Abstract
Trifolirhizin, a pterocarpan flavonoid, was isolated from the roots of Sophora flavescens, and its chemical structure was confirmed by1H and 13C NMR and MS spectra. Its anti-inflammatory activity was examined in lipopolysaccharide (LPS)-stimulated mouse J774A.1 macrophages. Trifolirhizin not only dose-dependently inhibited LPS-induced expression of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), but also inhibited lipopolysaccharide (LPS)-induced expression of cyclooxygenase-2 (COX-2). In addition, trifolirhizin showed in vitro inhibitory effects on the growth of human A2780 ovarian and H23 lung cancer cells. These results suggest that trifolirhizin possesses potential anti-inflammatory and anti-cancer activities.
Keywords: anti-inflammation, IL-6, COX-2, Sophora flavescens, TNF-α, trifolirhizin
INTRODUCTION
Inflammation plays an important role in a wide variety of chronic human diseases such as cardiovascular diseases and cancer. It has been demonstrated that pro-inflammatory cytokines, cyclooxygenase-2 (COX-2), and free radical species interact in a complex manner in an inflammation environment (1). For example, tumor necrosis factor-α (TNF-α) has been shown to be one of the major cytokines that mediates many crucial events for the initiation of both acute and chronic inflammation through regulating production of some other cytokines, up-regulation of adhesion molecule expression, and the activation of leukocyte-specific chemotactic cytokines (2). Interleukin-6 (IL-6) is another pro-inflammatory cytokine that promotes inflammatory events through the activation and proliferation of lymphocytes, differentiation of B cells, leukocyte recruitment and the induction of the acute-phase protein response in the liver (3). Pro-inflammatory cytokines such as TNF-α and IL-6 are also interlinked with the production of some small inflammatory mediators such as NO and prostaglandin (PGE2), and thus contribute inflammatory response. In addition, COX-2 has been identified as an important link in the inflammation cascade. Unlike COX-1, COX-2 is selectively induced by pro-inflammatory factors at the site of inflammation, and is responsible for the generation of inflammatory prostaglandins which results in inflammation response and production of pain (4). Inhibition of the expression and production of these powerful mediators by anti-inflammatory components might represent a possible preventive or therapeutic target, and may be used to develop anti-inflammatory nutraceuticals for health promotion and disease prevention.
The roots of S. flavescens (Leguminosae) have been traditionally used in East Asian countries as herb medicine and functional food ingredient for thousands of years because of its potential health beneficial properties such as improving mental heath, anti-inflammatory, antiashmatic, antithelmintic, free radical scavenging, and antimicrobial activities (5–9). Previous studies have isolated quinolizidine alkaloids, flavonoids, and triterpenoids from the roots of S. flavescens (5, 8, 10). In 2000, flavonoids isolated from S. flavescens showed antiproliferative activities against human myeloid leukemia HL-60 and human hepatocarcinoma HepG2 cells and induced apoptosis in both cell lines (10). Later in 2002, a prenylated flavonoid from this herb was able to down-regulate COX-2 in LPS-treated RAW 264.7 cells and exhibited in vivo anti-inflammatory effect (7). As part of our continuous effect to develop novel nutraceuticals for functional food utilization, this study was conducted to explore the possibility of discover additional natural anti-inflammatory flavonoids from the roots of S. flavescens. The anti-inflammatory activities were examined and estimated as the inhibitory capacity on LPS induced expression of the proinflammaroty cytokines TNF-α and IL-6, and COX-2 in macrophages. The pure flavonoid compound was also evaluated for its antiproliferative activity against A2780 ovarian and H23 lung cancer cells, as well as its scavenging capacity against the stable 2,2-diphenyl-1-picrylhydrazyl DPPH radical.
MATERIALS AND METHODS
General procedures and reagents
1H and 13C NMR spectra were obtained on a Bruker-AMX 500 instrument using DMSO-d6 as a solvent. Electrospray ionization (ESI) mass spectra were acquired in the positive ion mode on a LCQ DECA XP instrument (Thermo Finnigan, San Jose, CA, USA) equipped with an ion trap mass analyzer. Column chromatography was carried out on silica gel (200–300 mesh, Fisherscitific, US). Mouse J774A.1 macrophage cell line was obtained from ATCC. RNAqueous total RNA isolation kit was purchased from Ambion (Austin, TX). High-capacity cDNA archive kit and gene expression kit were obtained from Applied Biosystems (Foster City, CA). Bio-Rad protein assay reagent was purchased from Bio-Rad Laboratories (Hercules, CA). Western Lightning Chemiluminescence Reagent Plus was from Perkin-Elmer Life Sciences (Boston, MA).
