Abstract
The serotonin neurons of the dorsal and medial raphe nuclei project to all areas of the forebrain and play a key role in mood disorders. Hence, any loss or degeneration of serotonin neurons could have profound ramifications. In a monkey model of surgical menopause with hormone replacement and no neural injury, E and P decreased gene expression in the dorsal raphe nucleus of c-jun n-terminal kinase (JNK1) and kynurenin mono-oxygenase (KMO) that promote cell death. In concert, E and P increased gene expression of superoxide dismutase (SOD1), VEGF, and caspase inhibitory proteins that promote cellular resilience in the dorsal raphe nucleus. Subsequently, we showed that ovarian steroids inhibit pivotal genes in the caspase-dependent and caspase-independent pathways in laser captured serotonin neurons including apoptosis activating factor (Apaf1), apoptosis inducing factor (AIF) and second mitochondria-derived activator of caspases (Smac/Diablo). SOD1 was also increased specifically in laser captured serotonin neurons. Examination of protein expression in the dorsal raphe block revealed that JNK1, phosphoJNK1, AIF and the translocation of AIF from the mitochondria to the nucleus decreased with hormone therapy, whereas pivotal execution proteins in the caspase pathway were unchanged. In addition, cyclins A, B, D1 and E were inhibited, which would prevent re-entry into the cell cycle and catastrophic death. These data indicated that in the absence of gross injury to the midbrain, ovarian steroids inhibit the caspase-independent pathway and cell cycle initiation in serotonin neurons. To determine if these molecular actions prevented cellular vulnerability or death, we examined DNA fragmentation in the dorsal raphe nucleus with the TUNEL assay (terminal deoxynucleotidyl transferase nick end labeling). Ovarian steroids significantly decreased the number of TUNEL positive cells in the dorsal raphe. Moreover, TUNEL staining prominently colocalized with TPH immunostaining, a marker for serotonin neurons.
In summary, ovarian steroids increase the cellular resilience of serotonin neurons and may prevent serotonin neuron death in women facing decades of life after menopause. The survival of serotonin neurons would support cognition and mental health.
Keywords: serotonin, estrogen, progesterone, non-human primate, neuroprotection, apoptosis, necrosis, JNK1, apoptosis inducing factor, AIF, mitochondria, caspases, kynurenine mono-oxygenase, TUNEL, laser capture
Introduction
The serotonin system modulates a wide range of neural outcomes from emotion to intellect to metabolism and it is a target of pharmacotherapies, steroid hormones, cytokines, neuropeptides and trophic factors, all of which impact the generation and efficacy of serotonin neurotransmission. This laboratory has devoted effort toward understanding the actions of ovarian hormones in serotonin neurons and their terminal fields with a macaque model of surgical menopause. Serotonin neurons express estrogen receptor beta (ERβ) and progestin receptors (PR) [1; 2]. We found that estrogen (E), plus or minus progesterone (P) supplementation improves serotonin neural function by (1) increasing gene and protein expression of tryptophan hydroxylase (TPH2), the rate limiting enzyme in serotonin synthesis; (2) by increasing transport and binding of the serotonin reuptake transporter, or SERT; (3) by decreasing gene and protein expression of the 5HT1A somatodendritic autoreceptor, as well as 5HT1A binding and coupling to Gi protein and (4) by decreasing gene and protein expression of the serotonin degrading enzyme, monoamine oxidase A, or MAO-A [3; 4; 5; 6].
A large body of literature also indicates that the ovarian hormones, E and P are neuroprotective [7; 8; 9]. Animal models have shown that administration of E, prior to or coincident with trauma or global ischemia decreases tissue damage [10; 11]. Recent reports have found activation of caspases in the damaged tissue suggesting that the caspase-dependent pathway is involved in apoptosis resulting from ischemia [12; 13]. Moreover, E treatment decreases caspase activation in the damaged area of the brain [14]. In the clinical arena, some evidence suggests that E may delay the onset of overt neurodegeneration in Alzeheimer’s [15] or Parkinson’s disease [16; 17] although the precise mechanism(s) of action remain obscure.
Neurodegeneration is usually thought of in the context of severe deficits in motor or cognitive function. However recently, it has been suggested that even the psychopathologies may involve functional degeneration of critical central neural systems and many are thought to have a serotonergic etiology [18; 19]. The dorsal and medial raphe nuclei contain the serotonergic neurons that project to all areas of the forebrain and serotonin neurons modulate a wide range of autonomic functions, cognitive domains, affect or mood, anxiety, and stress-related disorders. Thus, any loss or degeneration of serotonin neurons could have profound ramifications.
