Abstract
Human prostate tumor cell invasion and metastasis is dependent in part on cell adhesion to extracellular matrix proteins and cell migration. We previously identified a synthetic D-amino acid tumor cell adhesion peptide called HYD1 (kikmviswkg) that supported adhesion of tumor cells derived from breast, prostate, ovary and pancreas tissue. Alanine substitution analysis and a peptide deletion strategy were used to determine the minimal element of HYD1 necessary for bioactivity in a prostate cancer cell line called PC3N. Bioactivity was measured by assays of cell adhesion, migration and ERK signaling. The most potent element of HYD1 necessary to support cell adhesion was kmvixw, the block to migration required xkmviswxx and activation of ERK signaling required ikmviswxx. The shortest sequence active in all three assays was iswkg. The HYD1 peptide contains overlapping elements required for adhesion, blocking migration and the activation of ERK signaling. These linear peptide sequences provide the starting point for development of novel compounds to target cancer cell adhesion and migration.
Keywords: prostate cancer, synthetic peptide, migration, cell adhesion, ERK signaling
INTRODUCTION
Novel therapeutics that target the molecular mechanisms of metastasis are needed given that most cancer deaths result from the consequences of metastatic tumors rather than the primary tumor itself.1,2 Cell adhesion receptors are molecular targets for the prevention of metastasis since they are required for metastasis and they possess the ability to integrate information from the extracellular environment into cellular signals necessary for cell motility and survival at distant sites.2–4 Integrins are cell adhesion molecules suitable for molecular targeting since they mediate a wide spectrum of cellular functions critical to cancer progression and metastasis.5–7 In addition, these molecules are associated with the progression of several epithelial tumors, including prostate cancer.8–10 Characterization of native extracellular matrix ligands or synthetic ligands to these cell surface receptors may prove beneficial in the development of antagonists for specific integrin functions.
Multiple studies have isolated biologically active peptides from defined regions within laminin chains and documented profound effects on biological events including cell migration and metastasis.11–18 The discovery of RGD, the tripeptide sequence found in many adhesive proteins such as fibronectin and vitronectin,19,20 has led to several studies showing that this cell adhesion peptide has anti-invasive and anti-metastatic effects both in vitro and in vivo.21,22 Peptides derived from the laminin β1 chain, YIGSR, and α5 chain, RLVSYNGIIFFLK, have also been effective at blocking experimental metastasis.11,18,23 An alternative to identifying biologically active regions from extracellular matrix proteins is to develop synthetic ligands using combinatorial chemistry techniques. The one-bead-one-compound combinatorial library method (OBOC)24–26 and the phage-display peptide library approach27–30 have been successfully used to identify peptide ligands for cell surface molecules. These techniques have the potential to produce novel peptides with antagonistic effects on the targeted cell surface receptor.
We have used the OBOC method to isolate peptide ligand mimetics that target the α6 integrin.31,32 HYD1, kikmviswkg, is a synthetic D-amino acid peptide that we have characterized from this approach. When immobilized, HYD1 acts as a ligand mimetic by supporting tumor cell adhesion.32 When introduced as a soluble ligand HYD1 completely blocks prostate tumor cell migration on laminin 322 (laminin 5) and alters the cellular signals elicited from a laminin 322 (laminin 5) matrix.33 The potent anti-migratory effect of HYD1 warranted further study of this peptide to determine the mimimal element required for bioactivity.
HYD1 is a linear peptide consisting of ten D-amino acids. Because HYD1 is relatively large in size compared to other bioactive cell adhesion peptides used in clinical studies, it is important to determine if the amino acid sequence contains a minimal motif that mediates the biological activity of the peptide. We have determined the minimal element of HYD1 by performing alanine substitution analysis and creating N- and C-terminal deletion peptides. We applied three endpoints of biological activity to determine the minimal element of HYD1 (kikmviswkg). The endpoints were cell adhesion to immobilized peptide variants of HYD1 (kikmviswkg) and the migration blocking and ERK signaling activity of these peptides.
MATERIALS AND METHODS
Cell culture conditions and bioactivity assays
The human prostate carcinoma cell line PC3N was grown in Iscove’s Modified Dulbecco’s Medium (Gibco BRL, Gaithersburg, MD.) plus 10% fetal bovine serum (Gibco BRL) and incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was supplemented with penicillin/streptomycin, 100 units/ml (Gibco BRL). Serum-Free medium was supplemented with 0.1% Bovine Serum Albumin (Sigma, St. Louis, MO.). PC3N cells are a variant of the human PC3 prostate carcinoma cell line.34 HaCaT cells35 were obtained from Dr. Norbert E. Fusenig (German Cancer Research Center, University of Heidelberg, Heidelberg, Germany).