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH•) and lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents were of the highest commercial grade and used without further purification.
Plant material
Roots of Sophora flavescens was collected from Shanxi Province, China, in October 2006, and authenticated by Dr. Zhihong Cheng.
Isolation and separation of Trifolirhizin
Air-dried roots of S. flavescens were ground, and refluxed and extracted three times for 4 h with methanol using a dried material/solvent ratio of 1:10 (w/v). The supernatant was collected by filtration, and the solvent was evaporated under reduced pressure to yield a brown solid residue. The residue was subjected to a silica gel column chromatography (CC) eluted with a mixture of chloroform-methanol of increasing polarity to afford three fractions. Fraction III, eluted by a mixture of chloroform-methanol (5:1, v/v), was further separated over silica gel CC eluted with chloroform-methanol (10:1, v/v), followed by recrystalliation in methanol to obtain the pure flavonoid compound, which was identified as trifolirhizin.
RNA isolation and real-time quantitative PCR
Mouse J774A.1 macrophages were pretreated with trifolirhizin (10 or 25 μM) for 2 h, then treated with lipopolysaccharide (LPS) at a final concentration of 0.5 μg/mL for 24 h. Total cellular RNA was isolated using the Ambion RNAqueous kit. Five μg of total RNA was used for first-strand cDNA synthesis using a High-Capacity cDNA Archive Kit. The mRNA levels of TNF-α and IL-6 were quantified using the specific gene expression assay kits for mouse TNF-α and IL-6 on iQ5 Multicolor Real-Time PCR Detection System. The mRNA values for each gene were normalized to internal control, β-actin mRNA. The ratio of normalized mean value for each treatment group to vehicle control group (DMSO) was calculated (11).
Western blot analysis
Mouse J774A.1 macrophage cells were pretreated with trifolirhizin (100 or 200 μM) for 2 h, then treated with LPS (0.5 μg/mL) for 24 h. Total cell lysates were prepared as previously described (12). The protein concentration was determined using the Bio-Rad Protein Assay reagent. The total cellular proteins (10 μg) were resolved on 10% Bis-Tris gels and transferred to Nitrocellulose membranes. Immunoblots were blocked overnight at 4 °C with 5% non-fat milk in Tris-buffered saline (TBS) and then incubated with antibodies to COX-2, or β-actin. Immunoreactive bands were detected using horseradish peroxidase-conjugated secondary antibody and the Western Lightning Chemiluminescence Reagent Plus. The density of the immunoblot bands was analyzed using Image J computer software (NIH).
Anti-proliferative activity estimation
The A2780 ovarian cancer or H23 lung cancer cells were plated in 96-well plates with a density of 1 × 104/well. The medium was replaced after 24 h. Following this incubation, trifolirhizin, dissolved in DMSO, was added to the wells in an appropriate series of concentrations. Ten microliters of MTT solution was added to each well. After 24 h incubation at 37 °C in a humidified 5% CO2 atmosphere, the absorbance at 570 nm was recorded using an ELISA plate reader. The control refers to incubations in the presence of vehicle only (DMSO, 0.5%) and was considered as 100% viable cells.
DPPH• scavenging capacity
The DPPH• scavenging capacity of trifolirhizin was evaluated using the high throughput assay described previously (13). Briefly, the assay was performed using a Victor3 multilabel plate reader (PerkinElmer, Turku, Finland) and 96-well plates. The reaction mixture contained 100 μL of 0.2 mM DPPH• in ethanol and 100 μL of standards, control, blank, or trifolirhizin. The absorbance of each reaction mixture at 515 nm was measured every minute for 40 min. The level of DPPH• scavenged was calculated as [(A0 − A1/A0)] × 100 (where A0 was the absorbance of the reagent blank, and A1 was the absorbance with trifolirhizin). All the measurements were conducted in triplicate.
Statistical analysis
All values are expressed as the mean ± SD of three independent determinations. Student’s t-test was employed to analyze the differences between sets of data. Statistics were performed using GraphPad Pro (GraphPad, San Diego, CA). Statistical significance was declared at P < 0.05.