This issue may be extremely important for menopausal women grappling with issues surrounding hormone therapy with estrogens or estrogens plus progestins. Women experience ovarian failure and loss of ovarian steroid production around 50 years of age. Thus, with extended life spans, a woman may live 35–40 years without ovarian steroids. If serotonin neurons are gradually dying due to lack of steroid supported gene expression, then geriatric depression, anxiety, fretfulness, decreased coping skills and increased vulnerability to stress can be predicted outcomes.
We use a model of hormone therapy in primates after surgical menopause, which does not involve a gross insult to the brain. So, although the neuroprotective effect of hormone therapy has been extensively studied in models of injury to the brain, the neuroprotective actions of hormone therapy in a non-injured environment are less understood. However, there is a continuum of lesser insults to the central nervous system over the course of life such as stress, illness and psychological trauma. Thus, we questioned the potential of ovarian steroids to increase the resilience of serotonin neurons to normal life stress. When we refer to “an effect of ovarian steroids” or “ an effect of hormone therapy”, it means that there was a response to E and E+P, or to mainly E+P. We did not include responses to E that were reversed by supplemental P treatment in the current analysis.
To address this question, we used laser capture of serotonin neurons and microarray platforms to seek changes in gene expression related to cell survival. We then determined if the changes in gene expression translated to changes in protein expression. Recently, we examined the dorsal and median raphe for chromosome fragmentation using a TUNEL assay.
How Neurons Die
After decades of research on neuronal death, a bewildering array of nomenclature describing different types of cell death and the morphological changes that accompany them preside in the literature. In an excellent review, Martin et al.,(1998) argue that that there is necrosis, or pathological cell death, and apoptosis, or physiological programmed cell death, as well as a hybrid continuum encompassing aspects of both in excitotic neuronal death. Both apoptosis and necrosis start with mitochondrial permeation, but apoptosis leads to chromatin condensation whereas necrosis requires lysosomal activation and leads to vacuole formation [20]. Morphological phenotypes have been designated apoptotic or necrotic as well as, endocytic-autophagic, nonlysosomal vesiculate [21] and nonapoptotic degenerative [22]. Based upon nuclear morphology, another scheme designates caspase-dependent programmed cell death (PCD) as classical apoptosis, and caspase-independent PCD as apoptosis-like PCD involving apoptosis inducing factor (AIF) or necrosis-like PCD involving autophagic death and paraptosis [23]. However, it is not clear how necrosis-like PCD is distinguished from canonical necrosis on a morphological basis. A discerning observation is that apoptotic cells are phagocytosed by nearby resident cells, typcially without generating an acute inflammatory response. Therefore, infiltration of neutrophils does not occur with apoptosis as it does with ischemic cellular necrosis. Thus, it seems prudent to refer to apoptosis as programmed cell death and necrosis as passive canonical cell death, which are different, but with the possibility of a continuum depending on the cell type and the insult. It also appears well established that there are indeed, caspase-dependent apoptosis and caspase-independent apoptosis, the latter of which involves AIF release from the mitochondria and recruitment of downstream nucleases [24]. Cell death involving unique morphological features requires additional study for definitive classification.
Mature differentiated neurons have permanently exited the cell cycle and forcing cell division on a mature neuron is a lethal event. An important aspect of neuronal apoptosis involves pathological re-initiation of the cell cycle. In a comprehensive review, Krantic et al., (2005) explain that entry of neurons into the cell cycle from quiescence or G0 phase is controlled by a family of cyclin-dependent kinases (CDKs). Activation of CDKs requires association with regulatory units called cyclins in a phase-specific manner. The induction of the cyclins is considered a prelude to cell death and it has been documented in various neurodegenerative diseases and models [25].