Cell adhesion experiments were performed by dissolving neuralite avidin (20 μg, Molecular Probes, Inc.) in 1 ml of distilled water and 100 μl of solution was added to each well of a tissue culture 96-multiwell plate (Falcon, Franklin Lakes, NJ). The solution of neuralite avidin was allowed to dry overnight. Use of neuralite avidin ensures maximum binding of the biotinylated peptide to the surface, avoiding the variability of peptide coating. The wells were then blocked with 100 μl of 1% BSA for 1 h. The wells were washed with HEPES buffer, and 50 μM of peptide were added in each well. After 1 h of peptide incubation, wells were washed with IDMEM without serum, and suspended PC3N cells (5 × 104) in serum-free IDMEM were added in each well. The cells were allowed to adhere for 60 min at 37°C. The wells were washed three times with HEPES buffer and fixed with 2.5% formaldehyde in PBS. The cells were then stained with 0.5% crystal violet in 20% (v/v) methanol/water and viewed under a microscope. The amount of bound cells was estimated by solubilizing the dye using 0.1 M sodium citrate and reading the absorbance at 562 nm. Triplicate determinations were done at each data point.
Laminin 322 (laminin 5) was obtained from conditioned media of HaCaT cells. Briefly, HaCaT cells were grown in DMEM/F12 serum free medium for one week in T175 cm2 tissue culture flasks. The media was collected and clarified by centrifugation at 40 for 15 minutes. The resulting supernatant was filtered through a 0.2 μm acetate filter in the presence of protease inhibitors (50uM PMSF, 50uM N-ethyl maliamide and 5mM EDTA). The resulting material is concentrated and frozen at −80°C and the resulting supernatant is used. This procedure is essential as it takes advantage of the known cold insoluble properties of a potential contaminant, fibronectin. For laminin 322 (laminin 5) coating of wells or coverslips, conditioned media was added to the surface for 2 hours at room temperature and washed 1 time with PBS (2.7 mM KCl, 1.5 mM KH2PO4, 138 mM NaCl, 8.1 mM Na2HPO4, pH 7.4) before use.
The cell migration assay was performed with PC3N cells grown to confluency on laminin 322 (laminin 5) coated square coverslips in IMDM (Gibco BRL, MD, USA) plus 10% fetal bovine serum (FBS). A scratch was made diagonally across the square coverslip with a plastic cell scraper (Fisherbrand Cat. # 08-773-2). The cover-slips were then rinsed in medium and placed in fresh medium containing 75 μg/ml peptide and 1% FBS for 12 hours at 37°C. The cells were fixed with chilled methanol for 10 min and post-fixed in chilled acetone. On drying, the cells were then stained with 0.5 μg/ml DAPI for 10 min. The coverslips were washed in PBS, post-fixed in Ethanol for 4 min and mounted using Prolong Antifade (Molecular Probes, OR, USA). The slides were visualized on a Zeiss Axiovert microscope. Images were collected using Axiocam camera at 10 × magnification. Quantification was done using Scion Image software.
The effect of HYD1 or HYDS peptide treatment on the cell cycle distribution was tested using PC3N cells grown to confluency using optimal growth conditions. The medium was replaced by fresh media containing 75 μg/ml peptide and 1% fetal bovine serum for 12 hours at 37°C. Cells were harvested with trypsin and suspended in Krishan buffer (0.1% sodium citrate, 0.02 mg/ml RNAse, 0.3% NP-40 and 0.05% propidium iodide) for 30 min on ice. DNA content was assessed with flow cytometry using a FACs Star Plus (Becton-Dickenson) and the percentage of cells in G0/G1, S, and G2/M phases evaluated using Cell Quest & ModFit cell cycle analysis software.
For cell signaling experiments, cells were incubated in serum-free media overnight and harvested with 5mM EDTA in PBS for 10 minutes. Cells were washed in serum-free media and added to laminin 322 (laminin 5) coated tissue culture plates for 1 hour at 37°C. The coated plates were blocked with 1% BSA for 30 minutes at room temperature. Cells were then treated with peptide in serum-free media for 10 minutes at 37°C. Two minutes before lysis, 0.5mM sodium orthovanadate was added into the media. Cells were lysed for 5 minutes in a modified RIPA lysis buffer containing 40 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 6 mM EDTA, 100 mM NaF, 1mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM PMSF, and 1 μg/ml leupeptin and aprotinin. Lysates were processed for SDS-PAGE after adjusting for equal loading by using the BCA protein assay kit (Rockford IL). Proteins resolved in the gel were electrotransferred to Millipore Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford MA). MAP Kinase antibodies, phospho-p44/42 (Thr202/Tyr204) and total p44/42, were purchased from Cell Signaling Technology Inc. (Beverly, MA). Phospho-p44/42 MAP Kinase (Thr202/Tyr204) antibody detects endogenous levels of p42 and p44 MAP kinase (Erk1 and Erk2) only when phosphorylated at Thr202 and Tyr204 of human Erk. The antibody does not cross-react with the corresponding phosphorylated residues of either JNK/SAPK or p38 MAP kinase.
Anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase for immunoblotting were obtained from Chemicon (Temecula, CA). Blots were developed using chemiluminescence (ECL Western Blotting Detection System, Amersham, Arlington Heights, IL) and band densities were analyzed by densitometry using NIH Image software (Scion). For stripping, membranes were incubated in stripping buffer (62.5 mM Tris, pH 6.75, 2% SDS, and 100 mM β-mercaptoethanol) at 50°C for 30 minutes followed by washing five times in TBS.