RESULTS AND DISCUSSION
Isolation and identification of Trifolirhizin
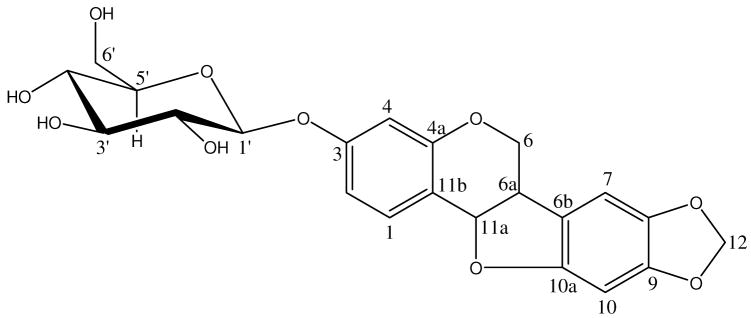
The CHCl3-methanol (5:1, v/v) fraction of the methanol extract of S. flavescens roots was further separated by silica gel column chromatography to obtain a pure flavonoid compound. After re-crystallization in methanol, needle crystals with light yellowish were collected. The molecular formula of the compound was established as C22H22O16 by ESI MS (m/z 447 [M + H]+ and 469 [M + Na]+). The structure of the pure compound was identified as trifolirhizin based on the spectroscopic analysis including 1H– and 13C–NMR spectroscopy, and electrospray ionization mass spectrometry (ESI MS). The chemical structure of trifolirhizin is shown in Figure 1. Its 1H and 13C NMR data, listed in Table 1, agreed well with the data reported previously (5, 14).
Figure 1.
Chemical structure of trifolirhizin.
Table 1.
NMR spectra data of trifolirhizin (400 MHz, DMSO-d6)
| No. C | 13C NMR | 1H NMR |
|---|---|---|
| 1 | 131.87 | 7.36 (d, J=8.8 Hz) |
| 2 | 110.36 | 6.71 (dd, J=2.4, 8.8 Hz) |
| 3 | 158.45 | |
| 4 | 104.00 | 6.55 (d, J=2.4 Hz) |
| 4a | 156.17 | |
| 6 | 65.83 | 4.28 (dd, J=4.0, 10.4 Hz); 3.68 (m) |
| 6a | 40.13 | 3.45 (m) |
| 6b | 118.23 | |
| 7 | 105.35 | 6.98 (s) |
| 8 | 141.12 | |
| 9 | 147.47 | |
| 10 | 93.26 | 6.52 (s) |
| 10a | 153.63 | |
| 11a | 77.63 * | 5.57 (d, J=7.2 Hz) |
| 11b | 114.14 | |
| 12 | 101.03 | 5.94 (d, J=0.8, 15.6 Hz) |
| 1′ | 100.26 | 4.84 (d, J=7.6 Hz) |
| 2′ | 73.15 | overlap |
| 3′ | 76.50 * | overlap |
| 4′ | 69.63 | overlap |
| 5′ | 77.04 * | overlap |
| 6′ | 60.63 | overlap |
Data can be exchanged in the column. Values in parentheses are multiplicity and coupling constants (Hz).
Trifolirhizin is a pterocarpan which belongs to a special group of isoflavonoids possessing two contiguous benzofuran and benzopyran rings. It was first isolated from Trifolium pretense L. in 1960 (15) and identified in S. flavescens by Yagi and co-workers in 1989 (5). Trifolirhizin has not been evaluated for its potential anti-inflammatory activity, anti-proliferative property against A2780 ovarian and H23 lung cancer cells, or free radical scavenging capacity, although it has been found to possess antifungal and anti tumor properties (5, 14).
It is well known that deregulated inflammation leads to the massive production of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6 by macrophages. Reduced overproduction of these inflammatory cytokines might be a potential target for development of preventive and therapeutic approaches against inflammation-related health problems. In this study, trifolirhizin was for the first time examined for its potential inhibitory effects on LPS-stimulated expression of pro-inflammatory cytokines (IL-6 and TNF-α) and COX-2 in macrophages, along with its antiproliferative activity and the potential to directly react with free radicals.