Vulnerable Neurons and Normal Life Stress
By the early 1990’s, studies of the degeneration, neuroprotection and regeneration of CNS neurons departed from descriptions of neurons as either dead or alive and the notion of neuronal health as a dynamic equilibrium that spans a spectrum between resilient and vulnerable neuronal states came to be accepted [26]. Thus, at any given time, neurons may be very healthy and functioning well including optimal DNA repair, or somewhere on a slippery slope where they are unhealthy and falling farther and farther behind in DNA repair but not dead. The concept of “neuroendangerment” has been proposed for these unhealthy neurons [27]. They are vulnerable and additional stresses could kill them. However, up to the point of no return, recovery may be possible. Many factors could render a neuron vulnerable. Endogenous factors such as imbalances in homeostasis, hormones, growth factors, cytokine milieu, genetics and ageing probably play important roles. External factors such as stress, diet, exercise, disease and medications are transduced into live or die signals for neurons in ways that are not fully understood. However, systemic infection and cytokines clearly contribute to neuroendangerment [28].
Depression and mood disorders have traditionally been viewed as neurochemical deficits. However, evidence is increasing that depression is accompanied by marked changes in the number or size of neurons and glia in discrete brain regions [27]. Stressful life events are one of the major predisposing risk factors for developing depression, and stress- induced atrophy occurs in hippocampal neurons of rodents [29]. In tree shrews, stress increased the incidence of apoptosis in the temporal cortex. Moreover, antidepressant treatment reduced apoptotic neurons to control unstressed levels [30]. The midbrain has not received the attention of the forebrain areas, yet it is the location of the serotonin and norepinephrine cell bodies, and these cells control many forebrain functions associated with stress responsivity [31].
We have begun to question whether the steroid hormones, E and P, play a role in serotonin neuron resilience or vulnerabily.
Nonhuman primate model
We utilize a non-human primate model of surgical menopause with hormone replacement. Adult female rhesus monkeys (Macaca mulatta) were ovariectomized following their use for in vitro fertilization (IVF) protocols by the surgical personnel of ONPRC according to accepted veterinary surgical protocol. Recently, the time between ovariectomy and initiation of hormone therapy has become a critical issue. Ideally, all the monkeys in a study should be ovariectomized for the same length of time. However, due to cost, we acquired animals that were ovariectomized in other programs and then released into the available pool for terminal studies. We aimed to use monkeys that were ovariectomized within a reasonably short time frame, but long enough to withdraw from elevated steroid secretion during ovarian hyperstimulation protocols. Thus, our animals were assigned between 3 and 8 months after ovariectomy and the average length of time equaled 5.5 months. All animals were born in China and were aged between 7 and 14 years by dental exam. The average age of the animals for which the age was known equaled 8.8 yrs for the animals perfused with 4% paraformaldehyde for immunocytochemical (ICC) and in situ hybridization (ISH) studies; 8.9 yrs for the animals perfused with RNA later for laser capture, RNA extraction and qRT-PCR studies; and 9.5 yrs for the animals that were not perfused and used for protein studies. They weighed between 4 and 8 kg, and were in good health. Animals were either treated with placebo (Ovx control group), or treated with E for 28 days (E only group), or treated with E for 28 days and then supplemented with P for the final 14 of the 28 days (E+P group). In some studies, we included a group treated with placebo for 14 days and then treated with P for 14 days (P only group).
The ovariectomized control monkeys received empty Silastic capsules (s.c.). The E-treated monkeys were implanted with a 4.5-cm E-filled Silastic capsules (i.d. 0.132 in.; o.d. 0.183 in.; Dow Corning, Midland, MI) filled with crystalline estradiol [1,3,5(10)-estratrien-3,17-b-diol; Steraloids, Wilton, NH]. The E+P-treated monkeys received an E-filled capsule and 14 days later, received one 6-cm capsule filled with crystalline P (4-pregnen-3,20 dione; Steraloids). The P-treated monkeys received a placebo capsule and then one 6-cm capsule filled with crystalline P on day 14. All capsules were placed in the periscapular area under ketamine anesthesia (ketamine HCl, 10 mg/kg, s.c.; Fort Dodge Laboratories, Fort Dodge, IA). The E concentrations achieved were similar to levels observed in the mid-follicular phase and in the mid-luteal phase. The P concentrations achieved were similar to levels observed in the mid-luteal phase. The levels achieved by the implants are noted for each experiment below.
The monkeys were euthanized at the end of the treatment periods according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine, given an overdose of pentobarbital (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta. Blood samples were obtained for measurement of steroid hormone levels.