Preparation of synthetic peptides
Solvents and chemicals were purchased from Aldrich (Milwaukee, WI) if not stated otherwise. The protected amino acids, and coupling reagents were purchased from NovaBiochem (San Diego, CA) or GL Biochem (Shanghai, China). Hydrophilic ethyleneglycol based linker Ebes was synthesized according to the previously published procedure.36 Mass spectrometry analyzes were performed using Bruker BiflexIII MALDI TOF spectrometer. The following HPLC systems were used to analyze and purify the products. The analytical reversed-phase HPLC (system A): Vydac C18 218TP54 (5 μm, 250 × 4.6 mm), linear gradient 0.05% TFA - acetonitrile 0–90% in 30 min, flow rate 1mL/min. Preparative reversed-phase HPLC (system B): Vydac C18 218TP1022 (10 μm, 250 × 22 mm), linear gradient 0.05% TFA-acetonitrile 0–90% in 30 min, flow rate 7 mL/min.
The D-amino acid peptides were synthesized on Rink Amide MBHA resin (loading 0.64 mmol/g) purchased from GL Biochem (Shanghai, China). The peptides were assembled using Linux powered parallel 96-peptide synthesizer developed in our laboratory and in cooperation with J-Kem Scientfic (St. Louis, MO). The peptides were synthesized using 3-fold excess of amino acid using O-Benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) N,N-diisopropylethylamine (DIEA) activation for 1.5 hr followed by removal of 9-fluorenylmethyloxycarbonyl (Fmoc) with 20% piperidine (2 times 10 min). The allyloxycarbonyl (Alloc) protecting group from Lys side chain amino group was removed using Pd[PPh3]4 0.2 eq and phenylsilane 20 eq 2 × 30 min.37 The peptides were biotinylated using 3-fold excess of d-biotin and HBTU/DIEA activation. The progress of the biotinylation was monitored by Kaiser test.38 The resin was washed and dried in vacuo before final cleavage and side chain deprotection. The peptides were cleaved from the resin using trifluoroacetic acid/triisopropylsilane/water 95:2.5:2.5 v/v/v for 3h at room temperature. The crude peptides were precipitated from cleavage mixtures by addition of 50-fold excess of chilled diethylether, the solvent was removed and the solid product was triturated three times with diethylether.
The peptides were purified on semi-preparative HPLC (system B) to final purity 90–99% determined using HPLC (UV 220 nm, 254 nm; system A) and the structure was confirmed by mass spectrometry. MS MALDI-TOF data (m/z) for peptides with general formula R-Ebes-Lys(Biotin)-NH2 where R is the D-amino acid sequence: kikmviswkg 1773.404 (M + H)+; aikmviswkg 1716.031 (M + H)+; kakmviswkg 1731.479 (M + H)+; kiamviswkg 1716.124 (M + H)+; kikaviswkg 1713.312 (M + H)+; kikmaiswkg 1745.169 (M + H)+; kikmvaswkg 1731.204 (M + H)+; kikmviawkg 1757.181 (M + H)+; kikmvisakg 1679.950 (M + Na)+; kikmviswag 1715.998 (M + H)+; kikmviswka 1787.124 (M + H)+; ikmviswkg 1645.412 (M + H)+; kmviswkg 1531.904 (M + H)+; mviswkg 1424. 226 (M + Na)+; viswkg 1272.824 (M + H)+; iswkg 1173.816 (M + H)+; kikmviswk 1716.208 (M + H)+; kikmvisw 1587.918 (M + H)+; kikmvis 1401.902 (M + H)+; kikmvi 1314.856 (M + H)+; kikmv 1201.900 (M + H)+; ikmviswk 1588.004 (M + H)+; ikmvisw 1459.902 (M + H)+; kmviswk 1474.936 (M + H)+; kmvisw 1346.774 (M + H)+; akmvisw 1417.197 (M + H)+; iamvisw 1402.275 (M + H)+; ikavisw 1399.276 (M + H)+; ikmaisw 1431.258 (M + H)+; ikmvasw 1417.715 (M + H)+; ikmviaw 1443.176 (M + H)+; ikmvisa 1344.793 (M + H)+.
