Abstract
Given the established importance of glial cell line-derived neurotrophic factor (GDNF) in maintaining dopaminergic neurotransmitter systems, the nigrostriatal system and associated behaviors of mice with genetic reduction of its high-affinity receptor, GDNF receptor (GFR)α-1 (GFRα-1+/−), were compared with wild-type controls. Motor activity and the stimulatory effects of a dopamine (DA) D1 receptor agonist (SKF 82958) were assessed longitudinally at 8 and 18 months of age. Monoamine concentrations and dopaminergic nerve terminals in the striatum and the number of dopaminergic neurons in the substantia nigra (SN) were assessed. The results support the importance of GFRα-1 in maintaining normal function of the nigrostriatal dopaminergic system, with deficits being observed for GFRα-1+/− mice at both ages. Motor activity was lower and the stimulatory effects of the DA agonist were enhanced for the older GFRα-1+/− mice. DA in the striatum was reduced in the GFRα-1+/− mice at both ages, and tyrosine hydroxylase-positive cell numbers in the SN were reduced most substantially in the older GFRα-1+/− mice. The combined behavioral, pharmacological probe, neurochemical and morphological measures provide evidence of abnormalities in GFRα-1+/− mice that are indicative of an exacerbated aging-related decline in dopaminergic system function. The noted deficiencies, in turn, suggest that GFRα-1 is necessary for GDNF to maintain normal function of the nigrostriatal dopaminergic system. Although the precise mechanism(s) for the aging-related changes in the dopaminergic system remain to be established, the present study clearly establishes that genetic reductions in GFRα-1 can contribute to the degenerative changes observed in this system during the aging process.
Keywords: monoamines, neuropharmacology, neurotrophic factors, nigrostriatal system, growth factor receptors, SKF 82958
Introduction
Neurotrophic factors promote survival of dopaminergic neurons and influence their metabolic and electrophysiological properties (Pothos et al., 1998; Wang et al., 2001). Glial cell-line derived neurotrophic factor (GDNF) promotes the survival of dopaminergic neurons in the substantia nigra (SN) during normal development and after neurotoxic lesions (Akerud et al., 2001; Borlongan et al., 2001). GDNF is a target-derived growth factor (Tomac et al., 1995) synthesized in the striatum (Trupp et al., 1997), and exerts its trophic effects on nigral neurons via a two-component receptor complex, GDNF receptor α-1 (GFRα-1) and c-Ret (Trupp et al., 1996). GFRα-1 functions as the ligand-binding, and c-Ret as the signal transduction, component of the receptor complex (Trupp et al., 1997). Consistent with its mRNA localization (Nosrat et al., 1997; Trupp et al., 1997; Glazner et al., 1998), GFRα-1 protein is expressed in brain areas with dopaminergic cell bodies, the SN and ventral tegmental area (VTA), and in their target areas, the striatum and nucleus accumbens, whereas c-Ret mRNA is expressed only in cell body areas (Glazner et al., 1998). Immunohistochemical mapping of rat brain indicates abundant expression of GFRα-1-like immunoreactivity on dopaminergic and other neurons in the pars compacta and pars reticulata of the SN and striatum (Perez-Navarro et al., 1999; Matsuo et al., 2000), suggesting that GDNF exerts neurotrophic effects on dopaminergic and non-dopaminergic cells.
Dopaminergic system dysfunction characterizes Parkinson’s disease (PD) and normal aging. The noted reduction of GDNF in the SN of PD patients (Chauhan et al., 2001; Mogi et al., 2001) suggests that either this trophic factor or its receptor complex might contribute to the dopaminergic system dysfunction associated with the disease, and perhaps normal aging. Genetic deficiency of GDNF in mice (GDNF+/− mice) has been reported to be associated with an accelerated aging-related decline in motor activity and nigrostriatal dopaminergic function (Boger et al., 2006), elevated extracellular dopamine (DA) levels (Airavaara et al., 2004), and impaired performance on a spatial version of the Morris water maze (Gerlai et al., 2001). In addition, studies on rats and non-human primates indicate that GDNF delivery reduces the dopaminergic neuron damage associated with acute lesions and normal aging (Bowenkamp et al., 1995; Choi-Lundberg & Bohn, 1995; Granholm et al., 1997; Mandel et al., 1997; Date et al., 1998; Gash et al., 1998; Kordower et al., 2000; Ericson et al., 2005). GDNF administration to the intact striatum of young adult rats via recombinant lentiviral vector reduced tyrosine hydroxylase (TH) mRNA levels in SN and VTA neurons and the optical density of the TH-positive fiber innervation in the striatum, but did not alter striatal DA neurotransmission (Rosenblad et al., 2003). A protective role for GDNF and its receptor complex is also suggested by increased expression of endogenous GDNF, GFRα-1 and c-Ret mRNA in several brain areas after systemic exposure to kainic acid (Reeben et al., 1998; Chen et al., 2001) and during ischemia (Arvidsson et al., 2001; Sarabi et al., 2001). Finally, the neuroprotective effect of GDNF during cerebral ischemia is reduced in GFRα-1+/− mice (Tomac et al., 2000).
The impact of reduced GFRα-1 signaling on dopaminergic system function was assessed in the present study using GFRα- 1+/− mice. The overall hypothesis was that the decline in dopaminergic systems accompanying the aging process would be exacerbated in GFRα-1+/− mice. Behavior was examined by spontaneous locomotor activity at 8 months (young adult) and 18 months (aged) of GFRα-1+/− and wild-type mice and their responses to a challenge with a DA D1 receptor agonist, SKF 82958 (SKF). Neurochemical assessment of DA and other monoamine neurotransmitters in striatal tissue, and immunohistochemical analysis of TH and dopamine transporter (DAT), were performed at both ages in additional groups of mice.
Materials and methods
Animals
Young adult GFRα-1+/− and wild-type mice (8 months old) obtained from Dr Barry Hoffer at the National Institutes on Drug Abuse were used for all experiments. Different groups of mice were used for the behavioral and the neurochemical and morphological assessments described below. The GFRα-1+/− mice were created by replacing the first coding exon of the murine GFRα-1 gene with a phosphoglycerate kinase (pgk) neo-resistance expression cassette in reverse orientation to GFRα-1 mRNA transcription, as described earlier (Tomac et al., 2000; Sarabi et al., 2003). Animals were housed in the MUSC Animal Care Facility under a 12:12 h light/dark cycle, with access to food and water ad libitum. All experiments were performed in accordance with the Society for Neuroscience Statement for the Use of Animals in Research, and were approved by the Institutional Animal Care and Use Committee, Medical University of South Carolina.
Assessment of locomotor activity
Locomotor activity (total distance traveled) was assessed with a Digiscan Animal Activity Monitor system (Omnitech Electronics Model RXYZCM (8); TAO, Columbus, OH, USA), details of which have been described previously (Halberda et al., 1997). Animals of each age (wild type; GFRα-1+/−) were assigned to one of three drug groups in a 2 (genotype) × 3 (drug dose) × 2 (age) factorial design, and were tested initially at 8 months of age, and then after aging to 18 months. After a 15-min habituation period, the mice were assigned to one of three groups for each genotype, injected intraperitoneally with saline or with SKF at 0.05, or 0.10 mg/kg, and returned to their respective activity monitors for an additional 30-min test to assess the effect of the DA D1 agonist. The resulting six groups included: (i) wild-type (n = 8) or (ii) GFRα-1+/− mice (n = 8) injected with saline; (iii) wild-type (n = 8) or (iv) GFRα-1+/− mice (n = 7) injected with the 0.05 mg/kg dose of SKF; and (v) wild-type (n = 8) or (vi) GFRα-1+/− mice (n = 6) injected with the 0.10 mg/kg dose of SKF. Data were analysed with a 2 (genotype) × 3 (dose) × 2 (age) ANOVA, with age as a repeated measure factor.
Tissue collection and monoamine assessment (high-performance liquid chromatography)
Aged (18 months) GFRα-1+/− (n = 4) and wild-type (n = 10) mice and young adult (8 months) GFRα-1+/− (n = 7) and wild-type (n = 7) mice were decapitated under halothane inhalation anesthesia, and brain tissues were collected. Brains were removed immediately, and the striatum (right hemisphere) was dissected according to previously published anatomical landmarks (Zaman et al., 2003). Samples were weighed in pre-weighed microcentrifuge tubes, and kept on dry ice before transfer into a −70 °C freezer until analysis. Levels of DA, serotonin and norepinephrine (NE) were determined using a previously described standard protocol (Hall et al., 1989). Data for the monoamines were analysed with ANOVAs across the four groups for each monoamine. Newman–Keuls post hoc analysis was used for comparison of individual means.