Effects of trifolirhizin on TNF-α and IL-6 mRNA expression in LPS-stimulated mouse J774A.1 macrophage cells
In the present study, the LPS-stimulated mRNA expression of representative pro-inflammatory molecules including TNF-α and IL-6 in mouse J774A.1 macrophage cells were examined by real-time RT-PCR. As shown in Figures 2A and 2B, trifolirhizin significantly inhibited LPS-induced increase in mRNA expression of TNF-α and IL-6 in a dose-dependent manner. At a concentration of 25 μM, trifolirhizin completely inhibited LPS-induced increase of TNF-α mRNA level.
Figure 2.

The effects of trifolirhizin on TNF-α (A), and IL-6 (B) mRNA levels in mouse J774A.1 macrophage cells. Cells (1 × 106/mL) were incubated with either vehicle, LPS (0.5 μg/mL), or LPS plus indicated concentrations of trifolirhizin. The mRNA levels of TNF-α and IL-6 in the culture medium were determined as described in Materials and Methods. Each column represents the mean ± SD of three independent experiments. *P<0.05 indicates a significant difference from the LPS treated control group.
The effects of trifolirhizin on the production of TNF-α and Il-6 were also examined by the ELSIA method. LPS-induced cells treated with trifolirhizin showed a significant decrease in TNF-α production in a dose-dependent matter (Figure 2C). This was in agreement with the observation that trifolirhizin dose-dependently suppressed LPS-induced mRNA expression of TNF-α. However, no significant inhibition was observed for IL-6 production under the same experimental conditions (data not shown).
In 2003, other herbal constituents including apigenin and ginsenoside were found to inhibit the same proinflammatory metabolites (16). It was observed that apigenin at a high dose of 37 μM significantly inhibited only LPS-induced levels of IL-6, without any significant effect on TNF-α concentration (16). It was also reported that ginsenoside Rb1 at a dose of 84 μM completely inhibited both TNF-α and IL-6 induction (16). These treatment concentrations were much higher than the trifolirhizin dose (25 μM) that effected the same observation in TNF-α mRNA levels in the present study. It does appear that trifolirhizin may be a better inhibitor of TNF-α compared with ginsenoside and apigenin. However, it needs to be pointed out that a strict quantitative comparison may lead to inaccurate conclusions due to the different cells and assays used in the different studies.
Xagorari and others (17) also observed that luteolin and quercetin, two natural flavonoids, possessed strong inhibitory activity against these proinflammatory cytokines. The mechanism by which trifolirhizin inhibits TNF-α expression at both mRNA and protein levels and IL-6 at the mRNA level is unclear at this point, but may be related to its ability to interfere with the transcription factor NF-κB (nuclear factor kappa B). NF-κB is responsible for the expression of these proinflammatory cytokines, and a down-regulation of its activity is a plausible explanation for the observed reduction in IL-6 and TNF-α levels. Further research is required to elucidate the exact mechanism involved in the inhibitory activity of trifolirhizin.
Effects of trifolirhizin on COX-2 expression in LPS-stimulated mouse J774A.1 macrophages
Trifolirhizin was examined for its effect on the expression of COX-2 protein in mouse J774A.1 macrophage cells stimulated with LPS. Trifolirhizin dose-dependently inhibited the LPS-stimulated COX-2 protein expression (Figure 3). At 0.1 and 0.2 mM concentrations, trifolirhizin suppressed LPS-induced COX-2 protein production by 14 and 28%, respectively, based on the density ratio of COX-2 versus internal control β-actin (Figure 3).
Figure 3.
The effect of trifolirhizin on COX-2 expression in mouse J774A.1 macrophage cells. Cells (1 × 106/mL) were incubated with either vehicle, LPS (0.5 μg/mL), or LPS plus indicated concentrations of trifolirhizin. COX-2 expression was determined by Western blot analysis as described in Materials and Methods.
Expression of COX-2 may be induced by proinflammatory cytokines, stress, and growth factors. COX-2 is one of the three cyclooxygenase isozymes responsible for the production of prostaglandins. Prostaglandins are the precursors of series-2 prostanoids which contribute significantly to the inflammatory response. Selective inhibition of COX-2 is a preferred way for controlling inflammation because of the increased risk of peptic ulcers, heart attacks, and thrombosis associated with the inhibition of the other two COX isozymes, COX-1 and COX-3. In addition to its well-established role in inflammation, COX-2 has also been implicated in human carcinogenesis. Inhibition of COX-2 may have the dual effect of controlling both inflammation and cancer, and is the mode of operation of coxibs, a class of NSAIDs. The transcription factor NF-κB is responsible for regulating the expression of COX-2. In the present study, the reduction of the LPS-induced COX-2 expression in macrophages by trifolirhizin may also be mediated through NF- κB, similar to that for the other proinflammatory cytokines studied.
