the axillary artery, the internal carotid, the vertebral and coronary arteries and the aorta (Wilkinson and Russell 1972).
GCA almost exclusively affects individuals older than 50 years of age and two thirds are women. Disease susceptibility has been associated with European descent. Prompt diagnosis and treatment are preconditions for the prevention of serious vascular complications, particularly visual loss. Up to now temporal artery biopsy is the gold standard for the diagnosis of GCA (Weyand and Gorenzy 2003).
Temporal artery biopsy is generally well tolerated with a complication rate in the range of 0.5% including facial nerve damage, infection, skin necrosis and ischemic stroke due to interruption of collateral flow (Ikard 1988).
More important biopsy results may be false negative in 9%–31% of patients with the clinical or autopsy diagnosis of GCA due to the segmental character of the vasculitis and pretreatment with steroids (Hall et al 1983; Nesher et al 2002; Salvarani et al 2002; Niederkohr and Levin 2007). Moreover in a considerable number of patients biopsies are unavailable due to several reasons like refusion of biopsy, collateral flow and others. The American College of Rheumatology (ACR) has proposed diagnostic criteria based on history, physical examination, and laboratory and biopsy findings (Hunder et al 1990). However these criteria are mainly research tools requiring exclusion of other diseases. They also have limitations in atypical manifestations of the disease (Karassa et al 2005).
Ultrasound (US) has been introduced as a diagnostic tool in patients suspected to suffer from GCA about 30 years ago (Brunholzl and Müller 1988). Initial studies used continous wave dopplersonography (CWDS) for the detection of stenoses and occlusions of the large arteries branching from the aorta and medium sized arteries like the STA and the occipital arteries but also for the exclusion of collateral flow and occlusion of the periorbital arteries (= Aa. supratrochleares, PA). Whereas CWDS of the PA has retained its diagnostic value CWDS of the STA has been replaced by high resolution color duplexsonography (CDS). CDS has greatly improved the non-invasive full length visualization of arterial wall abnormalities in medium sized arteries (Schmidt et al 1997). Several studies have demonstrated a hypoechogenic concentric thickening of the arterial wall, the so-called halo, as a typical finding in patients with different manifestations of GCA (Karassa et al 2005; Pfadenhauer and Weber 2003; Schmidt et al 1997). Halos and associated stenoses were found in the STA as well as in large arteries branching from the proximal aorta and were considered to be caused by inflammatory arterial wall edema (Schmidt et al 2002). A recently published metaanalysis including 2036 patients from 23 studies compared ultrasonography findings of the STA with biopsy results and diagnosis based on the ACR criteria. Using halo, stenosis and occlusion as ultrasound criteria sensitivity was found as high as 0.88 compared to biopsy and 0.87 compared to ACR criteria. Specificity was 0.78 and 0.96 (Karassa et al 2005). Most studies, however, have focused on the STA and have disregarded GCA associated abnormalities of other arteries.
Aim of this study is to evaluate the additional contribution of US diagnosis of other craniocervical arteries (carotid, vertebral and periorbital arteries) to the exclusive examination of the temporal arteries to the diagnosis of GCA and the distinction between arteritic and nonarteritic neuroophthalmological vascular complications (NOC).
Patients and methods
This prospective study included 182 patients with suspected GCA who were referred to the department of neurology for sonographic examination between January 1998 and August 2006 and whose sonographic evaluation were performed before biopsy by the same examiner (KP). He was not aware of the patient’s detailed clinical signs and case history. All patients were free from a prior diagnosis of GCA.
Patients with giant cell arteritis
149/182 patients (73% of them female, median age 75, range 52–91 yrs) received the diagnosis GCA, while being treated in the Departments of Rheumatology, Ophthalmology and Neurology. For this analysis we used only patients with the diagnosis of typical cranial GCA. Therefore 17 patients with atypical manifestations of the disease like isolated “large artery” (n = 9) and silent GCA (n = 8) were excluded. 13 of the remaining 132 GCA patients received the diagnosis without biopsy. In 7/13 patients biopsy seemed not necessary because of clear diagnostic signs of GCA. In 6/13 cases biopsy was impossible due to severe ICA stenosis and collateral flow (2), refusal of the patient (2), incorrect biopsy of a vein (1) or anticoagulation (1). In 89 of the 119 patients (75%) biopsies demonstrated GCA.