Global Changes in Survival Gene Expression with Hormone Therapy
Initially, we sought novel genes that are regulated by E and P in a block of tissue containing the dorsal raphe of rhesus monkeys using (1) the Affymetrix Human GeneChip array and then (2) the Affymetrix Rhesus GeneChip followed by quantitative (q) RT-PCR. Our approach was to use the chips as a screening or discovery tool, and then to use quantitative (q) RT-PCR on a statistically significant number of animals to confirm or refute the results obtained with the array. We found that E and P regulated genes in a number of pathways. Of current interest were genes related to cellular resilience, which could have a significant impact on the viability of serotonin neurons.
Nine ovariectomized adult female rhesus monkeys were either treated with placebo, E or E+P as described above (n=3/treatment). Serum E concentrations (pg/ml) equaled 11.6±0.7, 153±9.4, and 154± 8.9, respectively. Serum P concentrations (ng/ml) equaled 0.07±0.04, 1.05±0.07, and 7.7±0.3, respectively. The head of each animal was perfused with 3 liters of 1X cold RNA-later buffer (Ambion Inc., Austin, TX) and a small block of the midbrain was obtained that contained the dorsal raphe nucleus. The dorsal raphe block was immediately frozen in liquid nitrogen. RNA was obtained from the microdisected block using TriReagent (Sigma, St. Louis, MO) and further cleaned with a Qiagen RNAeasy column (Velencia, CA). The quality of the RNA from the Qiagen column was examined on an Agilent Bioanalyzer and found acceptable and of equal quality.
Labeled target cRNA was prepared from the 9 animals and hybridized to human Affymetrix HG_U95Av2 GeneChip arrays, which were available at the time. Later, the labeled cRNA was hybridized to the newly developed Affymetrix Rhesus GeneChips. Microarray assays were performed in the Affymetrix Microarray Core of the OHSU Gene Microarray Shared Resource.
Human GeneChip
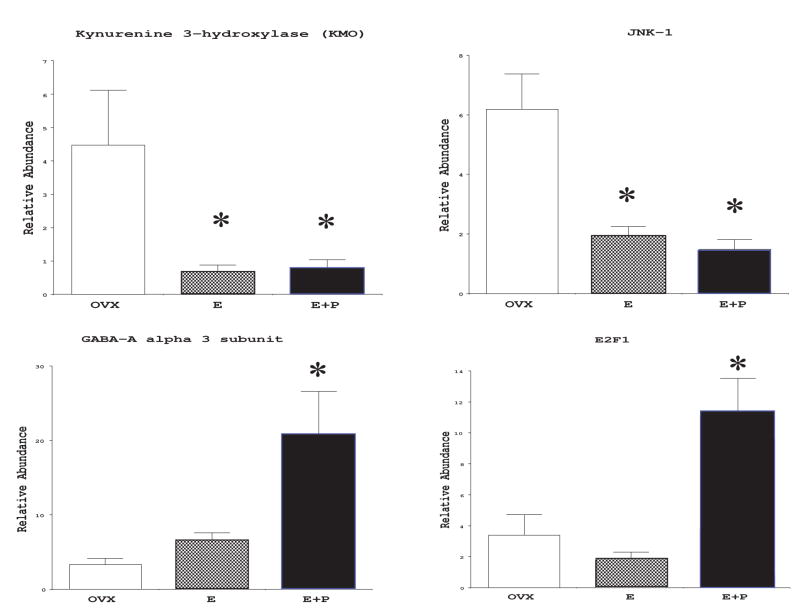
The human microarray reported a number of significant changes in gene expression in the raphe block with E and E+P treatment, and the expression of 4 interesting genes was confirmed with qRT-PCR (Figure 1). Two genes that are directly related to cell death decreased markedly with hormone therapy [32]. The expression of c-jun n-terminal kinase, or JNK1, showed an average 29-fold decrease on the microarray with E+P treatment. In the qRT-PCR assay, JNK-1 was significantly suppressed by E, and it remained significantly suppressed in the E+P treatment group. The second death gene of interest, kynurenin mono-oxygenase, or KMO, decreased ~4.5-fold on the microarray with E treatment. In the qRT-PCR assay, KMO was significantly suppressed by E, and it remained significantly suppressed in the E+P treatment group.
Figure 1.