Anti-proliferative activities of trifolirhizin
A growing body of evidence suggests a direct link between inflammation and cancer (1, 18). Various steps in tumorigenesis such as cellular transformation, promotion, proliferation, and metastasis have been found to be influenced by chronic inflammation (18). TNF-α has been associated with induction of reactive oxygen in the form of NO, which directly oxidizes DNA and lead to cancer development (19). It is well accepted that anti-inflammatory agents suppressing NF-κB or NF-κB-regulated products including TNF-α, IL-6 and COX-2 may also have a potential in the prevention and treatment of cancer (1, 18).
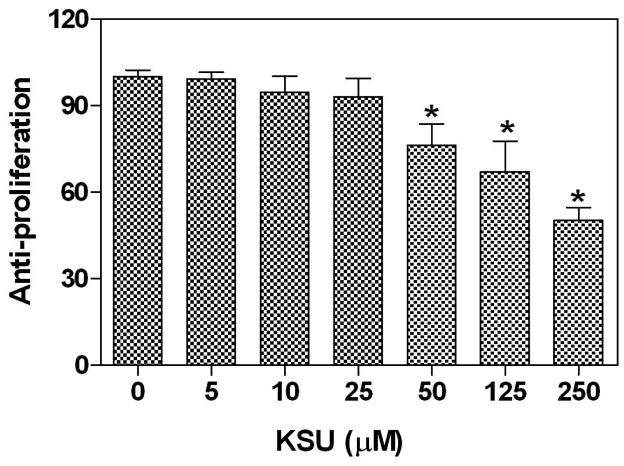
In the present study, trifolirhizin dose-dependently suppressed the expression of LPS-stimulated TNF-α, IL-6, and COX-2 in mouse macrophages (Figures 2 and 3), which are widely accepted in vitro models for investigating anti-inflammatory agents. It is interesting to further examine its possible anti-proliferative activities since trifolirhizin suppressed human myeloid leukemia HL-60 and hepatocarcinoma HepG2 cells in a previous study (10). Trifolirhizin was tested against human A2780 ovarian and H23 lung cancer cells using the MTT assay since these two cell lines are commonly used in our laboratory for screening and development of novel anti-proliferative agents. Trifolirhizin dose-dependently suppressed proliferation of both A2780 ovarian and H23 lung cancer cells (Figure 4). When exposed to trifolirhizin with concentrations less than 50 μM, no anti-proliferative activity was observed in either of the two cell lines. For A2780 ovarian cancer cells, significant anti-proliferative (50% growth inhibition) was achieved with concentration up to 100 μM. However, significant anti-proliferative effect was observed only with a trifolirhizin concentration of 250 μM for H23 lung cancer cells. These data demonstrated the potential anti-proliferative activity against caner cells and its possible selectivity.
Figure 4.
Dose effects of trifolirhizin on human cancer cell growth (A) A237 ovarian cancer cell and (B) H27 lung cancer cell. Cells were exposed to serial dilutions of trifolirhizin for 24 h. Each column represents the mean ± SD of three independent experiments.
Taking into account both anti-proliferative and anti-inflammatory data from this study, trifolirhizin might decrease tumorigenesis through inhibition of the inflammatory mediators. This is consistent with results from other studies that tested anticancer activity of natural anti-inflammatory flavonoids (20–23). Many different mechanisms have been proposed to explain these actions, but they are not mutually exclusive of each other. For example, although the results from this study seem to suggest that the antiproliferative activity of trifolirhizin may be related to its ability to inhibit the expression of some proinflammatory cytokines, another study by Aratanechemuge and others (14) found the antitumor effect of trifolirhizin might be explained by its ability to induce apoptosis in human promyelotic leukemia HL-60 cells. The actual mechanism may very well be a combination of several pathways. Considering in this study that both TNF-α and IL-6 mRNA expressions were suppressed, it is consistent to suggest that trifolirhizin acts at the transcription level.