The diagnoses of all patients were retrospectively confirmed by a experienced rheumatologist considering all diagnostic results and the clinical course especially the response to corticosteroid therapy.
All patients were studied in active phases of the disease. 120 GCA patients had received steroids before ultrasonographic examination (median 2 days both for patients with and without NOC, range 1–24 days).
In 47/132 patients (36%) neuroophthalmological vascular complications were found and confirmed by an experienced ophthalmologist (listed in Table 1). Parts of these data have been published previously (Pfadenhauer and Weber 2006).
Table 1.
Neuroophthalmological complications in 47 patients with giant cell arteritis
| Neuroophthalmological complication | Patients n = 47 | Biopsy positive n = 34 (72%) |
|---|---|---|
| anterior ischemic optic neuropathy | 26 | 19 (73%) |
| posterior ischemic optic neuropathy | 1 | 0 |
| central retinal artery/branch occlusion | 6 | 4 (67%) |
| N III palsy | 3 | 3 (100%) |
| transient diplopia | 4 | 3 (75%) |
| amaurosis fugax | 7 | 5 (71%) |
Patients with other diagnoses and neuroophthalmological complications (control group)
The control group consisted of 33/182 patients (58% of them female, median age 77 years, range 53–89 years) all with NOC and all initially considered to have GCA, but with other final diagnoses: arteriosclerotic vascular disease (n = 32) and Waldenström`s disease (n = 1).
Unilateral biopsy from the STA was taken in 14 patients. Biopsies were normal in 3 patients, arteriosclerosis and intimal fibrosis was found in 11 patients. The spectrum of neuroophthalmological complications included anterior ischemic optic neuropathy (n = 12), central retinal artery occlusion (n = 16), oculomotor nerve lesion (n = 2), posterior ischemic optic neuropathy (n = 1), transient diplopia (n = 1) and amaurosis fugax (n = 1).
Methods
Color duplex sonography (CDS) of the temporal artery
All examinations were done using a Siemens Sonoline Elegra ultrasound system. A linear array 13 MHz transducer (6–9 MHz) was used. Standard parameters for the B-mode were: emission-frequency 9 MHZ, dynamic range 50 dB, gain 70 dB. Axial resolution was 0.35 and lateral resolution 0.45 mm. Color doppler emission frequency was 5.1 MHz. For the doppler sonography a wall filter of 50 Hz and a pulserepetition frequency of 3125 Hz were used.
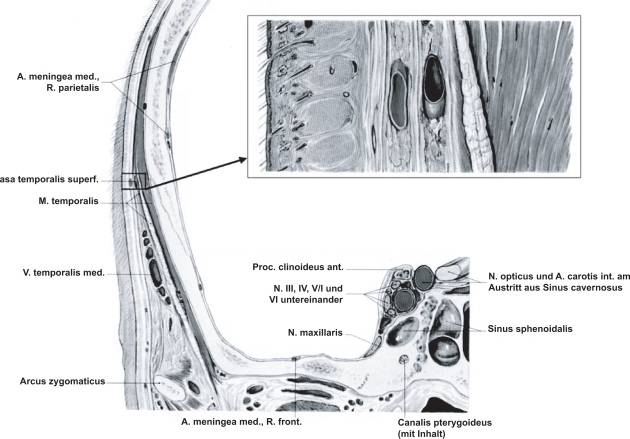
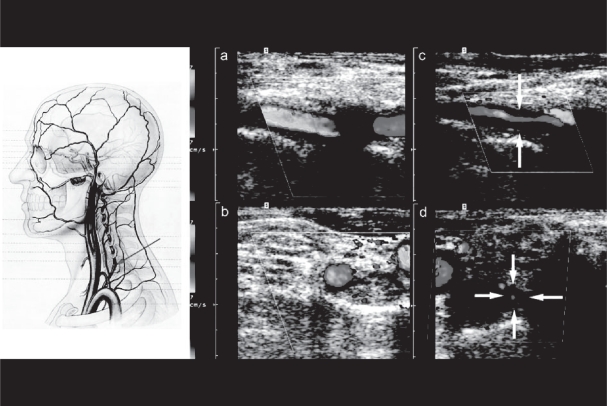
The superficial temporal artery and the frontal and parietal rami were examined as extensively as possible in transverse and longitudinal sections (Figures 1 and 2).