Histograms illustrating the relative abundance of mRNAs for KMO-1, JNK1, GABA-Aα3 and E2F1 in ovariectomized monkeys treated with placebo (OVX), E or E+P. There were 3 monkeys in each group and the bars on each column represent the standard error of the mean within the group. Asterisks illustrate that the treated group was significantly different from the placebo group by Student Newman Keul’s post-hoc pairwise comparison at p < 0.05 after the groups were found to differ significantly with ANOVA (JNK-1, p < 0.007; KMO, p < 0.05; GABA-Aα3, p < 0.02; E2F1, p < 0.009). The relative abundance of each transcript was determined with qRT-PCR and was normalized with GAPDH. (reprinted from [32])
The suppression of JNK-1 by E (with or without P) is of significant importance. JNK-1 (also known as MAP kinase 8), is believed to transduce a variety of extracellular stresses, UV radiation, heat shock, trophic factor deprivation or cytokines to selective cellular responses [33]. Ten isoforms of JNK have been identified in human brain by molecular cloning that result from alternative splicing of JNK genes [34]. Activation of JNKs lead to activation of bcl-xl and this can be blocked by overexpresssion of bcl-2 [35]. Thus, JNK1 is a pivotal protein that can initiate a cascade leading to mitochondrial permeability and the release of either cytochrome c or apoptosis inducing factor (AIF), in turn promoting either caspase dependent or independent cell death. JNK1 has demonstrated apoptotic activity in neurons and glia. In neurons, JNK-1 is activated by kainic acid and plays a critical role in the pathogenesis of glutamate neurotoxicity [36]. The ability of estrogen with or without P to markedly decrease expression of JNK1 suggests that this may be one mechanism by which ovarian hormones are neuroprotective.
KMO, is part of an alternate pathway for tryptophan metabolism. Serotonin neurons have a high affinity uptake mechanism for tryptophan and any regulation of this gene within serotonin neurons could be quite pivotal. KMO (EC 1.14.13.9), also known as kynurenine 3-hydroxylase, is a flavoprotein located on the outer membrane of mitochondria. It is distributed throughout the brain but information regarding its regulation is limited [37; 38; 39; 40]. 3-hydroxy-kynurenine (3HK) is the direct product of the enzyme and it is neurotoxic [41; 42; 43]. 3HK induced-neuronal cell death exhibits several features of apoptosis [42; 44; 45], but it does not act at receptors; rather it generates toxic free radicals/reactive oxygen species [45; 46]. Bcl-2, a typical anti-apoptotic gene product, and cyclosporine, which inhibits apoptotic mitochondrial damage, reduced 3HK-induced neurotoxicity in cell culture [47].
Neurotoxic 3HK is converted to three other neurotoxic kynurenines called xanthurenic acid, quinolinic acid and 3-hydroxy-anthranilic acid via kynureninase, kynurenine aminotransferase and 3-hydroxy anthranilic acid oxidase, respectively [39; 48]. All are neurotoxic, however their toxicity proceeds through different mechanisms. [38; 42; 45; 49; 50; 51]
Thus, KMO-1 resides at the branch point in the metabolic pathway of tryptophan and regulates the balance of neuroprotective to neurotoxic kynurenine metabolites. The suppression of this gene by E (with or without additional P) in the raphe region suggests a critical mechanism for estrogenic neuroprotection of serotonin neurons. We have shown that E increases expression of tryptophan hydroxylase at gene and protein levels. The concomitant decrease in KMO indicates that E pushes tryptophan metabolism toward serotonin and away from neurotoxic quinolones.
Another interesting gene, E2F1, showed increased expression with E+P treatment (Figure 1). E2F1 is a transcription factor with different, perhaps context dependent roles. Most studies report that E2F1 functions as an apoptosis-inducing factor [52; 53]. However, transfection of E2F1 into cortical neurons reduced NFkB [54]. We have shown that serotonin neurons contain a large reservoir of NFkB in the cytoplasm and that nuclear translocation increases without ovarian hormones [55]. Thus, an increase in E2F1 could be beneficial in serotonin neurons where NFkB may mediate stress responses. E2F1 may block the activity of p53, which can initiate catastrophic entry into the cell cycle. Moreover, the integrity of the E2F1/Rb complex is neuroprotective in the presence of free oxygen radicals [56]. More study of the role of this factor in serotonin neurons is warranted.