Measurement of GDNF and GFRα-1 levels in brain tissues
Fresh frozen cortical tissues were collected from 8-month-old and 18-month-old GFRα-1+/− and wild-type mice for analysis of GDNF and GFRα-1 tissue levels. Tissue samples were homogenized in 5–10 volumes of lysis buffer (RIPA buffer, 1% NP40, 0.25% sodium deoxycholate, 150 mm NaCl, 50 mm Tris-Cl, pH 7.4); after protein quantitation using a BCA protein assay (Pierce, Rockford, IL, USA), equal protein amounts (20 µg) were loaded and separated onto 4–12% NuPAGE gradient gels (Invitrogen, Carlsbad, CA, USA). After transfer onto nitrocellulose membranes, proteins were visualized using antibodies against GDNF (R&D Systems, Minneapolis, MN, USA; 1 : 500 dilution) or GFRα-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1 : 500 dilution), horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) and an ECL detection kit (Pierce, Rockford, IL, USA). Quantification of major products was done using a FluorChem 9900 system (Alpha Innotech, San Leandro, CA, USA). Data were analysed with a 2 (genotype) × 2 (age) ANOVA.
Morphological analysis of the dorsolateral and ventromedial striatum and of the SN and VTA
After dissection of the striatum from the right hemisphere for the monoamine measurements described above, the remaining brain tissues from mice of the four groups – aged (18 months) GFRα-1+/− (n = 4) and wild-type (n = 6) mice and young adult (8 months) GFRα-1+/− (n = 5) and wild-type (n = 6) mice – were prepared for histological analysis. Tissue was immediately immersed in 4% paraformaldehyde (in 0.1 m phosphate buffer at pH 7.4) for 48 h, and then transferred into 30% sucrose at 4 °C. Forty-five-micrometer coronal cryosections taken through the left striatum, and bilaterally from the SN and VTA, were collected into 24-well plates containing phosphate buffer. Every sixth section from the striatum and midbrain was processed for immunohistochemical localization of TH (Pel-Freez, Rogers, AR, USA), using our routine protocol (Zaman et al., 2003). In brief, the free-floating sections were treated for 15 min with H2O2 (30%), methanol (100%), and 0.01 m Tris-buffered saline (TBS) (pH 7.6; 1 : 2 : 7, respectively) to quench endogenous peroxidase activity. After a wash with TBS, sections were then treated for 20 min with sodium m-periodate (0.1 m) in TBS. Sections were washed in TBS and incubated for 30 min in 10% normal goat serum (Sigma, St Louis, MO, USA) in TBS + 0.25% Triton X-100 to block non-specific binding sites, and this was followed by 48 h of incubation with the TH antibody (1 : 1000; rabbit anti-TH; Pel-Freez) in 3% normal goat serum/TBS + 0.25% Triton X-100 at 4 °C. Sections were then incubated with a goat anti-rabbit biotinylated secondary antibody (1 : 200; Vector Laboratories, Burlingame, CA, USA) for 1 h, and then in avidin–biotin complex for 1 h (ABC kit; Vector Laboratories). The reaction was developed by a common chromagen, 3′,3′-diaminobenzidine (Sigma, St Louis, MO, USA), and 0.05% of 3% H2O2. For the SN and VTA, the reaction was enhanced with nickel ammonium sulfate (2.5%; Sigma). Adjacent sections were stained with cresyl violet for evaluation of neuronal morphology.
Immunofluorescent labeling was used for co-localization of DAT protein on three to four representative sections through the SN as another marker for dopaminergic neurons and fibers to complement the TH marker. Free-floating sections were washed in phosphate-buffered saline (PBS, pH 7.4) and incubated in a cocktail of 10% normal donkey serum, 0.5% fish skin gelatin and 0.3% Triton X-100 for 1 h at room temperature to block non-specific binding and permeabilize the membrane; sections were incubated in primary antibodies (for TH, 1 : 1000; sheep anti-TH; Abcam, Cambridge, MA, USA; and for DAT, 1 : 500; rabbit anti-DAT; Millipore, Billerica, MA, USA) in blocking cocktail for 48 h at 4 °C. Sections were washed in PBS with Tween-20 (0.05%) and incubated in secondary antibodies – donkey anti-rabbit fluorescein isothiocyanate (1 : 200) and donkey anti-sheep tetramethyl rhodamine isothiocyanate (1 : 100) (Jackson Immuonoresearch) – for 2 h at room temperature. Section were washed in PBS, mounted on glass slides, and protected with ProLong Gold (Invitrogen). Images were acquired with a Zeiss confocal microscope (LSM 510 META), using appropriate bandpass filters for fluorescein isothiocyanate and tetramethyl rhodamine isothiocyanate.
Morphometric analysis
Quantitation of dopaminergic fiber innervation density was determined from every sixth section through the dorsolateral and ventro-medial (shell and core) striatum, according to a previously described routine protocol (Boger et al., 2006). In brief, four to six images of TH-stained sections of the two brain areas per animal were digitized in gray scale using a 20× lens. All samples were digitized in the same time frame, to ensure a constant light intensity across all groups. The density of TH-immunostained fibers was determined using NIH Image Software (Scion Image, Fredrick, Maryland, USA). The software measures gray scale values within the range of 0–256, with 0 representing white, and 256 black. Measurements were performed blinded, and the values from the four to six images were averaged to obtain one value per animal for each brain area. Subtracting background-staining values from these calculated means provided staining intensities for every sixth section through the two brain areas for each animal. Data were analysed with 2 (genotype) × 2 (age) ANOVAs for each brain area and Newman–Keuls post hoc tests.
Quantitative estimates of total numbers of TH-positive neurons in the SN and VTA were determined by unbiased stereological cell counts from serial sections passing through each nuclear group. The method is described in detail by Gundersen et al. (1988) and West (1993). The optical fractionator system used in the present study was Stereo Investigator stereological software (MicroBrightfield, Colchester, VT, USA) coupled to a Prior H128 computer-controlled x–y–z motorized stage. Outline contours were drawn at low magnification (10×), and the outlined region was measured with a systematic random design of dissector-counting frames. The counting frame area was 2500 µm2, and the sampling grid area was 100 × 100 µm (Zaman et al., 2004). The counting brick was approximately 20 µm thick after excluding the upper and lower guard zones of 2.5 µm each (Granholm et al., 2002). TH-positive cells were counted using a 40× objective lens with a 1.4 numerical aperture. The first section from the SN in the rostral position was selected randomly; subsequently, every sixth section was counted, and the total number of neurons was calculated. Outline contours for SN cell counts included areas predominantly containing TH-labeled dopaminergic neurons (i.e. SN pars compacta and lateralis), and excluded the SN pars reticulata area, which contains mostly TH fiber network. Similarly, cell counts were determined for the VTA area as defined by the Mouse Brain Stereotaxic Atlas (Franklin et al., 1997). Data from each group were analysed with 2 (genotype) × 2 (age) ANOVAs.