Figure 1.
Anatomy of the superficial temporal artery located between two layers of the fascia temporalis.
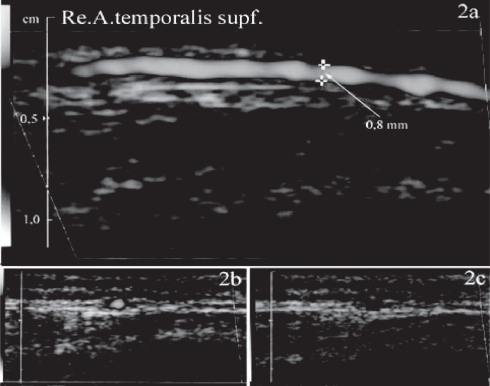
Figure 2.
a–c Color duplexsonography (power mode 2a,b, B-mode 2c) of the normal STA (longitudinal 2a and cross sections 2b,c) located between two layers of the fascia temporalis identified as two hyperechogenic parallel lines. In a normal STA wall thickness cannot be measured.
Absent flow in the STA was considered to be an occlusion of the artery, segmental increase of blood flow velocity with wave forms demonstrating turbulence were classified as stenosis. Periarterial hypoechogenic areas (Figure 3) were classified as “halo” according to the suggestions from Schmidt et al (1997).
Figure 3.
Longitudinal and transverse sections of the STA in a patient with biopsy-proven giant cell arteritis demonstrating hypoechogenic concentric mural thickening (the so-called Halo).
Color duplex sonography (CDS) of the carotid and vertebral arteries
Examination of the carotid and vertebral arteries was performed in transverse and longitudinal sections using a Siemens Sonoline Elegra ultrasound system and a 7.5 MHz linear array high resolution transducer (6–9 MHz). Standard parameters for the B-mode were: Emission-frequency 7.2 MHZ, dynamic range 50 dB, gain 34 dB. Color doppler emission frequency was 5.1 MHz. For the doppler sonography a wall filter of 50 Hz and a pulserepetition frequency of 868 Hz were used.
For the classification of the degree of stenosis of the carotid arteries we used generally accepted doppler sonographic and morphologic criteria (Hennerici and Meairs 2001). The results were categorized into: normal (N), plaque and stenosis <50% (P) and stenosis ≥50% (St). Abnormalities of the vertebral arteries were also classified according to generally accepted criteria (Hennerici and Meairs 2001).
Doppler sonography of the periorbital arteries (DSPA)
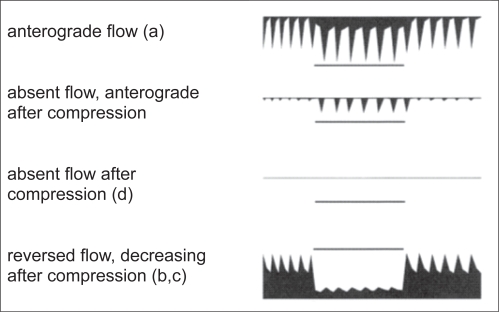
Doppler frequency shifts were recorded from the periorbital arteries using a directional continuous wave doppler device and a 8 MHz ultrasound probe (Figure 4). Compression maneuvers of the temporal and facial artery were carried out to detect reversed flow in the PA. According to accepted criteria (Hennerici and Meairs 2001) the results were categorized into: anterograde flow (A), anterograde reduced flow below half of the contralateral signal amplitude (B), absent flow only before compression maneuvers (C), absent flow (incompressible flow) before and after compression maneuvers (Ci), reversed flow (D) (Figures 5 and 6). Abnormal flow patterns B, C and D were considered as stenosis related if >70% ipsilateral ICA stenosis was present. Flow patterns Ci and D without associated >70% ipsilateral ICA stenosis were considered as GCA typical abnormalities.