RESULTS
Minimal element of HYD1 to support cell
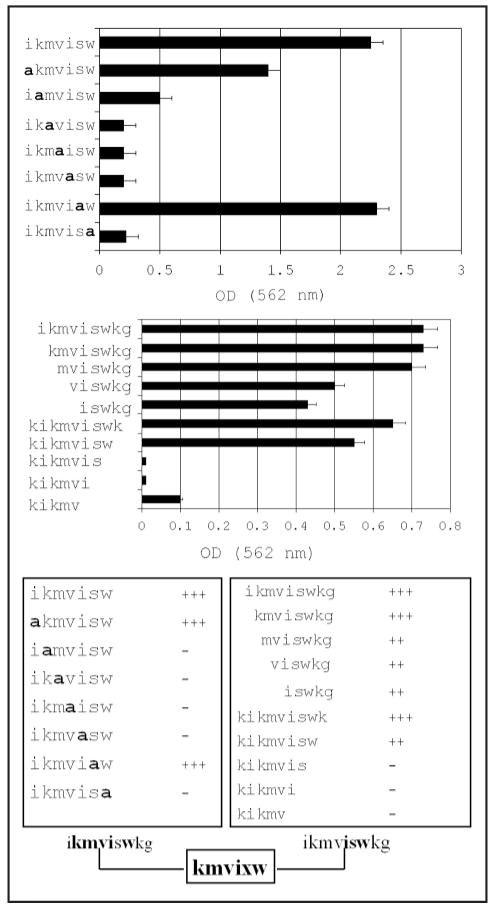
Adhesion is kmvixw. HYD1 (kikmviswkg), when immobilized, supports tumor cell adhesion at concentrations as low as 2 μg/well and maximal cell adhesion occurred at 10 μg/well.32 Since variants of HYD1 have different molecular weights, cell adhesion to 50 μM of each variant was used in the assay. To determine the minimal element of HYD1 required for cell adhesion, we systematically truncated the N and C terminus one amino acid at a time (Fig. 1A). Most of the adhesion activity was retained with the deletion of lys-glycine from the C-terminus. However the peptide no longer supported cell adhesion if the next residue, tryptophan was removed. Deletion of the N-terminus on the other hand, was tolerated very well until the third residue, methionine was removed, leading to gradual decrease in cell binding. When HYD1 was truncated from both termini, we have found that ikmvisw peptide retained full cell adhesion activities. Alanine substitution analysis of this peptide indicated that the N-terminal isoleucine and one serine in the C-terminal region were not critical amino acids for cell adhesion (Fig. 1B). Taken together (Fig. 1C), these data show that the minimal element necessary for cell adhesion is kmvixw.
Figure 1.
The minimal element mediating cell adhesion to immobilized HYD1 is kmvixw. The ability of PC3N cells to adhere within 60 minutes to either immobilized variants of HYD1 containing systematic alanine substitution (A) at the indicated residues or (B) deletion variants of the HYD1 peptide. The residue that was substituted by alanine (a) is as indicated. Similarly, the sequence of the variant forms in the deletion analysis is as indicated. The amount of bound cells was estimated by releasing the cell bound crystal violet dye using 0.1 M sodium citrate and reading the absorbance at 562 nm. All determinations were done in triplicate and the error bars represent one standard deviation about the mean. The data obtained from both the alanine substitution and deletion analysis is collated in (C).
Blocking migration required xkmviswxx
HYD1 completely blocks random haptotaxis on laminin 322 (laminin 5) without toxicity or influencing cell division.33 We tested whether HYD1 or HYDS influenced the cell cycle distribution of the PC3N cells as determined by DNA analysis using flow cytometry. PC3N cells treated with HYD1 had the following distribution: G0–G1: 47%, S: 37.8%, G2-M: 15.0% and Sub G: 0.5%. PC3N cells treated with the scrambled peptide (HYDS) had the following distribution: G0–G1: 49%, S: 37.3%, G2–M: 13.0% and Sub G: 0.8%. Treatment with the active peptide HYD1 did not alter the cell cycle distribution as compared to treatment with the inactive scrambled peptide, HYDS.
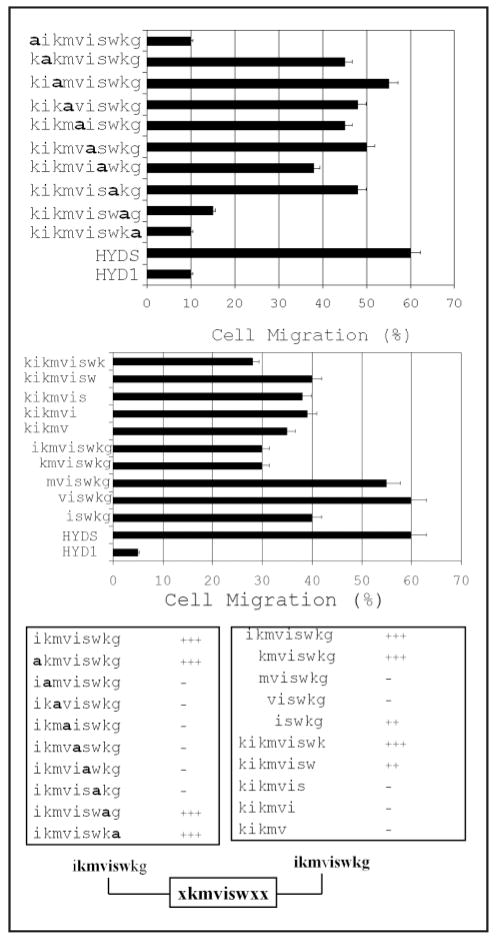
The inhibition of haptotaxis by the HYD1 peptide and its derivatives is independent of disrupting adhesion to laminin 322 (laminin 5) since the cells were grown on a ligand before treatment with the peptide. Alanine-substitution analysis of HYD1 revealed that the N-terminal lysine and the final two C-terminal residues, a lysine and glycine, were not critical residues for blocking haptotaxis (Fig. 2A). All other alanine-substitution mutants resulted in cell migration, i.e., a loss of peptide activity. These data suggest that the active sequence for blocking cell migration was kmvisw.