Results
Aged GFRα-1+/− mice are hypoactive but hypersensitive to a DA D1 receptor agonist
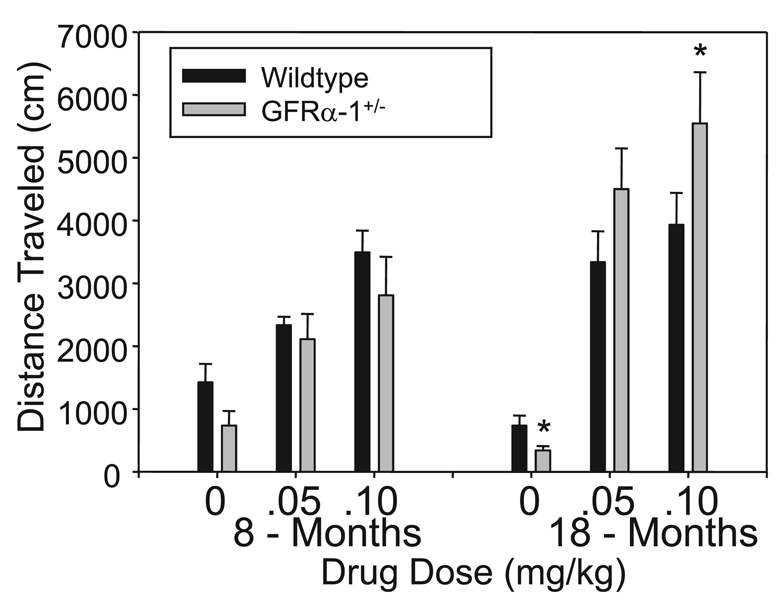
Locomotor activities of young and aged wild-type and GFRα- 1+/− mice following injections of saline or SKF are summarized in Fig. 1. The 2 (genotype) × 3 (drug dose) × 2 (age) anova established that age influenced the effect of both genotype (age × genotype: F1,39 = 4.408, P = 0.05) and SKF (age × SKF: F2,39 = 4.344, P = 0.05) on motor activity. Subsequent follow-up anova on data generated when the mice were young adults indicated that SKF clearly elevated motor activity (SKF: F2,39 = 18.549, P = 0.01). Although the mean motor activity levels in Fig. 1 appear to be lower for young GFRα-1+/− than for wild-type mice, particularly for those injected with saline, this was not supported statistically (F1,39 = 3.655, P = 0.06). anova of data generated after the animals had aged indicated that the effect of SKF on motor activity was influenced by genotype (SKF × genotype: F2,39 = 3.157, P = 0.05), with the aged GFRα-1+/− mice exhibiting a greater response to the drug. Subsequent post hoc drug group comparisons at the older age indicated that motor activity was lower for GFRα-1+/− than for wild-type mice injected with saline (t14 = 2.57, P = 0.01), but was higher for GFRα-1+/− than for wild-type mice injected with a high dose (0.10 mg/kg) of SKF (t12 = 2.08, P = 0.03).
FIG. 1.
Locomotor activity of young adult (8 ± 1 months) and aged (18 ± 2 months) GFRα-1+/− and wild-type mice over a 30-min interval following injections of saline or the D1 receptor agonist SKF 82958 (SKF). Following saline control injections, GFRα-1+/− mice were less active than wild-type controls at both ages, and activity declined with age. SKF elevated motor activity at both ages, and to a greater extent in the older than in the young adult animals. Importantly, despite the lower basal activity levels for the older GFRα-1+/− mice following saline injections, their response to the D1 agonist was greater than that of comparable aged wild-type mice at the highest SKF dose. Follow-up anova results are indicated (*P < 0.05). (See Results for further information on statistical analysis.)
GFRα-1 protein level is lower in the cortex of aged GFRα-1+/− mice
The amounts of GDNF and GFRα-1 in samples of cortical tissue from GFRα1+/− and wild-type mice at the two ages are summarized in Table 1. The amounts of GDNF did not differ according to either age or genotype; however, the GFRα-1 levels for GFRα1+/− mice depended on an interaction of genotype and age (F1,17 = 4.131, P = 0.05). When the GFRα-1 levels in the cortex of 8-month-old wild-type mice were used as a baseline, levels were 19% lower in the 18-month-old GFRα-1+/− mice, and there were no significant differences from those of aged wild-type mice or younger GFRα-1+/− mice.
TABLE 1.
GFR α-1 and GDNF levels in cortical tissue from GFRα-1 +/− and wild-type mice
| GDNF level* | GFRα-1 level* | |||
|---|---|---|---|---|
| Genotype | 8 months | 18 months | 8 months | 18 months |
| Wild type | 1.00 ± 0.029 | 1.02 ± 0.38 | 0.84 ± 0.015 | 0.81 ± 0.75 |
| GFRα-1 + /− | 1.01 ± 0.020 | 0.99 ± 0.026 | 0.82 ± 0.015 | 0.68 ± 0.016 |
Iintegrated density normalized to the control.
DA level is reduced in the striatum of aged GFRα-1+/− mice
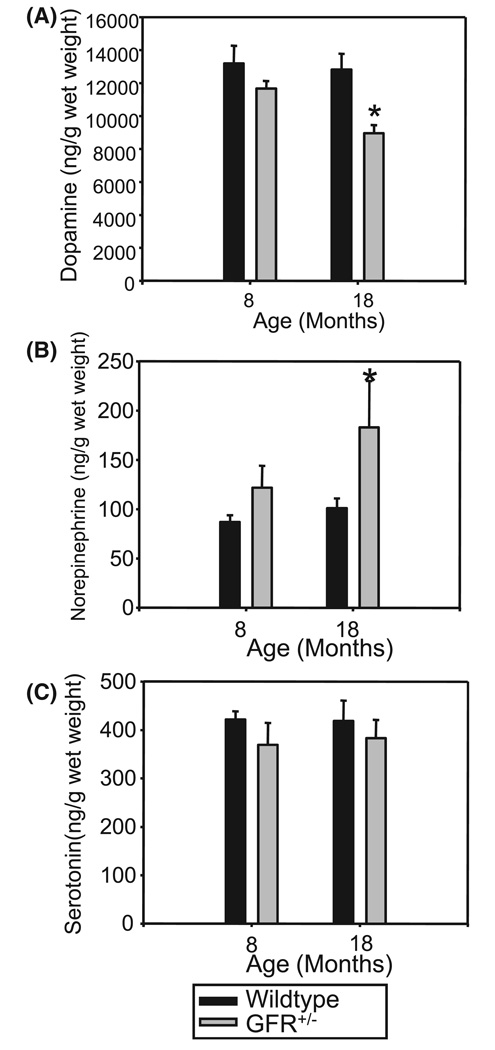
Monoamine levels in striatal tissue (ng/g tissue) of young adult and aged GFRα-1+/− and wild-type mice are shown in Fig. 2. anovas established group differences for DA (F3,20 = 3.887, P = 0.02) and NE (F3,20 = 3.404, P = 0.04), but not serotonin (F3,20 = 0.44, P > 0.05). Post hoc comparison of means indicated that the older GFRα-1+/− mice had lower tissue levels of DA than younger mice of either genotype, and higher levels of NE than all other groups (P = 0.05, Newman–Keuls test).
FIG. 2.
Brain monoamine levels (ng/g tissue) in striatal tissue of young adult and aged GFRα1+/− and wild-type mice. Dopamine levels (A) were reduced in older GFRα-1+/− mice as compared to all other groups (*P = 0.02). Norepinephrine (NE) levels (B) varied according to genotype, with NE levels being significantly higher (P < 0.05) in the striatum of GFRα-1+/− mice than in that of wild-type mice for both age groups. Serotonin concentrations (C) did not differ across genotype or the ages assessed.
Morphological alterations in the nigrostriatal and mesolimbic system of aged GFRα-1+/− mice
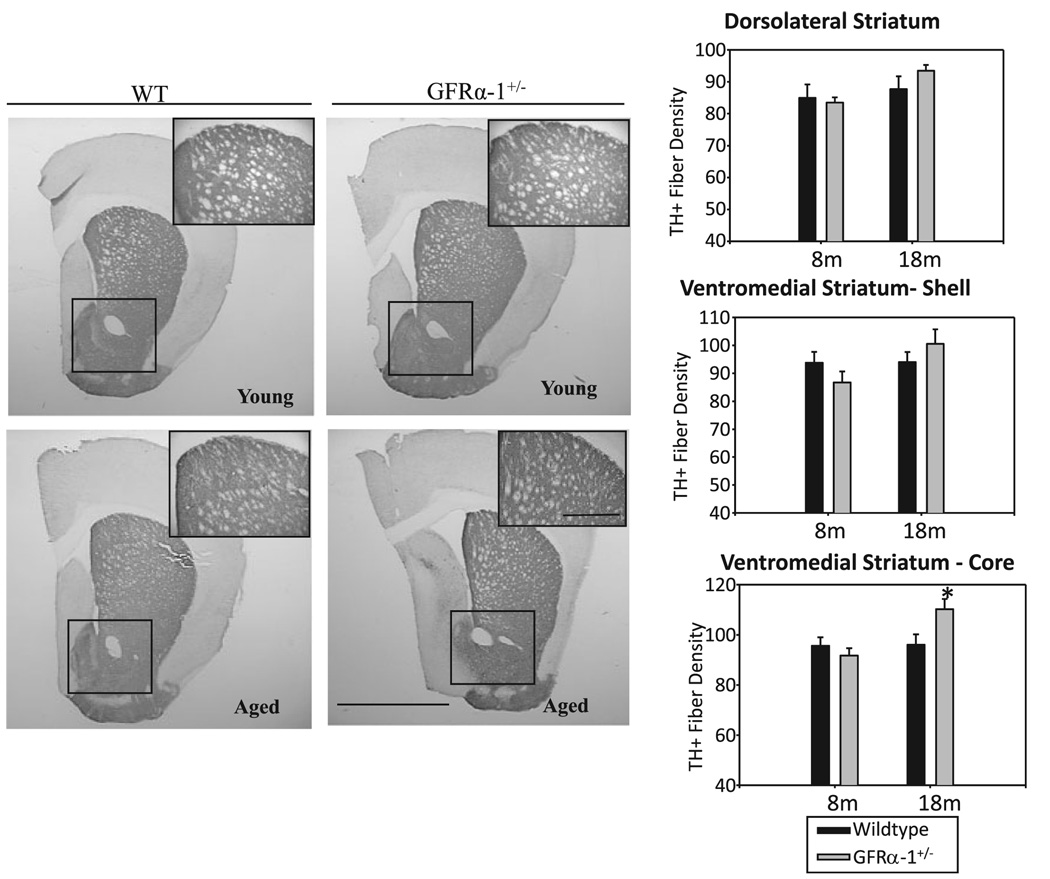
Immunohistochemical assessments of the nigrostriatal dopaminergic system for GFRα-1+/− and wild-type mice at the two ages are summarized in Fig 3–Fig6. Quantification of TH-positive fiber density in the dorsolateral striatum, and the core and shell areas of the ventromedial striatum, is summarized in Fig. 3. The 2 (genotype) × 2 (age) anovas of data from each region indicated that TH-positive fiber density in the core of the ventromedial striatum depended on a genotype × age interaction (F1,18 = 5.129, P = 0.03). Post hoc Newman–Keuls tests indicated that TH-positive fiber density was significantly elevated for older GFRα-1+/− mice in comparison to all other groups (P = 0.05). TH-positive fiber density in the shell region of the ventromedial striatum or the dorsolateral striatum was unaffected by genotype, age, or their interaction.