DPPH• scavenging capacity of Trifolirhizin
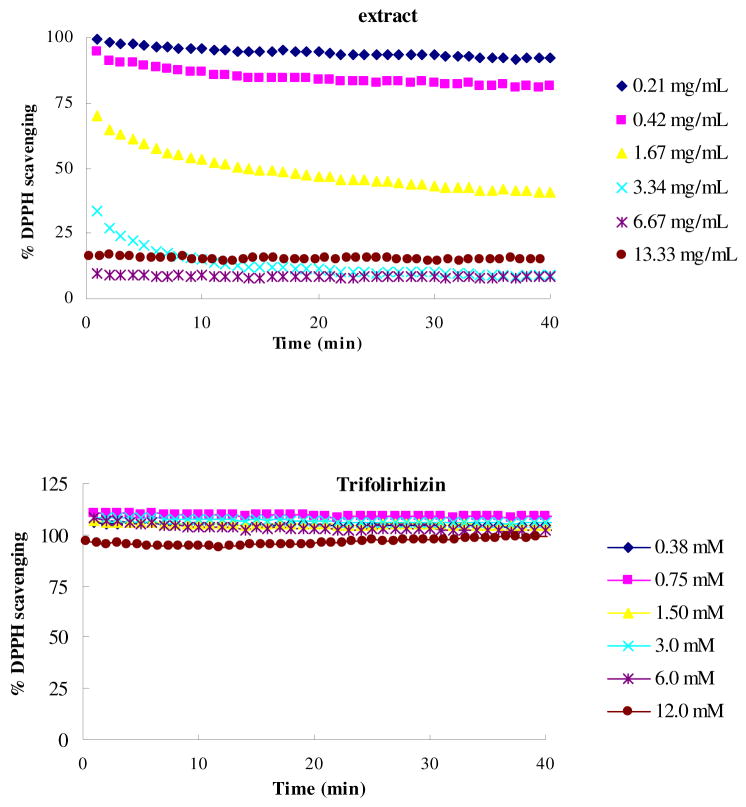
The capacity of trifolirhizin to directly react with and quench free radicals was evaluated and compared with that of the crud methanol extract of the S. flavescens. The crud extract showed DPPH• scavenging capacity in a dose-dependent manner (Figure 5A), but no significant DPPH• scavenging capacity was observed for pure trifolirhizin at concentrations up to 12 mM (Figure 5B).
Figure 5.
Dose and time effects of antioxidants-DPPH• reactions. The final concentration of DPPH• was 100 μM. (A) Reactions with methanol extract of Sophora flavescens; 0.25, 0.20, 0.17, 0.14, 0.11, and 0 represent the concentrations (mg/mL) of the extract in the initial reaction mixture, respectively. (B) Reactions with trifolirhizin; 1.00, 0.67, 0.50, 0.40, 0.33, 0.25, and 0 represent the concentrations of trifolirhizin in the initial reaction in mM.
Growing evidence suggests that free radicals and free radical mediated oxidative stress are closely correlated with the development of inflammation by increasing activation of transcription factors important in regulation of pro-inflammatory cytokines (24, 25). As a result, expression of these genes stimulates the secretion of pro-inflammatory cytokines. In the present study, no significant free radical scavenging activity was observed for trifolirhizin with concentrations of up to 12 mM. This observation was in consistent with the previous studies showing that trifolirhizin might not act as an active scavenger of free radicals (6, 8, 9). This may be partially explained by the lack of phenolic hydroxyl groups in trifolirhizin molecule (Figure 1). This result was also supported by another study showing that trifolirhizin did not have detectable ONOO− or DPPH• scavenging activities (9). Taken together these previous observations with our present data, it may be concluded that the anti-inflammatory and antiproliferative effects of trifolirhizin were not mediated through its direct interaction with free radicals.
In summary, trifolirhizin from S. flavescens roots was not only able to inhibit LPS-induced TNF-α, IL-6 and COX-2 expression in macrophages, but also inhibited cancer cell growth. These findings clearly indicate that trifolirhizin possesses anti-inflammatory and anti-cancer activities and may have application in the prevention and treatment of inflammation and cancer.
Acknowledgments
This work was supported by grants to Dr. Zhou from the National Institutes of Health (R21AI068432, R01AT004148-01), GlaxoSmithKline research fund, A.D. Williams fund and Jeffress Memorial Trust grant.