Figure 2.
The minimal element to block cell migration is xkmviswxx. (A) PC3N cells were grown to a monolayer on laminin 322 and cell migration was induced by scratching the monolayer surface. The cells were incubated with the indicated alanine substitution sequences of HYD1 at a concentration of 50 μM in the presence of 1% FBS for 12 hours following the scratch. (B) PC3N cells were grown to a monolayer on laminin 322 and cell migration was induced by scratching the monolayer surface. The cells were incubated with the indicated deletion forms of the HYD1 sequences at a concentration of 50 μM in the presence of 1% FBS for 12 hours following the scratch. In both (A and B), the data represent the percent of cells in a microscopic field migrating into the scratch. Three fields were analyzed per sample and the error bars represent one standard deviation about the mean. The data obtained from both the alanine substitution and deletion analysis is collated in (C).
Analysis using the deletion variants of HYD1 showed that minimal deletion from either end of the peptide resulted in at least partial loss of activity (Fig. 2B). Using the negative control scrambled peptide, HYDS, to indicate 100% cell migration, several truncated peptides including, ikmviswkg, kmviswkg and kikmviswk were effective at blocking cell migration by approximately 50%. These data are consistent with the results found in the adhesion assays in that both the N- and C-terminal regions of HYD1 are responsible for its bioactivity. Taken together (Fig. 2C), these data show that the minimal element necessary to maximally block cell migration on laminin 322 (laminin 5) is xkmviswxx.
Activation of ERK signaling required ikmviswxx
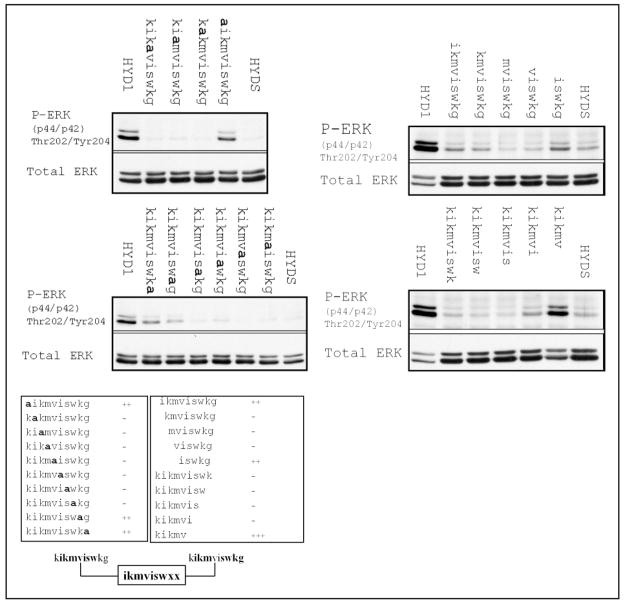
HYD1, when introduced as a soluble ligand, completely blocks random hapotaxis on laminin 322 and enhanced cellular signals are coupled with the loss of cell motility.33 HYD1 induces activation of ERK that is maximal at 10 min post-treatment.33 Using this endpoint as an indicator of HYD1 bioactivity, we tested the truncated and alanine-substituted variants of HYD1 for their ability to activate ERK. Activation of ERK at 10 min post-treatment was conserved with alanine-substitution only at the N-terminal lysine and C-terminal glycine (Fig. 3A). All other alanine-substitution resulted in a loss of ERK activation, suggesting that the interior residues, ikmviswk, are critical. Deletion analysis of HYD1 revealed that both N- and C-terminal regions are involved in activation of ERK (Fig. 3B). Specifically, the N-terminal 5 amino acid residues, kikvm, and the C-terminal 5 amino acid residues, iswkg, alone induced partial activation of ERK. The N-terminal region, kikvm, induced a stronger signal than iswkg. In addition, some of the truncated mutants were able to partially activate ERK (Fig. 3B), however the amount of activation was substantially reduced in comparison to the full-length peptide. In addition, mixing the two terminal regions of HYD1, kikmv and iswkg, enhanced ERK activation compared to the response of these two peptides alone (data not shown). These data showed that both the N- and C-terminal regions of HYD1 contained an element that can induce ERK activation. Taken together, the results (Fig. 3C) indicated that activation of ERK signaling required ikmviswxx.
Figure 3.