FIG. 3.
Immunohistochemical localization of tyrosine hydroxylase (TH) in the dorsolateral and ventromedial striatum of young and aged wild-type (WT) and GFRα-1+/− mice. Insets show the magnified view of the dorsal striatum from the respective images, and the boxed area shows the ventromedial striatum (scale bar 125 µm, inset scale bar 10 µm). TH-positive fiber density in the dorsolateral striatum and the shell of the ventromedial striatum did not vary according to genotype or the ages examined. However, the core of the ventromedial striatum of the aged GFRα-1+/− mice exhibited a significant increase (*P < 0.05) in TH-positive fiber density as compared to all other groups.
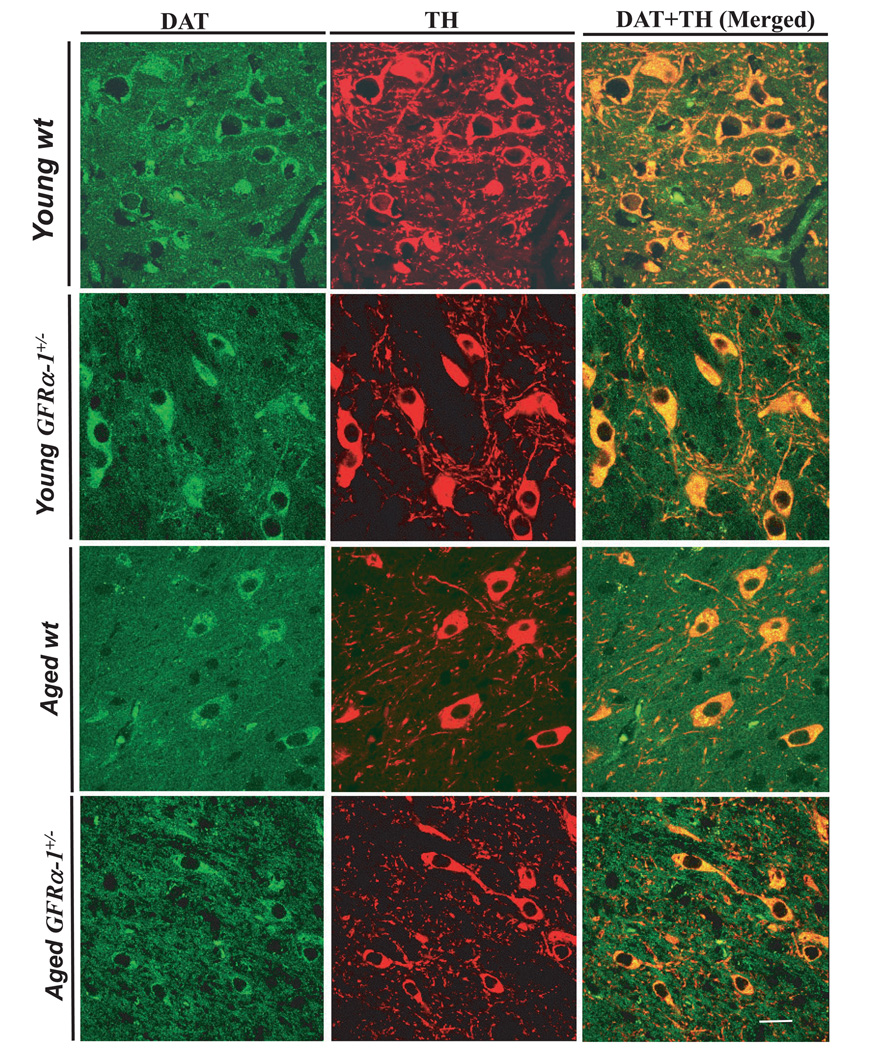
FIG. 6.
Photomicrograph showing representative fields obtained using confocal microscopy at 63× oil magnification. Images of dopamine transporter (DAT), tyrosine hydroxylase (TH) and co-localization (merged images) from examples of respective age and genotypes (labeled in the left-hand margin) are presented in the right-hand panel. All DAT-positive cells express TH. There was 100% co-localization of DAT-positive and TH-positive neurons. Scale bar: 10 µm.
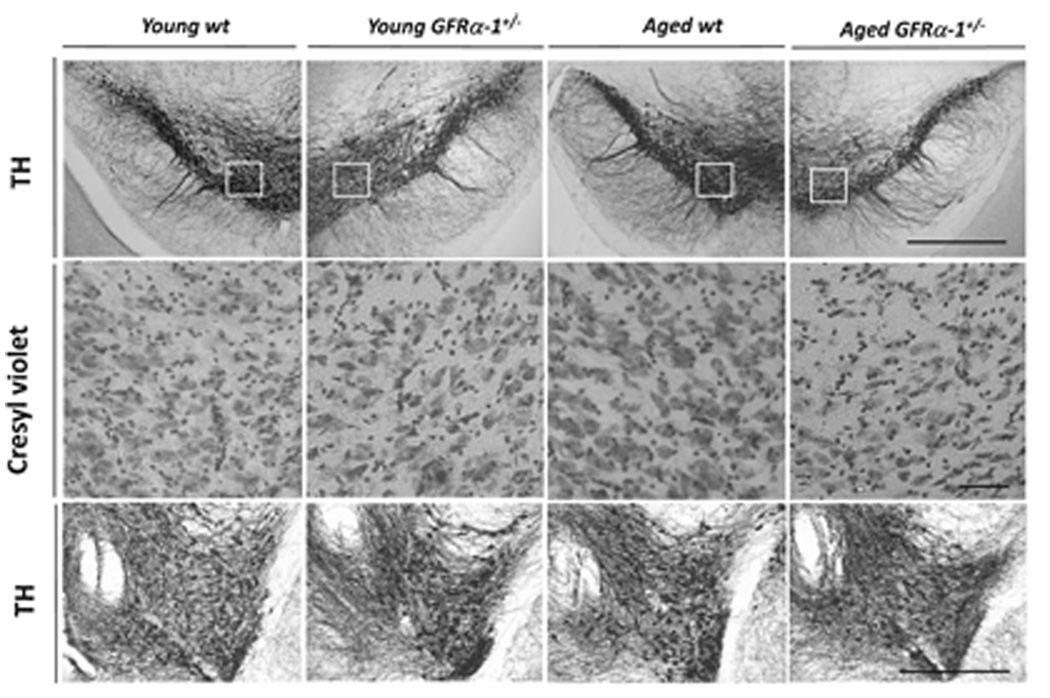
Figure 4 summarizes photomicrographs of TH immunostaining in sections from the SN (top row) and VTA (bottom row) of young wild-type and GFRα-1+/− mice (first two columns), and aged wild-type and GFRα-1+/− mice (last two columns). Evaluation of the photomicrographs of the two genotypes suggests that there was no TH-immunoreactive cell loss for young GFRα-1+/− mice, a conclusion supported by comparison of the adjacent sections stained with cresyl violet noted in the middle row of Fig. 4. Evaluation of the photomicrographs taken from the SN of aged mice, however, clearly indicates fewer TH-positive neurons for aged GFRα-1+/− mice than for wild-type mice of either age. This interpretation is also supported by comparing the adjacent cresyl violet-stained sections in the middle row. This TH-positive neuronal loss for aged GFRα-1+/− mice was more evident in the dorsal than in the ventral tier of the SN pars compacta neurons, and was not observed for the VTA, which is shown in the bottom row of Fig. 4 for the four groups.