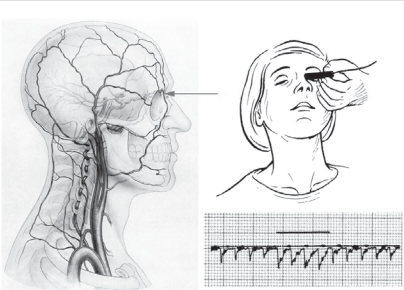
Figure 4.
Continuous wave-dopplersonography of the periorbital (supratrochlear) arteries demonstrating anterograde flow increased after compression of the STA and facial artery.
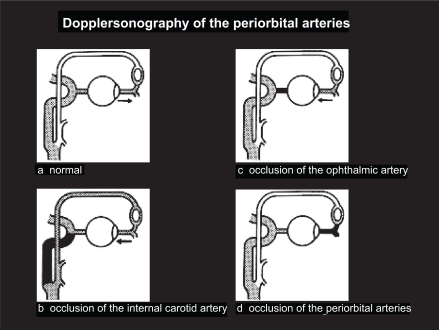
Figure 5.
Summary of DSPA findings of the periorbital arteries: (a) anterograde normal; (b) reversed flow associated with severe stenosis of the ipsilateral ICA; (c) reversed flow not associated with severe stenosis of the ipsilateral ICA; (d) no flow, persisting after compression of the temporal and facial arteries.
Figure 6.
Doppler-sonography of the periorbital arteries: doppler-frequency spectra according to the different abnormalities shown in Figure 5.
Statistical analysis
Specifity and sensitivity of ultrasound diagnosis were calculated according to generally accepted rules. For statistical analysis Fisher’s exact test was used, significance was assumed at p values < 0.05.
Results
Giant cell arteritis patients (Tables 2 and 3)
Table 2.
Ultrasonographic findings in 47 patients with giant cell arteritis and neuroophthalmological complications
| Neuroophthalmological complication | Dopplersonography periorbital arteries | CDS temporal artery | CDS carotid artery | CDS vertebral artery | ||||
|---|---|---|---|---|---|---|---|---|
| anterior ischemic optic neuropathy (n = 26) | abn GCA | n = 8 (31%) | halo | n = 15 (58%) | halo | n = 1 (4%) | halo | n = 3 (12%) |
| abn ICA occl | n = 3 (12%) | halo + stenosis | n = 8 (31%) | plaques | n = 20 (77%) | halo+stenosis | n = 1 (4%) | |
| normal | n = 15 (58%) | normal | n = 3 (12%) | stenosis ≥50% | n = 4 (15%) | normal | n = 22 (85%) | |
| normal | n = 1 (4%) | |||||||
| posterior ischemic optic neuropathy (n = 1) | normal | n = 1 (100%) | halo | n = 1 (100%) | plaques | n = 1 (100%) | normal | n = 1 (100%) |
| central retinal artery/branch occlusion (n = 6) | abn GCA | n = 3 (50%) | halo + stenosis | n = 4 (67%) | plaques | n = 6 (100%) | halo | n = 1 (17%) |
| normal | n = 3 (50%) | normal | n = 2 (33%) | normal | n = 5 (83%) | |||
| N III palsy (n = 3) | abn GCA | n = 2 (67%) | halo | n = 1 (33%) | plaques | n = 3 (100%) | normal | n = 3 (100%) |
| normal | n = 1 (33%) | halo + stenosis | n = 2 (67%) | |||||
| transient diplopia (n = 4) | normal | n = 4 (100%) | halo | n = 2 (50%) | plaques | n = 2 (50%) | normal | n = 4 (100%) |
| normal | n = 2 (50%) | normal | n = 2 (50%) | |||||
| amaurosis fugax (n = 7) | abn GCA | n = 2 (29%) | halo | n = 4 (57%) | plaques | n = 4 (57%) | normal | n = 7 (100%) |
| normal | n = 5 (71%) | halo + stenosis | n = 1 (14%) | normal | n = 3 (43%) | |||
| normal | n = 2 (29%) | |||||||
Abbreviations: abn GCA, abnormalities typical for GCA; abn ICA occl, abnormalities typical for internal carotid artery occlusion; CDS, color duplex sonography
Table 3.