The minimal element to activate ERK signaling is ikmviswxx. (A) PC3N cells were placed on laminin-5 coated tissue culture plates in the presence of serum-free media for 1 hour followed by the addition of HYD1 (kikmviswkg), alanine-substitution mutants of HYD1, or a scrambled version of HYD1, HYDS (wiksmkivkg) at a concentration of 50μM in serum-free media for 10 minutes. (B) PC3N cells were placed on laminin 322 coated tissue culture plates in the presence of serum-free media for 1 hour followed by the addition of HYD1 (kikmviswkg), the indicated deletion forms of HYD1, or HYDS (wiksmkivkg) at a concentration of 50μM in serum-free media for 10 minutes. In both (A and B), cell lysates were analyzed for phosphorylation of ERK Thr-202/Tyr-204. Blots were stripped and reprobed for total ERK. The data obtained from both the alanine substitution and deletion analysis is collated in (C).
DISCUSSION
Our previous data characterized the cell adhesion peptide, HYD1 (kikmviswkg), as a bioactive peptide. Specifically, when immobilized, HYD1 was an adhesion agonist by supporting tumor cell adhesion.32 In addition, when introduced in soluble form to prostate tumor cells growing on laminin 322 (laminin 5), HYD1 completely blocked random haptotaxis. Coupled to the loss of cell motility, HYD1 enhanced cell signaling on laminin 322 (laminin 5) resulting in transient activation of ERK.33 The objective of this study was to determine the minimal element within the linear 10 amino acid structure of HYD1 that mediates these biological activities. We utilized both alanine substitution analysis of the peptide and N- and C-terminal truncation of HYD1 to determine the minimal elements.
Analysis of the peptide variants suggests the minimal element necessary to support prostate tumor cell adhesion was kmvixw. However, we found that deletion of amino acids from both the N- and C-terminal regions of the peptide severely attenuated its ability to activate ERK and block cell migration on laminin 322 (laminin 5). Alanine-substitution revealed that kmviswxx is the minimal element necessary to induce both ERK activation and block cell migration. This is consistent with the idea that the bioactive regions of HYD1 could be sensitive to structural changes produced with amino acid deletion when HYD1 is used in soluble form, such as with the signaling and migration assays, whereas when the peptide is immobilized a smaller motif is sufficient. Nevertheless, the interior motif ikmvisw was critical in each biological assay tested. One observation that is more difficult to explain is that the N-terminal 5 residues of HYD1, kikmv, was totally inactive in both cell adhesion assay and cell migration blocking assay but potent in activating ERK signaling. At the present time, it may be possible that kikmv and iswkg bind to two different sites on the same receptor or bind to two totally different receptors that interact. We have shown that HYD1 interacts with the integrin alpha subunits, α6 and α3.33
Cell surface molecules such as integrins are suitable therapeutic targets because they are critical mediators for several aspects of cancer progression.5,7 In particular, integrins are attractive anti-metastatic targets because they directly mediate adhesion events necessary in metastasis while also integrating information from the extracellular environment into cellular signals that are required for cell motility and survival at distant sites in the body. There are currently several integrin inhibitors, both antibody and peptide-based, under investigation for cancer therapy.39,40 Vitaxin, a humanized αvβ3 antibody, is currently in Phase II trials.41–44 The peptide-based inhibitor of αvβ3/αvβ5, Cilengitide, is in Phase II trial for advanced solid tumors and has shown promise for use in combination therapy.45–48 A few other αvβ3 and α5β1-blocking peptides have been developed but have not yet entered clinical trials.49 These current therapies are focused on αv and α5 integrin subunits that bind vitronectin or fibronectin. We have shown that HYD1 interacts with integrin alpha subunits that bind laminin, i.e., α6 and α3.33 Given the importance of these receptors in epithelial tumor progression,8–10,50–52 HYD1 may provide insight for the development of a new class of integrin inhibitors.
It is important to note that we have not tested the minimal peptide derivatives that support adhesion, block migration or activate signaling (kmvixw, xkmviswxx or ikmviswxx, respectively) for their ability to bind to the α6 or α3 integrin. This is primarily because these derivatives all contain kmvixw; a sequence which is the “core” of the full-length sequence (ikmviswg) of HYD1. We have reported that the parent peptide, HYD1, interacts with α6 and α3 integrins as determined by affinity precipitation reactions.33 Since the derivatives all contain the “core sequence” it would be unlikely that we would be able to measure a difference in integrin binding with the addition of “flanking” sequences.
Another important consideration is that migration alterations could simply be due to cell death or cell growth arrest. We showed previously that the migration blocking effects of the parent peptide, HYD1, were not due to cell toxicity. Specifically, the inhibitory effect of the parent peptide was reversible by removing the peptide and adding soluble laminin-5, indicating a reversible process. In addition, video microscopy illustrated that the membrane surfaces were still active, anoikis or necrosis was not observed and cell division still occurred.33 However, the question of whether cell cycle arrest is involved was not addressed previously. We find that alterations in cell cycle distributions as determined by FACS analysis, do not change in response to the peptide treatment conditions.