FIG. 4.
The top row shows tyrosine hydroxylase (TH)-positive neurons in the substantia nigra (SN) pars compacta of the SN of young and old wild-type (wt) and GFRα-1+/− mice. Adjacent sections were stained with cresyl violet, and are presented in the middle row. The magnified view of cresyl violet staining is from the boxed area in TH-stained sections from respective groups in the SN. Cresyl violet-stained sections revealed fewer neurons in the SN pars compacta of aged GFRα-1+/− mice. The bottom row shows TH-positive neurons in the ventral tegmental area of young and aged wild-type and GFRα-1+/− mice. There was no significant cell loss in this area of aged wild-type or GFRα-1+/− mice. Scale bar: 25 µm.
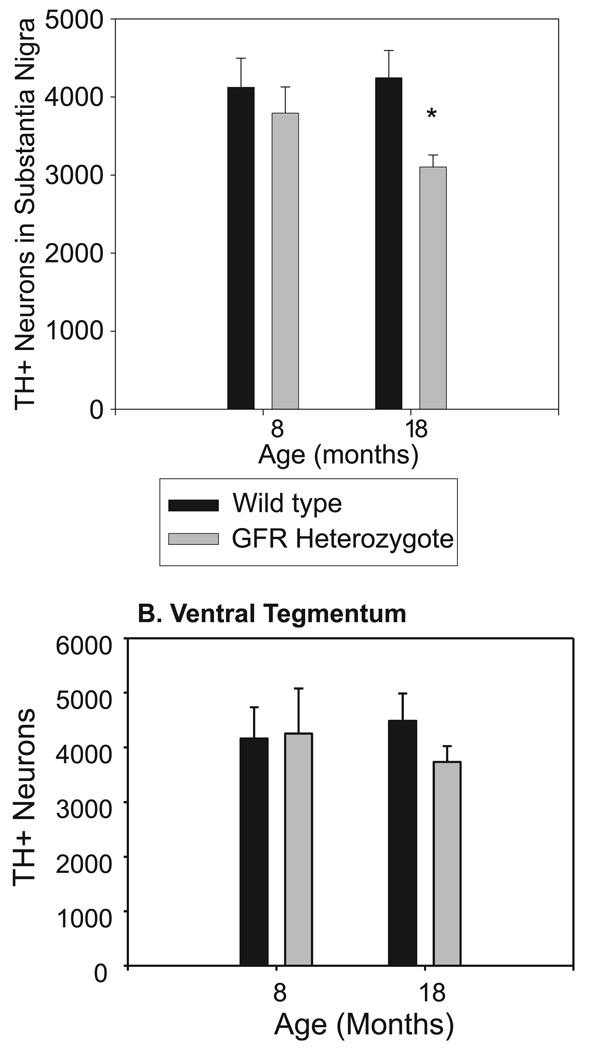
Figure 5 summarizes estimates of the number of TH-positive neurons in the SN (top graph) and VTA (bottom graph). A genotype × age anova on data from the VTA indicated no significant effects of genotype, age, or their interaction. Analysis of data from the SN, however, indicated that the TH-positive cell count for aged GFRα-1+/− mice was reduced (F1,16 = 4.574, P = 0.05), and that it was substantially lower for the aging GFRα-1+/− mice than for young or aged wild-type mice (25% and 30%, respectively), or for young GFRα-1+/− mice (18%).
FIG. 5.
Stereological cell counts in the substantia nigra pars compacta stained with tyrosine hydroxylase (TH) show fewer TH-positive neurons in aged GFRα-1+/− mice (30% cell loss; *P < 0.038). Although the number of TH-positive neurons in the ventral tegmental area was reduced (24%) in aged GFRα-1+/− mice as compared to aged wild-type mice, the difference was not significant.
The co-localization of DAT and TH is noted in Fig. 6, and confirms that the reduction in TH immunostaining accompanies loss of DAT. DAT located in dopaminergic terminals and cell bodies is independent of TH synthesis or accumulation. It is a reliable marker for functionally viable dopaminergic neurons. The magnified images in the figure are from the SN pars compacta. DAT activities for the four genotype × age groups as labeled in the left margin are summarized in the green panels on the left of Fig. 6. TH immunostaining for the different genotype × age groups are presented in the middle panels (red). Examination of the TH images for the young adult wild-type group (top panel) indicates a dense network of TH-positive fibers in addition to the neurons in the SN pars compacta. In comparison, images from aged GFRα-1+/− mice (bottom panel) have not only reduced TH-positive fiber density, but also smaller neurons than aged wild-type or young adult mice of either genotype. The confocal images are merged in the panels on the right side of Fig. 6 to demonstrate the co-localization of neurons positive for both DAT (green) and TH (red) for the four groups of mice. We could not find cells in any group that were positive for DAT and not for TH. This co-localization of DAT and TH further confirmed that the loss of TH-positive neurons, measured by unbiased stereological cell counts, was indeed functional neuronal loss.
Discussion
Five findings of this study indicate the importance of the high-affinity GDNF receptor, GFRα-1, in maintaining normal function of the nigrostriatal dopaminergic system. First, motor activity declined with aging and was lower for the older GFRα-1+/− mice than for their wild-type age-matched controls, which is consistent with hypofunction of either the nigrostriatal and/or mesolimbic dopaminergic systems. Second, the enhanced locomotor stimulation produced by the DA D1 receptor agonist SKF 82958 in the older GFRα-1+/− mice is consistent with the upregulation of this receptor system that occurs with prolonged reductions in synaptic DA levels (Narang & Wamsley, 1995). Third, the reduction of striatal DA levels commonly noted during the aging process (Collier et al., 2007) was even greater in GFRα-1+/− mice. Fourth, the number of TH-positive neurons in the SN was lower for GFRα-1+/− mice than for wild-type mice, most notably for the older GFRα-1+/− mice. Finally, TH immunostaining in the core of the ventromedial striatum was elevated in the older GFRα-1+/− mice. The combined behavioral, pharmacological, neurochemical and morphological measures provide evidence of abnormalities in GFRα-1+/− mice indicative of an exacerbated aging-related decline in dopaminergic system function. The noted deficiencies, in turn, suggest that GFRα-1, the primary signaling mechanism for GDNF, is necessary for GDNF to maintain normal function of the nigrostriatal dopaminergic system. Although the precise mechanism(s) for the aging-related changes in the dopaminergic system are not clear, the present study clearly establishes that genetic reductions in GFRα-1 can contribute to the degenerative changes observed in this system during the aging process.
GFRα-1 deficiency, aging and motor activity reduction
Motor activity declined with age for both genotypes, and was lower for older GFRα-1+/− mice than for age-matched wild-type controls. The decline in motor activity with age is consistent with a number of reported studies on rats (Roth, 1986; Emerich et al., 1993; Dorce & Palermo-Neto, 1994; Hebert & Gerhardt, 1998; Yurek et al., 1998; Ingram, 2000; Leussis & Bolivar, 2006) and several mouse strains (Boger et al., 2006; Colebrooke et al., 2006; Golan et al., 2006; Kopp et al., 2006; Serradj & Jamon, 2007; Zambrana et al., 2007) using the characteristic 20–24 months of age criterion for senescence. Although the older mice in the present study were tested at 18 months of age, motor activity at this age was clearly lower than that obtained when they were young adults, and the reduction was greater for the GFRα-1+/− mice. The observed lower motor activity for GFRα-1+/− mice is similar to that reported for GDNF+/− mice (Zaman et al., 2003; Boger et al., 2006), and provides additional evidence that this neurotrophic factor is involved in the maintenance of neural systems mediating motor behavior. The lower motor activity for GFRα-1+/− mice is not likely to be due to compromised motor function, as their motor activity was equal to or greater than that of wild-type controls after DA D1 agonist injections. Perhaps a more likely explanation for the lower motor activity for GFRα-1+/− mice in our study is reduced arousal. Arousal, indexed as heightened motor activity, is commonly observed when mice are introduced to a novel environment (Misslin et al., 1981), declines with aging (Willig et al., 1987; Emerich et al., 1993; Dorce & Palermo-Neto, 1994; Shukitt-Hale et al., 2001), and is associated with declining dopaminergic system function (Fink & Smith, 1980; Dulawa et al., 1999). These results and a report that GDNF infusion improves motor function deficits in aged non-human primates, probably due to improved DA levels and release in the striatum and SN (Grondin et al., 2003), indicate the importance of this trophic factor in aging-related decline in motor activity. Alternatively, the ascending NE system with cell bodies in the locus coeruleus mediates exploratory activity (Harro et al., 1995), and dysfunction of this system might also mediate the aging-related changes observed in GFRα-1+/− mice. Interestingly, locus coeruleus NE neurons degenerate with age in GDNF+/− mice (Zaman et al., 2003), again indicating the importance of this trophic factor for systems associated with aging-related changes in motor activity.