Summary of the ultrasonographic findings in 47 patients with giant cell arteritis and 33 patients with other diagnoses and neuroophthalmological complications and 85 patients with giant cell arteritis without neuroophthalmological complications
| Diagnosis | Patients | Dopplersonography periorbital arteries | CDS temporal artery | CDS carotid artery | CDS vertebral artery | ||||
|---|---|---|---|---|---|---|---|---|---|
| giant cell arteritis with NOC | 47 | abn GCA | n = 15 (32%) | halo | n = 23 (49%) | halo | n = 1 (2%) | halo | n = 4 (9%) |
| abn ICA occl | n = 3 (6%) | halo + stenosis | n = 15 (32%) | plaques | n = 36 (77%) | halo + stenosis | n = 1 (2%) | ||
| normal | n = 29 (62%) | normal | n = 9 (19%) | stenosis ≥50% | n = 4 (9%) | stenosis ≥ 50% | n = 0 | ||
| normal | n = 6 (13%) | normal | n = 42 (89%) | ||||||
| giant cell arteritis without NOC | 85 | abn GCA | n = 4 (5%) | halo | n = 36 (42%) | halo | n = 5 (6%) | halo | n = 4 (5%) |
| abn ICA occl | n = 2 (2%) | halo + stenosis | n = 17 (20%) | plaques | n = 54 (64%) | halo + stenosis | n = 2 (2%) | ||
| normal | n = 79 (93%) | normal | n = 32 (38%) | stenosis ≥ 50% | n = 6 (7%) | stenosis ≥ 50% | n = 6 (7%) | ||
| normal | n = 20 (24%) | normal | n = 73 (86%) | ||||||
| other diagnoses with NOC | 33 | abn GCA | n = 0 | halo | n = 3 (9%) | halo | n = 0 | halo | n = 0 |
| abn ICA occl | n = 3 (9%) | halo + stenosis | n = 0 | plaques | n = 23 (70%) | halo + stenosis | n = 0 | ||
| normal | n = 30 (91%) | normal | n = 30 (91%) | stenosis ≥ 50% | n = 9 (27%) | stenosis ≥ 50% | n = 2 (6%) | ||
| normal | n = 1 (3%) | normal | n = 31 (94%) | ||||||
Abbreviations: abn GCA, abnormalities typical for GCA; abn ICA occl, abnormalities typical for internal carotid artery occlusion; CDS, color duplex sonography
Color duplex sonography of the temporal arteries was abnormal with halos and halos and stenosis in 91/132 GCA patients (69%). There was a significant difference (p = 0.03) between the patients with NOC (38/47; 81%) and without NOC (53/85; 62%). The rate of halo combined with stenosis was different with 32% in NOC patients compared to 20% in the non NOC cases (not significant, p = 0.14).