The fact that HYD1 consists of all D-amino acids (10-mer) makes it resistant to proteolysis. Very recently, Lam and his colleagues described the use of OBOC combinatorial library method to optimize α4β1 integrin binding peptides into a peptidomimetic molecule (Kd~2pM) that can be used to image a lymphoma xenograft in vivo with high specificity.53 This same strategy can be applied to HYD1 by modifying the nonessential amino acid residues with various organic moieties. Highly potent HYD1 mimics can potentially be used as prostate cancer targeting/imaging agents or as therapeutics to block metastasis.
In summary, the minimal element for optimal bioactivity of the cell adhesion peptide HYD1 (kikmviswkg) was determined. When immobilized as an adhesive substrate, the minimal element for potent activity is kmvixw. In solution, the minimal element necessary to block cell migration and activate cell signaling through ERK is ikmviswxx. The shortest sequence active in all three assays was iswkg. Work is currently underway to further optimize this peptide into useful tumor targeting agents.
Figure 4.
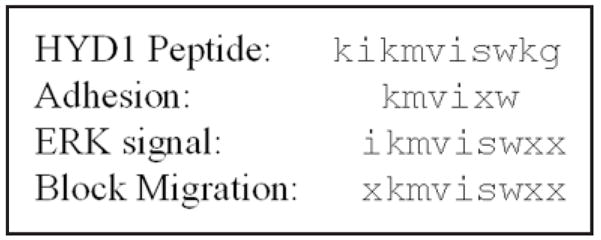
The optimal sequences for cell adhesion, ERK signaling and blocking cell migration. The results indicating the minimal sequences from Figures 1, 2 and 3 are aligned for comparative purposes.
Acknowledgments
The work was supported by grants provided by: NIH, T32 CA 09213, CA75152, CA 23074, CA56666 and Department of Defense Program Grant DAMD17-03-1-0110 to J.M. We appreciate the helpful discussions of the results with Drs. Bowden, Nagle and Vagner and the technical assistance of Dr. Man Ling Chen.
ABBREVIATIONS
- OBOC
one-bead-one compound combinatorial library method
- PBS
Phosphate buffered saline
- FBS
fetal bovine serum
- PMSF
phenyl methyl sulfonyl floride
- HYD1
hybrid d-amino acid peptide (kikmviswkg)
References
- 1.Chambers AF, MacDonald IC, Schmidt EE, Morris VL, Groom AC. Clinical targets for anti-metastasis therapy. Adv Cancer Res. 2000;79:91–121. doi: 10.1016/s0065-230x(00)79003-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi T. Cancer metastasis: Characterization and identification of the behavior of metastatic tumor cells and the cell adhesion molecules, including carbohydrates. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:39–64. doi: 10.2174/1568006053005038. [DOI] [PubMed] [Google Scholar]
- 4.Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–57. [PubMed] [Google Scholar]
- 5.Jin H, Varner J. Integrins: Roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 8.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–28. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 9.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–6. [PubMed] [Google Scholar]
- 10.Rosendahl A, Neumann K, Chaloupka B, Rothmund M, Weinel RJ. Expression and distribution of VLA receptors in the pancreas: An immunohistochemical study. Pancreas. 1993;8:711–8. doi: 10.1097/00006676-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–6. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- 12.Ponce ML, Hibino S, Lebioda AM, Mochizuki M, Nomizu M, Kleinman HK. Identification of a potent peptide antagonist to an active laminin-1 sequence that blocks angiogenesis and tumor growth. Cancer Res. 2003;63:5060–4. [PubMed] [Google Scholar]
- 13.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, Roller PP, Kleinman HK, Yamada Y. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270:20583–90. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin alpha 1 chain Ile-Lys-Val-Ala-Val (IKVAV)-containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–80. [PubMed] [Google Scholar]
- 16.Skubitz AP, Letourneau PC, Wayner E, Furcht LT. Synthetic peptides from the carboxy-terminal globular domain of the A chain of laminin: Their ability to promote cell adhesion and neurite outgrowth, and interact with heparin and the beta 1 integrin subunit. J Cell Biol. 1991;115:1137–48. doi: 10.1083/jcb.115.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SY, Nomizu M, Yamada Y, Kleinman HK. Liver metastasis formation by laminin-1 peptide (LQVQLSIR)-adhesion selected B16-F10 melanoma cells. Int J Cancer. 1997;71:436–41. doi: 10.1002/(sici)1097-0215(19970502)71:3<436::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–4. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 19.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 20.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 21.Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467–70. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- 22.Gehlsen KR, Argraves WS, Pierschbacher MD, Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988;106:925–30. doi: 10.1083/jcb.106.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engbring JA, Kleinman HK. The basement membrane matrix in malignancy. J Pathol. 2003;200:465–70. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 24.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–4. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 25.Lam KS, Lebl M, Krchnak V. The “one-bead-one-compound” combinatorial library method. Chem Rev. 1997;97:411–48. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 26.Lam KS, Liu R, Miyamoto S, Lehman AL, Tuscano JM. Applications of one-bead one-compound combinatorial libraries and chemical microarrays in signal transduction research. Acc Chem Res. 2003;36:370–7. doi: 10.1021/ar0201299. [DOI] [PubMed] [Google Scholar]