GFRα-1 deficiency, aging and DA D1 agonist hypersensitivity
The enhanced response of the older GFRα-1+/− mice to the DA D1 agonist suggests upregulation of DA D1 receptor activity, perhaps related to prolonged reductions in DA exposure. Synaptic DA influences the number and/or function of DA D1 and D2 receptors (Gnegy & Costa, 1980), with partial dopaminergic system lesions producing compensatory increases in DA turnover (Greenwood et al., 1991), sprouting of striatal fibers (Ho & Blum, 1998), and hypersensitivity (Reader & Dewar, 1999), as well as upregulation of D1 and D2 receptor expression in the striatum (Raisman et al., 1985; Narang & Wamsley, 1995; Betarbet & Greenamyre, 2004). Similar compensatory increases are reported for aged animals with SN neuronal loss, whose surviving neurons increased DA synthesis and turnover (Greenwood et al., 1991; Stokes et al., 1999). In addition, previous work from our group indicates that GDNF+/− mice have increased levels of D2 receptor mRNA expression in the striatum at 12 months (Boger et al., 2004). Finally, PD patients are reported to have higher striatal levels of D1 and D2 receptors (Raisman et al., 1985; Seeman et al., 1987), and hypersensitivity of DA receptors is hypothesized to account for the dyskinesias of striatal origin during l-3,4-dihydroxy-phenylalanine therapy of PD patients (Bernheimer et al., 1973). Collectively, these studies indicate that prolonged deficiency of DA in the striatum leads to D1 receptor hypersensitivity. The reduced activity of both young adult and aged GFRα-1+/− mice in our experiment is consistent with reduced DA levels in the mesolimbic or nigrostriatal system. Although altered drug metabolism cannot be ruled out, the hypersensitivity to the DA D1 agonist in the aged mice is consistent with compensatory increases in DA D1 receptor activity, perhaps related to prolonged reductions in their exposure to DA. This interpretation is consistent with the reduced tissue level of DA and the reduction in TH-positive cells in the SN of the older GFRα-1+/− mice in our experiment, and with reports that the extent and duration of DA reduction influences the response to DA D1 agonists (Herrera-Marschitz et al., 1985; Starr et al., 1987; Narang & Wamsley, 1995; Bishop & Walker, 2003). Thus, the enhanced stimulatory effect of the DA D1 agonist on aged GFRα-1+/− mice relative to age-matched wild-type or younger mice of either genotype is probably due to upregulated activity of DA D1 receptors resulting from the long-term reduction in available DA.
GFRα-1 deficiency, aging and striatal DA system abnormalities
The reduced DA concentration in the striatum and the enhanced TH-positive fiber density in the core of the ventromedial portion of this structure, combined with the lower cell number in the pars compacta of the SN of GFRα-1-deficient aged mice, implicate the importance of GFRα-1 and GDNF in maintaining the normal structure and function of dopaminergic systems with aging. The aging-related reduction in striatal DA levels and the reduced TH-positive cell number in the SN of the GFRα-1-deficient aged mice are consistent with previous reports indicating a decline in the nigrostriatal dopaminergic system with aging (Greenwood et al., 1991; Dorce & Palermo-Neto, 1994; Stark & Pakkenberg, 2004). The striatal DA level was lower in GFRα-1+/− mice of both ages; however, the TH-positive cell number in the SN was reduced for only the older GFRα-1+/− mice (30%). Our evaluation of TH and DAT co-localization in histological sections from all groups indicated differences according to age and genotype. Given that the sodium- and chloride-dependent DA uptake protein is expressed by SN dopaminergic neurons, it has been proposed that DAT co-localization establishes the dopaminergic nature of TH-positive neurons, and indicates that the TH-positive neurons contain an essential part of the functionally viable machinery (Betarbet et al., 1997). The SN dopaminergic neurons express many transcription factors (e.g. Pitx3, Engrailed-1/2, Nurr1 and Lmx1a/b) that are essential for the generation, survival and maintenance of these neurons during development and throughout life (Alavian et al., 2008). Because we did not use these markers to label the SN dopaminergic neurons in the present study, we cannot rule out the possibility that the neurons not staining for TH and DAT observed in the older GFRα-1+/− mice still survived but lost the ability to express TH. The loss of DAT expression in the neurons suggests that they were not functionally viable. Despite the 30% TH-positive cell loss in the SN of older GFRα-1+/− mice, TH-positive fiber density in projection areas for these dopaminergic neurons was increased by 14% in the ventromedial striatum core region, suggesting the possibility of a compensatory sprouting of dopaminergic terminals in the striatum. Dopaminergic fiber sprouting is an established important compensatory response following partial dopaminergic denervation, and can derive from dopaminergic neurons of either the SN or the VTA (Song & Haber, 2000; Hansen et al., 1995; Ho & Blum, 1998). The significantly increased TH-positive fiber density in the core region of the ventromedial striatum for the older GFRα-1+/− mice could reflect compensatory increased branching of projections from the surviving SN dopaminergic neurons in this group. Another possible explanation for the increased TH-positive fiber density in the older GFRα-1+/− mice is increased sprouting of NE neurites into the striatum as a result of reduced numbers of dopaminergic terminals, an interpretation consistent with the elevated NE levels observed in this genotype × age group. A similar effect has been reported for transmitter loss in other brain regions, such as the hippocampus (Crutcher et al., 1981; Gage et al., 1983). Future studies utilizing tract-tracing will be necessary to establish the cellular origin of the sprouted fibers observed in the present study. Although further investigation will be necessary to establish the detailed mechanisms, the reductions in striatal DA levels and TH-positive neurons in the SN provide a reasonable mechanism for upregulation of DA receptors and resultant hypersensitivity to the DA D1 agonist for the older GFRα-1+/− mice as compared to wild-type mice, and is consistent with the reported relationship between DA level and motor activity after acute 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine exposure (Bezard et al., 2001; Kurosaki et al., 2004). The lower striatal DA levels and morphological alterations in aged GFRα-1+/− mice than in wild-type mice and young GFRα-1+/− mice also provide a potential mechanism for the hypoactivity of the older GFRα-1+/− mice as well as their enhanced response to the DA D1 agonist.
GFRα-1, GDNF and the aging process
GDNF and its receptor components (c-Ret and GFRα-1) have both been reported to be elevated in spinal motor neurons of aged rats (Bergman et al., 1999), and GDNF has been reported to be elevated in the striatum of aged primates (Collier et al., 2005). The present study further defines the relationship of this growth factor and its primary receptor to the aging process. At the ages assessed (8 and 18 months), GDNF levels in the cortex did not differ according to either genotype or age. In contrast to the lack of changes in cortical GDNF levels, GFRα-1 protein levels in the cortex were lower for aging GFRα-1+/− mice than for either their younger counterparts or for age-matched wild-type mice. At the younger adult age (8 months), GFRα-1 protein levels were similar to those of wild-type mice. This result is consistent with an earlier report (Sarabi et al., 2003) that the basal level of GFRα-1 mRNA was similar for GFRα-1+/− and wild-type mice. In that study, the increase in GFRα-1 mRNA produced by ischemic challenge was attenuated for the GFRα-1+/− mice, as it was for the older GFRα-1-deficient mice in our experiment. The results suggest that the aging process can impact on GFRα-1 protein levels, and that further study of the interaction of GFRα-1 function and the aging process is warranted.