There was a P-induced increase in GABA-Aα 3 subunit expression (Figure 1), which may not directly impact neuronal survival, but it could play an important role in neurotransmission in the dorsal raphe where GABA interneurons impinge on serotonin neurons. The α3 subunit contributes to the benzodiazepine binding site, and this site also binds to P metabolites [57; 58; 59; 60]. Thus, P, perhaps through a genomic mechanism, facilitates nongenomic mechanisms of action by increasing the receptor that in turn, binds to its metabolites. Up-regulation of this subunit could facilitate the anti-anxiety effect of progestins in women. Moreover, a genetic association study found a link between a polymorphism in this subunit and depression in women [61].
Rhesus GeneChip
The RNA from the 9 dorsal raphe blocks was then labeled and hybridized to Affymetrix Rhesus GeneChips when they became available. Hormone therapy changed the expression of a number of pivotal genes in the dorsal raphe block of tissue that play a role in cell survival [62]. With the Rhesus GeneChip and new GeneSifter software, we were able to apply ANOVA to gene expression in the 3 treatment groups and determine significant differences. Figure 2 illustrates the pathways and the genes that were significantly altered as determined by ANOVA (p < 0.05). Genes illustrated in red print were increased and genes illustrated in blue print were decreased. Genes illustrated in green boxes were previously confirmed by qRT-PCR on this RNA preparation and include JNK1, KMO and E2F1 [32]. Different genes were chosen for confirmation of the microarray results with qRT-PCR. The increase in SOD1, VEGF and BIRC4 observed on the microarray was confirmed with qRT-PCR (Figure 3).
Figure 2.
Diagrammatic representation of hormone therapy-induced changes in gene expression in the dorsal raphe block. Genes that exhibited a significant increase (p< 0.05, ANOVA) are shown in RED and genes that exhibited a significant decrease (p< 0.05, ANOVA) are shown in BLUE. Green blocks outline gene changes that were previously confirmed with qRT-PCR. Other information is shown in black. Arrows indicate downstream drive or activation, whereas t-bars indicate downstream blockade. Significant increases in expression were observed in vascular endothelial growth factor (VEGF), fibroblast growth factor receptor 2 (FGF-R2), nerve growth factor receptor associated protein (NGFR-AP), Ras protein-specific quanine nucleotide-releasing factor 1(RAS GRF), MAP kinase kinase 5 (MAP2K5), superoxide dismutase (SOD1) and in 2 ubiquinases (UCHL1 and UBCH7). Also, gene expression significantly increased for 3 proteins called baculovirus IAP repeat containing 4 (BIRC4 or XIAP), BCL2-related protein (MCL-1) and bifunctional apoptosis regulator (BFAR) that are capable of inhibiting caspases. Significant decreases in expression were observed in members of the cytokine signaling pathway including chemokine ligand 12 (CXCL12), TGF-β receptor 3 (TGFβ3), tumor necrosis factor α induced protein (TNFAIP6), member 21 of the TNF superfamily (TNFRSF21), the prostaglandin F receptor (PTGFR) and Fas associated factor 1 (FAF1). Significant decreases were also observed in downstream effectors of cell death including p21-activated kinase 2 (PAK), MAP kinase kinase 4 (MAP2K4) and nibrin (NBSI). The pro-apoptosis gene PDCD4 (programmed cell death 4) was significantly decreased as well. With qRT-PCR we previously found that expression of kynurenin mono-oxygenase (KMO), which produces neurotoxic metabolites of serotonin, and c-jun n-terminal kinase (JNK), which is an apoptosis effector, were significantly decreased in this mRNA preparation (green outline). We also previously found that E2F1 was significantly increased in this mRNA preparation (green outline). (reprinted from [62])
Figure 3.