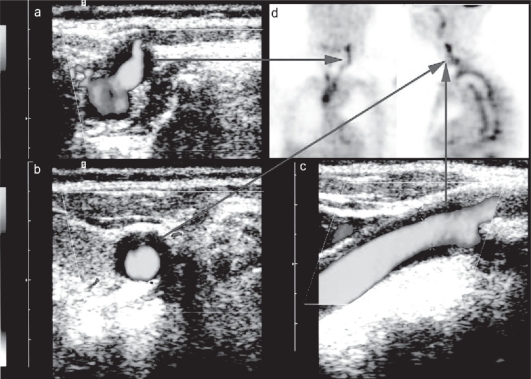
Arteriosclerotic carotid disease was detected in 85% NOC and 71% non NOC patients. Carotid artery halos considered to represent GCA were present in 1 (2%) patient with NOC and 5 (6%) patients without NOC. In 5 of the 6 cases the halos were bilateral. In 1 patient (Figure 7) GCA was proven by a positive FDG-PET demonstrating high FDG uptake not only in the aorta but also in the carotid arteries corresponding to the CDS findings.
Figure 7.
CDS (power mode) with longitudinal (c) and transverse (a,b) sections of the left carotid artery in a patient with biopsy-proven giant cell arteritis demonstrating hypoechogenic mural thickening (the so-called Halo). 18-FDG-PET in the same patient demonstrating increase 18-FDG-uptake in the arterial wall of the carotid arteries and the aorta (d).
Vertebral artery halos were found in 11 patients always involving the VO-V2 segment of the artery (Figure 8) and were unilateral in 7 cases. 5 NOC positive and 6 NOC negative patients were affected. Arteriosclerotic vertebral artery occlusive disease was detected in 6 NOC negative patients.
Figure 8.
CDS (color mode) with longitudinal (a,c) and transverse (b,d) sections of a normal (a,c) and an abnormal left vertebral artery in a patient with biopsy-proven giant cell arteritis (c,d) demonstrating severe hypoechogenic mural thickening (the so-called Halo) and stenosis.
Dopplersonography of the periorbital arteries in 132 GCA cases showed a significantly higher rate (p < 0.0001) of abnormal findings of GCA associated abnormalities in NOC positive patients (15/47; 32%) than in NOC negative patients (4/85; 5%). Bilateral abnormalities of PA and severe carotid artery stenosis were similar in NOC (8/15 resp 4/47) and in non NOC patients (2/4 resp 6/85).
Two cases may illustrate the varying findings in patients with the final diagnosis GCA: In a patient with unilateral AION reversed flow was detected in both periorbital arteries, CDS and digital substraction angiography of the carotid arteries excluded a stenosis of the carotid arteries and biopsy demonstrated active GCA. In another patient with bilateral blindness due to AION halos of both temporal arteries and typical DSPA findings indicated GCA whereas biopsy showed sclerosis of the intima.
In summary the sensitivity of ultrasonographic findings of the investigated craniocervical arteries in GCA patients was 70% relative to the final diagnosis made by an expert. In the NOC positive GCA patients even 38/47 (81%) had pathological findings. In all 28 cases with GCA typical abnormalities in 2 or more different arteries the final diagnosis was GCA (temporal and periorbital arteries (14), temporal, vertebral und periorbital arteries (5), temporal and vertebal arteries (4), temporal and carotid arteries (4) and carotid and vertebral arteries (1)).
Biopsies of 30/119 CGA patients (33%) revealed negative or unclear results. Altogether in 36/132 CGA patients (27%) biopsy was not available, negative or ambiguous. 15 of these patients (42%) had positive ultrasound results with GCA typical findings of the craniocervical arteries.
Patients with other diagnoses and NOC (control group) (Table 3)
Color duplex sonography of the temporal arteries was abnormal with halos in 3 of the 33 patients (9%), corresponding to a specifity of 91%. Halo and stenosis were found in no patient.
In 1 patient with an unilateral occlusion of the central retinal artery and the final diagnosis of Waldenström`s disease a bilateral halo of the common trunk of the STA without stenosis was found whereas biopsy showed arteriosclerosis. In 2 patients with generalized arteriosclerotic occlusive disease unilateral halos in a small segment of the temporal artery were detected. In 1 patient biopsy showed severe occlusive fibrosis of the intima layer.