GFRα-1/GDNF deficiency, aging and striatal dopaminergic system abnormalities
In addition to aging-related changes in GFRα-1, noted above, the protective effects of intranigral injections of GDNF against 6-hydroxydopamine neurotoxicity has been reported to be reduced for aged as compared to young adult rats (Fox et al., 2001). Although the report can be interpreted as showing possible aging-related changes in the growth factor signaling pathway at the receptor level, aging-related reductions in the GDNF increase in response to the striatal 6-hydroxydopamine lesion might also be a possibility (Yurek & Fletcher-Turner, 2001). The protective effect of GDNF has also been shown in age-related macular degeneration, which is caused by oxidative damage (Dong et al., 2007). These reports suggest the possibility that the dopaminergic system abnormalities noted for the older GFRα-1+/− mice might be mediated by an aging-related inability to compensate for oxidative stress and inflammatory irritants. Alternative possibilities include the contribution of prolonged insufficient activity of high-affinity GFRα-1 in GFRα-1+/− mice, or perhaps developmental alterations imposed by the deficiency that become evident only after normal DA function subsides with the aging process. Finally, as is common to all transgenic experiments, the possible impact of unforeseen gene changes other than those for GFRα-1 itself might contribute to the dopaminergic system changes. A conditional GFRα-1 knockout study, or time-dependent experiments with GDNF antisense, may be able to resolve which alternative is true, but these models have not yet been developed for the GDNF family of growth factors. Although the mechanism remains to be established, the current results indicate that GFRα-1 is important for maintaining the numbers of DA neurons, and also suggest that GFRα-1 expression is not the primary regulator of GDNF protein production in the brain.
Conclusions
The combined behavioral, pharmacological, neurochemical and morphological changes in dopaminergic systems of GFRα-1+/− mice in this study indicate an exacerbated aging-related decline in dopaminergic system function. The reductions in motor activity and enhanced response to the DA D1 agonist are consistent with the observed reductions in striatal DA concentrations and TH-positive SN neuron number. These deficiencies, in turn, suggest that GFRα-1 is necessary for GDNF to maintain normal function of the nigrostriatal dopaminergic system, as it is probably the primary signaling mechanism for this growth factor. Although the precise mechanism(s) for age-related alterations in the dopaminergic transmitter system in the brain are not clear, the present study clearly establishes that genetic reductions in GFRα-1 can contribute to the degenerative changes observed in this system during the aging process.
Acknowledgements
The authors would like to thank Ms C. Umphlet and Mr S. Surgener for excellent technical assistance. We are grateful to Dr J. McGinty for useful discussions, and critical reading of the manuscript. The work was supported by grants from the NIH [AG13494, NS039787 (G. Gerhardt), AG013494 (G. Gerhardt), AG15239 (A.-C. Granholm), and AG023630 (A.-C. Granholm)] and from the army (DAMD17-99-1-9480).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- GDNF
glial cell-line derived neurotrophic factor
- GFR
glial cell-line derived neurotrophic factor receptor
- NE
norepinephrine
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- SKF
SKF 82958
- SN
substantia nigra
- TBS
Tris-buffered saline
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur. J. Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Scholz C, Simon HH. Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov. Disord. 2008;23:319–328. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Airaksinen MS, Saarma M, Lindvall O. Stroke induces widespread changes of gene expression for glial cell line-derived neurotrophic factor family receptors in the adult rat brain. Neuroscience. 2001;106:27–41. doi: 10.1016/s0306-4522(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Bergman E, Kullberg S, Ming Y, Ulfhake B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J. Neurosci. Res. 1999;57:153–165. doi: 10.1002/(SICI)1097-4547(19990715)57:2<153::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Greenamyre JT. Regulation of dopamine receptor and neuropeptide expression in the basal ganglia of monkeys treated with MPTP. Exp. Neurol. 2004;189:393–403. doi: 10.1016/j.expneurol.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT. Dopaminergic neurons intrinsic to the primate striatum. J. Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine-lesioned macaque model of Parkinson’s disease. J. Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Walker PD. Combined intrastriatal dopamine D1 and serotonin 5-HT2 receptor stimulation reveals a mechanism for hyperlocomotion in 6-hydroxydopamine-lesioned rats. Neuroscience. 2003;121:649–657. doi: 10.1016/s0306-4522(03)00516-5. [DOI] [PubMed] [Google Scholar]
- Boger HA, Granholm AC, Jin L, Nelson ME, Page GP, McGinty JF. Striatal gene expression profile of 12 months old GDNF heterozygous mice. Soc. Neurosci. Abstr. 2004;725:17. [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp. Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Zhou FC, Hayashi T, Su TP, Hoffer BJ, Wang Y. Involvement of GDNF in neuronal protection against 6-OHDA-induced parkinsonism following intracerebral transplantation of fetal kidney tissues in adult rats. Neurobiol. Dis. 2001;8:636–646. doi: 10.1006/nbdi.2001.0410. [DOI] [PubMed] [Google Scholar]
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J. Comp. Neurol. 1995;355:479–489. doi: 10.1002/cne.903550402. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J. Chem. Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Chen AC, Eisch AJ, Sakai N, Takahashi M, Nestler EJ, Duman RS. Regulation of GFRalpha-1 and GFRalpha-2 mRNAs in rat brain by electroconvulsive seizure. Synapse. 2001;39:42–50. doi: 10.1002/1098-2396(20010101)39:1<42::AID-SYN6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res. Dev. Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Colebrooke RE, Humby T, Lynch PJ, McGowan DP, Xia J, Emson PC. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson’s disease. Eur. J. Neurosci. 2006;24:2622–2630. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Dung Ling Z, Carvey PM, Fletcher-Turner A, Yurek DM, Sladek JR, Jr, Kordower JH. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp. Neurol. 2005;191 Suppl. 1:S60–S67. doi: 10.1016/j.expneurol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher KA, Brothers L, Davis JN. Sympathetic noradrenergic sprouting in response to central cholinergic denervation; a histochemical study of neuronal sprouting in the rat hippocampal formation. Brain Res. 1981;210:115–128. doi: 10.1016/0006-8993(81)90889-1. [DOI] [PubMed] [Google Scholar]
- Date I, Aoi M, Tomita S, Collins F, Ohmoto T. GDNF administration induces recovery of the nigrostriatal dopaminergic system both in young and aged parkinsonian mice. Neuroreport. 1998;9:2365–2369. doi: 10.1097/00001756-199807130-00039. [DOI] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Hackett SF, Campochiaro PA. Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J. Neurochem. 2007;103:1041–1052. doi: 10.1111/j.1471-4159.2007.04839.x. [DOI] [PubMed] [Google Scholar]
- Dorce VA, Palermo-Neto J. Behavioral and neurochemical changes induced by aging in dopaminergic systems of male and female rats. Physiol. Behav. 1994;56:1015–1019. doi: 10.1016/0031-9384(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, McDermott P, Krueger P, Banks M, Zhao J, Marszalkowski J, Frydel B, Winn SR, Sanberg PR. Locomotion of aged rats: relationship to neurochemical but not morphological changes in nigrostriatal dopaminergic neurons. Brain Res. Bull. 1993;32:477–486. doi: 10.1016/0361-9230(93)90294-l. [DOI] [PubMed] [Google Scholar]
- Ericson C, Georgievska B, Lundberg C. Ex vivo gene delivery of GDNF using primary astrocytes transduced with a lentiviral vector provides neuroprotection in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2005;22:2755–2764. doi: 10.1111/j.1460-9568.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Mesolimbic and mesocortical dopaminergic neurons are necessary for normal exploratory behavior in rats. Neurosci. Lett. 1980;17:61–65. doi: 10.1016/0304-3940(80)90062-2. [DOI] [PubMed] [Google Scholar]
- Fox CM, Gash DM, Smoot MK, Cass WA. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 2001;896:56–63. doi: 10.1016/s0006-8993(00)03270-4. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, Watson C. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gage FH, Bjorklund A, Stenevi U. Reinnervation of the partially deafferented hippocampus by compensatory collateral sprouting from spared cholinergic and noradrenergic afferents. Brain Res. 1983;268:27–37. doi: 10.1016/0006-8993(83)90387-6. [DOI] [PubMed] [Google Scholar]