This research was also supported by a grant from USDA National Research Initiatives with a federal grant number of 20043550314852, a grant from National Science Foundation with a federal grant number of CBET-0650650, a grant from the Maryland Grain Producers Utilization Board (MGPUB) with a MGPUB grant proposal number of 208198, and a grant from the Maryland Soybean Board.
References
- 1.Macarthur M, Hold GL, El-Omar EM. Inflammation and cancer II. Role of chronic inflammation and cytokines gen polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointes t Liver Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Cerami A. The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 3.Pecoits-Filho R, Lindholm B, Axelsson J, Stenvinkel P. Update on interleukin-6 and its role in chronic renal failure. Nephrol Dial Transplant. 2003;18:1042–1045. doi: 10.1093/ndt/gfg111. [DOI] [PubMed] [Google Scholar]
- 4.Crofford L-J, Lipsky P-E, Brooks P, Abramson S-B, Simon LS, van de Putte LBA. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43:4–13. doi: 10.1002/1529-0131(200001)43:1<4::AID-ANR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Yagi A, Fukunaga M, Okuzako N, Mifuchi I, Kawamoto F. Antifungal substances from Sophora flavescens. Shoyakugaku Zasshi. 1989;43:343–347. [Google Scholar]
- 6.Kuroyanagi M, Arakawa T, Hirayama Y, Hayashi T. Antibacterial and antiandrogen flavonoids from Sophora flavescens. J Nat Prod. 1999;62:1595–1599. doi: 10.1021/np990051d. [DOI] [PubMed] [Google Scholar]
- 7.Kim DW, Chi YS, Son KH, Chang HW, Kim JS, Kang SS, Kim HP. Effects of sophoraflavanone G, a prenylated flavonoid from Sophora flavescenes, on cyclooxygenase-2 and in vitro inflammatory response. Arch Pharm Res. 2002;25:329–335. doi: 10.1007/BF02976635. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JS, Lee SA, Hong SS, Lee KS, Lee MK, Hwang BY, Ro JS. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch Pharm Res. 2005;28:190–194. doi: 10.1007/BF02977714. [DOI] [PubMed] [Google Scholar]
- 9.Jung HJ, Kang SS, Hyun SK, Choi JS. In vitro free radical and ONOO− scavengers from Sophora flavescens. Arch Pharm Res. 2005;28:534–540. doi: 10.1007/BF02977754. [DOI] [PubMed] [Google Scholar]
- 10.Ko WG, Kang TH, Kim NY, Lee SJ, Kim YC, Ko GI, Ryu SY, Lee BH. Lavandulylflavonoids: a new class of in vitro apoptogenic agents from Sophora flavescens. Toxicol In Vitro. 2000;14:429–433. doi: 10.1016/s0887-2333(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Jarujaron S, Gurley EC, Chen L, Ding H, Studer E, Pandak WM, Jr, Hu W, Zou T, Wang J, Hylemon PB. HIV protease inhibitors increase TNF-alpha and IL-6 expression in macrophages: Involvement of the RNA-binding protein HuR. Atherosclerosis. 2007;195:e134–e143. doi: 10.1016/j.atherosclerosis.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H, Pandak WM, Jr, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005;68:690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Z, Morre J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 14.Aratanechemuge Y, Hibasami H, Katsuzaki H, Imai K, Komiya T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncology Rep. 2004;12:1183–1188. [PubMed] [Google Scholar]
- 15.Hietala PK. A countercurrent distribution method for separation of chemical compounds. Ann Acad Sci Fennicae, Series A II Chemica. 1960;100:54–62. [Google Scholar]
- 16.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb1 (ginseng), and parthenolide (feverfew) Food Chem Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 17.Xagorari A, Papapetropoulous A, Mauromatics A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–187. [PubMed] [Google Scholar]
- 18.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? . Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 20.Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12:3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 21.Nair HK, Rao KVK, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. 2004;11:63–69. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DJL, Lamb JH, Verschoyle RD, Howells LM, Butterworth M, Lim CK, Ferry D, Farmer PB, Gescher AJ. Characterization of metabolites of the putative cancer chemopreventive agent quercetin and their effect on cyclo-oxygenase activity. Br J Cancer. 2004;91:1213–1219. doi: 10.1038/sj.bjc.6602091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Zhou Q, Shi X, Jiang B. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2006;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 24.Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49:506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- 25.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]