Histograms illustrating the relative expression of SOD1, VEGF and BIRC4 in the dorsal raphe block as determined with qRT-PCR (n=3 animals/treatment). There was a significant difference between treatment groups for each gene (p < 0.05, ANOVA). Post hoc pairwise comparison indicated that SOD1 and VEGF were significantly higher in the E+P group than the OVX control group, and that BIRC4 was significantly higher in the E and E+P groups than in the OVX control groups. These changes in expression confirm the expression as reported by the microarray. (reprinted from [62])
A number of genes involved in cell survival were increased by E or E+P. There was a significant increase in superoxide dismutase (SOD1) on the microarray, which was confirmed with qRT-PCR (Figure 4). This critical enzyme scavenges free oxygen radicals (ROS) and thus, plays an important role in cell survival [63]. Hormone therapy induced a significant increase in several genes in the MAPK pathway such as VEGF, FGF-receptor 2, NGF receptor associated protein, RAS GRF, and MAP2K5. The increase observed in VEGF on the microarray was confirmed with qRT-PCR (Figure 4) and VEGF has a newly recognized neuroprotective role [64]. The proteins encoded by RAS GRF and MAP2K5 lead to activation of ERK and ERK5 which transcribe genes essential to cell survival [65; 66; 67]. The encoded FGF-receptor 2 protein and the NGF receptor-associated protein are involved in signal transduction of their respective trophic factors and hence would be pro-survival. Hormone therapy also increased the expression of 3 genes that code for endogenous caspase inhibitors called BIRC4 (also known as XIAP), MCL-1 and BFAR. The increase in BIRC4 was confirmed with qRT-PCR (Figure 4). This effect would decrease caspase activation and apoptosis along the caspase dependent pathway [68]. Hormone therapy also increased the expression of two ubiquinases that shuttle α-synuclein and PARK 2 to the proteosome [69], and this action could protect neurons from neurodegeneration associated with the accumulation of these proteins.
Figure 4.
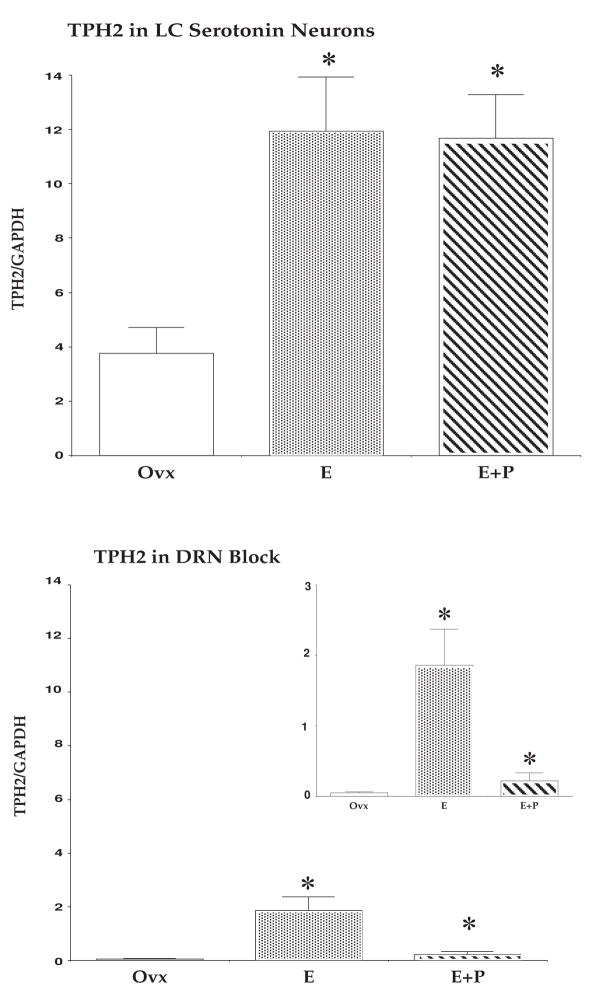
Histograms illustrating the relative expression of TPH2 in the dorsal raphe block compared to laser-captured serotonin neurons (n=3 animals/treatment). There was an enrichment of TPH2 relative to GAPDH in the laser capture pools (top) compared to the dorsal raphe blocks (bottom) illustrated by plotting the data on the same scale. The insert illustrates the relative expression of TPH2 in the dorsal raphe block on a smaller x-axis scale. There was a significant difference between the treatment groups in the laser capture pools (ANOVA p <0.02) and in the dorsal raphe blocks (ANOVA p < 0.01). Asterisks indicate a significant difference from the Ovx control group as determined by posthoc pairwise comparison (SNK p < 0.05). (reprinted from [62])
Furthermore in the raphe block, hormone therapy decreased the expression of a number of pivotal genes in the stress-related, cytokine-signaling pathway. The expression of chemokine ligand 12 (CXCL12), TGF-β receptor 3 (TGFβ3), tumor necrosis factor α induced protein (TNFAIP6), member 21 of the TNF superfamily (TNFRSF21) and the prostaglandin F receptor (PTGFR) were significantly decreased. Fas associated factor 1 (FAF1) decreased significantly and the protein encoded by this gene binds to Fas antigen and can initiate apoptosis or enhance apoptosis initiated through Fas antigen. Intermediaries PAK and MAP2K4 which code for proteins that can activate JNK1 were also decreased. Thus, hormone therapy decreased gene expression in an intercellular signaling pathway that mediates various kinds of stresses.