- Gash DM, Gerhardt GA, Hoffer BJ. Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and nonhuman primates. Adv. Pharmacol. 1998;42:911–915. doi: 10.1016/s1054-3589(08)60895-9. [DOI] [PubMed] [Google Scholar]
- Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, Powell-Braxton L, Phillips HS. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur. J. Neurosci. 2001;14:1153–1163. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J. Comp. Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Costa E. Catecholamine receptor supersensitivity and subsensitivity in the central nervous system. Essays Neurochem. Neuropharmacol. 1980;4:249–282. [PubMed] [Google Scholar]
- Golan H, Stilman M, Lev V, Huleihel M. Normal aging of offspring mice of mothers with induced inflammation during pregnancy. Neuroscience. 2006;141:1909–1918. doi: 10.1016/j.neuroscience.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp. Brain Res. 1997;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol. Behav. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Tatton WG, Seniuk NA, Biddle FG. Increased dopamine synthesis in aging substantia nigra neurons. Neurobiol. Aging. 1991;12:557–565. doi: 10.1016/0197-4580(91)90087-z. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hall ME, Hoffer BJ, Gerhardt GA. Rapid and sensitive determination of catecholamines in small tissue samples by high pressure liquid chromatography coupled with dual-electrode coulometric electrochemical detection. LC/GC. 1989;7:258–265. [Google Scholar]
- Hansen JT, Sakai K, Greenamyre JT, Moran S. Sprouting of dopaminergic fibers from spared mesencephalic dopamine neurons in the unilateral partial lesioned rat. Brain Res. 1995;670:197–204. doi: 10.1016/0006-8993(94)01244-c. [DOI] [PubMed] [Google Scholar]
- Harro J, Oreland L, Vasar E, Bradwejn J. Impaired exploratory behaviour after DSP-4 treatment in rats: implications for the increased anxiety after noradrenergic denervation. Eur. Neuropsychopharmacol. 1995;5:447–455. doi: 10.1016/0924-977x(95)00015-h. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, Forster C, Ungerstedt U. Rotational behaviour elicited by intracerebral injections of apomorphine and pergolide in 6-hydroxy-dopamine-lesioned rats. I: Comparison between systemic and intrastriatal injections. Acta Physiol. Scand. 1985;125:519–527. doi: 10.1111/j.1748-1716.1985.tb07750.x. [DOI] [PubMed] [Google Scholar]
- Ho A, Blum M. Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J. Neurosci. 1998;18:5614–5629. doi: 10.1523/JNEUROSCI.18-15-05614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med. Sci. Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Kopp C, Ressel V, Wigger E, Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav. Brain Res. 2006;167:165–174. doi: 10.1016/j.bbr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kurosaki R, Muramatsu Y, Kato H, Araki T. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2004;78:143–153. doi: 10.1016/j.pbb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc. Natl Acad. Sci. USA. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo A, Nakamura S, Akiguchi I. Immunohistochemical localization of glial cell line-derived neurotrophic factor family receptor alpha-1 in the rat brain: confirmation of expression in various neuronal systems. Brain Res. 2000;859:57–71. doi: 10.1016/s0006-8993(99)02442-7. [DOI] [PubMed] [Google Scholar]
- Misslin R, Haberkorn E, Ropartz P. Responses to novelty and changes in behavior across a 3-week postoperative period in hippocampallesioned mice. Physiol. Behav. 1981;27:413–418. doi: 10.1016/0031-9384(81)90325-5. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Kogure O, Kuno S, Ichinose H, Nagatsu T. Glial cell line-derived neurotrophic factor in the substantia nigra from control and parkinsonian brains. Neurosci. Lett. 2001;300:179–181. doi: 10.1016/s0304-3940(01)01577-4. [DOI] [PubMed] [Google Scholar]
- Narang N, Wamsley JK. Time dependent changes in DA uptake sites, D1 and D2 receptor binding and mRNA after 6-OHDA lesions of the medial forebrain bundle in the rat brain. J. Chem. Neuroanat. 1995;9:41–53. doi: 10.1016/0891-0618(95)00064-e. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Hoffer BJ, Olson L. Cellular and developmental patterns of expression of Ret and glial cell line-derived neurotrophic factor receptor alpha mRNAs. Exp. Brain Res. 1997;115:410–422. doi: 10.1007/pl00005711. [DOI] [PubMed] [Google Scholar]
- Perez-Navarro E, Arenas E, Marco S, Alberch J. Intrastriatal grafting of a GDNF-producing cell line protects striatonigral neurons from quinolinic acid excitotoxicity in vivo. Eur. J. Neurosci. 1999;11:241–249. doi: 10.1046/j.1460-9568.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J. Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman R, Cash R, Ruberg M, Javoy-Agid F, Agid Y. Binding of [3H]SCH 23390 to D-1 receptors in the putamen of control and parkinsonian subjects. Eur. J. Pharmacol. 1985;113:467–468. doi: 10.1016/0014-2999(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Reader TA, Dewar KM. Effects of denervation and hyperinnervation on dopamine and serotonin systems in the rat neostriatum: implications for human Parkinson’s disease. Neurochem. Int. 1999;34:1–21. doi: 10.1016/s0197-0186(98)00048-5. [DOI] [PubMed] [Google Scholar]
- Reeben M, Laurikainen A, Hiltunen JO, Castren E, Saarma M. The messenger RNAs for both glial cell line-derived neurotrophic factor receptors, c-ret and GDNFRalpha, are induced in the rat brain in response to kainate-induced excitation. Neuroscience. 1998;83:151–159. doi: 10.1016/s0306-4522(97)00361-8. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur. J. Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Roth GS. Effects of aging on mechanisms of alpha-adrenergic and dopaminergic action. Fed. Proc. 1986;45:60–64. [PubMed] [Google Scholar]
- Sarabi A, Chang CF, Wang Y, Hoffer BJ, Morales M. Time course study of GFRalpha-1 expression in an animal model of stroke. Exp. Neurol. 2001;170:283–289. doi: 10.1006/exnr.2001.7714. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Chang CF, Wang Y, Tomac AC, Hoffer BJ, Morales M. Differential expression of the cell line-derived neurotrophic factor (GDNF) receptor GFRalpha1 in heterozygous Gfralpha1 null-mutant mice after stroke. Neurosci. Lett. 2003;341:241–245. doi: 10.1016/s0304-3940(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Reynolds GP, Bird ED, Riederer P, Jellinger K, Tourtellotte WW. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neuropsychopharmacology. 1987;1:5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Serradj N, Jamon M. Age-related changes in the motricity of the inbred mice strains 129/sv and C57BL/6j. Behav.Brain Res. 2007;177:80–89. doi: 10.1016/j.bbr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Casadesus G, Cantuti-Castelvetri I, Joseph JA. Effect of age on object exploration, habituation, and response to spatial and nonspatial change. Behav. Neurosci. 2001;115:1059–1064. doi: 10.1037//0735-7044.115.5.1059. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J. Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92. doi: 10.1007/s00441-004-0972-9. [DOI] [PubMed] [Google Scholar]
- Starr BS, Starr MS, Kilpatrick IC. Behavioural role of dopamine D1 receptors in the reserpine-treated mouse. Neuroscience. 1987;22:179–188. doi: 10.1016/0306-4522(87)90208-9. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J. Neurosci. Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc. Natl Acad. Sci. USA. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac AC, Grinberg A, Huang SP, Nosrat C, Wang Y, Borlongan C, Lin SZ, Chiang YH, Olson L, Westphal H, Hoffer BJ. Glial cell line-derived neurotrophic factor receptor alpha1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience. 2000;95:1011–1023. doi: 10.1016/s0306-4522(99)00503-5. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol. Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Willig F, Palacios A, Monmaur P, M’Harzi M, Laurent J, Delacour J. Short-term memory, exploration and locomotor activity in aged rats. Neurobiol. Aging. 1987;8:393–402. doi: 10.1016/0197-4580(87)90033-9. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Zaman V, Li Z, Middaugh L, Ramamoorthy S, Rohrer B, Nelson ME, Tomac AC, Hoffer BJ, Gerhardt GA, Granholm A. The noradrenergic system of aged GDNF heterozygous mice. Cell Transplant. 2003;12:291–303. [PubMed] [Google Scholar]
- Zaman V, Nelson ME, Gerhardt GA, Rohrer B. Neurodegenerative alterations in the nigrostriatal system of trkB hypomorphic mice. Exp. Neurol. 2004;190:337–346. doi: 10.1016/j.expneurol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zambrana C, Marco EM, Arranz L, de Castro NM, Viveros MP, de la Fuente M. Influence of aging and enriched environment on motor activity and emotional responses in mice. Ann. NY Acad. Sci. 2007;1100:543–552. doi: 10.1196/annals.1395.060. [DOI] [PubMed] [Google Scholar]