Arteriosclerotic carotid artery disease was found in 32 (97%) patients including 9 patients (27%) with stenoses exceeding 50%. 6% of the vertebral arteries were affected by arteriosclerotic stenosis ≥50%.
GCA typical abnormalities were neither found in the carotid and vertebral nor in the periorbital arteries.
Discussion
Sensitivity and specifity of ultrasound diagnosis of the cranio-cervical arteries as examined in this study for the diagnosis of GCA is in line with the results of other studies (Brunholzl and Müller 1988; Schmidt et al 2002) and a metaanalysis based on 23 studies and 2036 patients (Karassa et al 2005).
The relatively low sensitivity of our ultrasonographic findings may be explained in part by steroid pretreatment of most patients before the US examination. Therefore the typical ultrasonographic findings were possibly no more detectable.
Our data from an hospital based cohort of GCA patients show significantly higher rates of halos and halos in combination with stenoses in the temporal arteries and abnormal DSPA findings in patients with NOC than in patients without NOC. This suggests a more severely disturbed hemodynamic situation in NOC positive GCA patients.
Typical ultrasonographic findings in the temporal and periorbital arteries clearly help to distinguish between arteritic and nonarteritic NOC. Color duplex sonography of the temporal arteries showed by far the highest rates (69%) of GCA typical abnormalities followed by the periorbital arteries (14%). In the GCA patients with neuroophthalmological complications the abnormalities were more frequent in the temporal (81%) than in the periorbital arteries (32%), too.
In accordance with other studies (Karassa et al 2005) a low rate (9%) of false positive halos of the STA was found in the non GCA patients. However, no halo combined with stenosis and no false positive GCA typical DSPA abnormalities were detected in these patients.
The most significantly different finding in the GCA patients with and without NOC was the of abnormal DSPA results in the NOC patients (32% vs 5%). In another study including 27 biopsy proven GCA patients typical DSPA abnormalities were found in two thirds of the NOC positive patients (Brunholzl and Müller 1988).
Clinical, arteriographic and autopsy studies have demonstrated GCA of the carotid arteries in up to 19% of the 166 patients studied (Caselli et al 1988). However, in our study only in 6 (5%) patients hypoechogenic concentric mural thickening of the carotid arteries was found. Carotid arteriosclerotic disease was different with ≥ 50% stenoses in 8% of the GCA and 27% of the non-GCA patients. The carotid arteries were normal in 20% of all GCA and 3% of all non GCA patients. This clearly points to a more severe arteriosclerotic disease in the non GCA patients. Unlike in the carotid arteries the detection of structural vertebral artery abnormalities is compromised by its more complicated anatomy and partial inaccessibility to CDS, which decreases the accuracy in detecting less extensive arterial wall disease. However in this study GCA typical abnormalities of the vertebral arteries were more often seen in the vertebral than in the carotid arteries. This is supported by previous histopathological observations by Wilkinson and Russell (1972) who reported GCA of the vertebral arteries in 75%–100 % of their autopsy cases.
Conclusion
Whereas CDS cannot definitely differentiate between inflammatory and degenerative arterial wall disease and has limitations of spatial resolution, ultrasonography of the temporal arteries clearly contributes to the diagnosis of GCA particularly in subjects with neuroopthalmological complications. In addition CDS allows full length visualization of the temporal arteries in contrast to biopsy, the selection of abnormal temporal artery segments for biopsy and follow-up studies to explore the response to steroid treatment.
In experienced hands color duplex sonography of the superficial temporal, carotid and vertebral arteries and doppler sonograpy of the periorbital arteries are valuable tools in the atraumatic diagnosis of GCA. They are complementary methods and should be part of the evaluation of all patients suspected to suffer from giant cell arteritis, but at present cannot replace biopsy in all patients.