- 27.Cwirla SE, Peters EA, Barrett RW, Dower WJ. Peptides on phage: A vast library of peptides for identifying ligands. Proc Natl Acad Sci USA. 1990;87:6378–82. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin JJ, Panganiban LC, Devlin PE. Random peptide libraries: A source of specific protein binding molecules. Science. 1990;249:404–6. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- 29.Rodi DJ, Makowski L, Kay BK. One from column A and two from column B: The benefits of phage display in molecular-recognition studies. Curr Opin Chem Biol. 2002;6:92–6. doi: 10.1016/s1367-5931(01)00287-3. [DOI] [PubMed] [Google Scholar]
- 30.Aina OH, Sroka TC, Chen ML, Lam KS. Therapeutic cancer targeting peptides. Biopolymers. 2002;66:184–99. doi: 10.1002/bip.10257. [DOI] [PubMed] [Google Scholar]
- 31.Pennington ME, Lam KS, Cress AE. The use of a combinatorial library method to isolate human tumor cell adhesion peptides. Mol Divers. 1996;2:19–28. doi: 10.1007/BF01718696. [DOI] [PubMed] [Google Scholar]
- 32.DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, Cress AE. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001;61:3308–13. [PubMed] [Google Scholar]
- 33.Sroka TC, Pennington ME, Cress AE. Synthetic D-amino acid peptide inhibits tumor cell motility on laminin-5. Carcinogenesis. 2006;27:1748–57. doi: 10.1093/carcin/bgl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran NL, Nagle RB, Cress AE, Heimark RL. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with Stromal cells. Am J Pathol. 1999;155:787–98. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, Fusenig NE. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur J Cell Biol. 1998;75:273–86. doi: 10.1016/S0171-9335(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 36.Song A, Wang X, Zhang J, Marik J, Lebrilla CB, Lam KS. Synthesis of hydrophilic and flexible linkers for peptide derivatization in solid phase. Bioorg Med Chem Lett. 2004;14:161–5. doi: 10.1016/j.bmcl.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 37.Grieco P, Gitu PM, Hruby VJ. Preparation of ‘side-chain-to-side-chain’ cyclic peptides by Allyl and Alloc strategy: Potential for library synthesis. J Pept Res. 2001;57:250–6. doi: 10.1111/j.1399-3011.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–8. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 39.Kerr JS, Slee AM, Mousa SA. The alpha v integrin antagonists as novel anticancer agents: An update. Expert Opin Investig Drugs. 2002;11:1765–74. doi: 10.1517/13543784.11.12.1765. [DOI] [PubMed] [Google Scholar]
- 40.Tucker GC. Alpha v integrin inhibitors and cancer therapy. Curr Opin Investig Drugs. 2003;4:722–31. [PubMed] [Google Scholar]
- 41.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: A humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–61. [PubMed] [Google Scholar]
- 42.Mikecz K. Vitaxin applied molecular evolution. Curr Opin Investig Drugs. 2000;1:199–203. [PubMed] [Google Scholar]
- 43.Patel SR, Jenkins J, Papadopolous N, Burgess MA, Plager C, Gutterman J, Benjamin RS. Pilot study of vitaxin-an angiogenesis inhibitor-in patients with advanced leiomyosarcomas. Cancer. 2001;92:1347–8. doi: 10.1002/1097-0142(20010901)92:5<1347::aid-cncr1456>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 44.Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16:125–32. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- 45.Smith JW. Cilengitide merck. Curr Opin Investig Drugs. 2003;4:741–5. [PubMed] [Google Scholar]
- 46.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39:917–26. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 47.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–72. [PubMed] [Google Scholar]
- 48.Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Cilengitide (EMD 121974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral Oncol. 2004;40:228–30. doi: 10.1016/j.oraloncology.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–87. [PubMed] [Google Scholar]
- 50.Carey TE, Laurikainen L, Ptok A, Reinke T, Linder K, Nair TS, Marcelo C. Culture conditions affect expression of the alpha 6 beta 4 integrin associated with aggressive behavior in head and neck cancer. Adv Exp Med Biol. 1992;320:69–79. doi: 10.1007/978-1-4615-3468-6_10. [DOI] [PubMed] [Google Scholar]
- 51.Rabinovitz I, Mercurio AM. The integrin alpha 6 beta 4 and the biology of carcinoma. Biochem Cell Biol. 1996;74:811–21. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- 52.Weinel RJ, Rosendahl A, Neumann K, Chaloupka B, Erb D, Rothmund M, Santoso S. Expression and function of VLA-alpha 2, -alpha 3, -alpha 5 and -alpha 6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992;52:827–33. doi: 10.1002/ijc.2910520526. [DOI] [PubMed] [Google Scholar]
- 53.Li P, Liu R, Marik J, Wang W, Takada Y, Lam KS. Identification of high-affinity peptidomimetics against a4b1 integrin using combinatorial chemistry. Nature Chemical Biology. 2006;2:381–9. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]