Because GCA typical abnormalities were neither found in the carotid and vertebral nor in the periorbital arteries in non GCA patients with NOC, our results support the hypothesis, that in patients with typical CDS findings in the STA and other craniocervical arteries (carotid, vertebral or periorbital arteries) biopsy taking is not obligatory. In those patients the response of clinical symptoms, serum markers of inflammation and ultrasonographic abnormalities to adequate steroid treatment can be taken as diagnostic confirmation of GCA.
Footnotes
This work was not supported by grants or industry supports.
Disclosure
The results of this article are parts of the M.D. thesis of Christoph Behr, Ludwig-Maximilian-University Munich, in preparation.
References
- Brunholzl C, Müller HR. Doppler sonography fiindings in arteritis temporalis. Ultraschall Med. 1988;9:232–6. doi: 10.1055/s-2007-1011633. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Hunder GG, Whisnant JP. Neurologic disease in biopsy proven giant cell (temporal) arteriitis. Neurology. 1988;38:352–9. doi: 10.1212/wnl.38.3.352. [DOI] [PubMed] [Google Scholar]
- Hall S, Persellin S, Lie JT, et al. The therapeutic impact of temporal artery biopsy. Lancet. 1983;2:1217–20. doi: 10.1016/s0140-6736(83)91269-2. [DOI] [PubMed] [Google Scholar]
- Hennerici MG, Meairs SP. Cerebrovascular ultrasound: theory, practice and future developments. Cambridge: University Press; 2001. [Google Scholar]
- Hunder GG, Bloch DA, Michel BA. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- Ikard RW. Clinical efficacy of temporal artery biopsy in Nashville, Tennessee. South Med J. 1988;81:1222–4. doi: 10.1097/00007611-198810000-00005. [DOI] [PubMed] [Google Scholar]
- Karassa FB, Matsagas MI, Schmidt WA, et al. Meta-Analysis: Test performance of Ultrasonography for giant-cell arteritis. Ann Intern Med. 2005;142:359–69. doi: 10.7326/0003-4819-142-5-200503010-00011. [DOI] [PubMed] [Google Scholar]
- Nesher G, Shemesh D, Mates M, et al. The predictive value of the halo sign in color Doppler ultrasonography of the temporal arteries for diagnosing giant cell arteritis. J Rheumatol. 2002;29:1224–6. [PubMed] [Google Scholar]
- Niederkohr RD, Levin LA. A Bayesian analysis of the true sensitivity of a temporal artery biopsy. Invest Ophthalmol Vis Sci. 2007;48:675–80. doi: 10.1167/iovs.06-1106. [DOI] [PubMed] [Google Scholar]
- Pfadenhauer K, Weber H. The contribution of duplexsonography of the temporal and occipital artery in the diagnosis of temporal arteritis. Results of a prospective study in 67 biopsy controlled patients. J Rheumatol. 2003;30:2177–88. [PubMed] [Google Scholar]
- Pfadenhauer K, Weber H. Ultrasonography of the temporal, perorbital and carotid arteries in the diagnosis of giant cell arteritis and its neuroophthalmological complications. Ultraschall Med. 2006;27:329–35. doi: 10.1055/s-2006-926555. [DOI] [PubMed] [Google Scholar]
- Salvarani C, Silingardi M, Ghirarduzzi A, et al. Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med. 2002;137:232–8. doi: 10.7326/0003-4819-137-4-200208200-00006. [DOI] [PubMed] [Google Scholar]
- Schmidt WA, Kraft HE, Vorpahl K, et al. Color Duplex Ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337:1336–42. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- Schmidt WA, Natusch A, Möller DE. Involvement of peripheral arteries in giant cell arteritis: A color Doppler sonography study. Clin Exp Rheumatol. 2002;20:309–18. [PubMed] [Google Scholar]
- Weyand CM, Gorenzy JJ. Giant cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;329:505–15. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- Wilkinson IMS, Russell RWR. Arteries in the head and neck in giant cell arteritis: a pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27:378–87. doi: 10.1001/archneur.1972.00490170010003. [DOI] [PubMed] [Google Scholar]