The nibrin (NBS1) gene product is thought to be involved in DNA double-strand break repair and DNA damage-induced checkpoint activation. The decrease in nibrin may indicate inhibition of the cell cycle, which is neuroprotective for neurons [70]. PDCD4 (programmed cell death 4) encodes a protein localized to the nucleus in proliferating cells and the expression of this gene is modulated by cytokines. The gene product is thought to play a role in apoptosis, but the specific role has not yet been determined [71].
It is likely that the gene changes observed in the block of tissue are global changes that occur in many cell types, which is an important data set, but we were specifically concerned about the changes that occur in serotonin neurons. Moreover, although the changes on the microarray were deemed significant by ANOVA, we noticed that the absolute differences in signal intensity between the groups were not robust, suggesting that masking of differences may occur when multiple cell types are examined.
Survival Gene Expression in Serotonin Neurons with Hormone Therapy
With the advent of technology to microdissect individual neurons, we sought novel genes related to neuronal survival that are regulated by E and P in laser captured serotonin neurons of rhesus monkeys using the Rhesus Affymetrix cDNA array and quantitative (q) RT-PCR [62].
Nine ovariectomized female rhesus macaques were acquired and treated with placebo, E and E+P as described above. Serum E concentrations (pg/ml) equaled <20, 153±10.9, and 187±3.2, respectively. Serum P concentrations (ng/ml) equaled 0.22±0.003, 0.21±0.03 and 7.1±1.2, respectively. The left ventricle of the heart was cannulated and the head of each animal was perfused with 3 liters of 1X cold RNA-later buffer plus 20% sucrose. The midbrain block was obtained and temporarily frozen at −80C. Later, the midbrain block was placed in a cryostat (Microm HM500OM) and brought to −20C. Thin sections (7 μm) through the dorsal raphe nucleus were thaw mounted onto plain glass slides and frozen at −80C. The next morning, the sections were processed in a rapid, RNAse free ICC assay for tryptophan hydroxylase (TPH). Then, the slides were immersed in xylene for 2 minutes and dried under vacuum for 1 hr prior to laser capture. Serotonin neurons appeared darkly stained and were captured with an Arcturus Laser Dissection Microscope (PixCell II). After capture to the microcap film (Capsure macro-211), the films were removed from the caps and immersed in lysis buffer. Up to 10 films with 1000–3000 pulses each were collected into one microtube containing 200 μl of lysis buffer. Approximately 150,000 laser pulses were conducted for a pool (12–15 microtubes were pooled).
The tubes containing the lysis buffer and films were vortexed to dislodge the captured material from the films. Each tube was adjusted to 350 μl of lysis buffer and then 350 μl of 70% ethanol was added and mixed well. The samples were extracted with the RNAeasy microRNA kit from Qiagen according to the directions. The final eluates were pooled and evaporated in a Speedvac. The RNA was suspended in 12 μl of TE (0.01MTris+0.005M EDTA). The quantity of RNA in the resuspended sample was determined with the Ribogreen Quantitation Kit (Molecular Probes, Eugene, OR) or with a Nanodrop Spectrophotometer (ND 1000 V3.3, Wilmington, DE). The integrity of the RNA was examined with the Agilent Bioanalyzer using the pico-chip according to the directions of the manufacturer.
Labeled target cRNA was prepared from 6 pools of laser captured serotonin neurons (n=2 animals/treatment group). The cRNA was hybridized to Rhesus Affymetrix GeneChip arrays. Microarray assays were performed in the Affymetrix Microarray Core of the OHSU Gene Microarray Shared Resource.
GeneSifter software calculated the mean signal intensity and the standard error of the mean for each treatment group. Probe sets that were undetectable in all treatment groups were eliminated. The detectable probe sets were filtered and those probes sets exhibiting a 2 fold or greater change between the treatment groups were subjected to KEGG analysis. Probe sets with robust signal intensities that showed a consistent change with E and E+P (both decreased or both increased) and probe sets that were consistent across multiple representations on the chip were recorded.
Another laser capture pool was made from the 6 animals used for the microarrays, plus an additional 3 animals, yielding 9 laser capture pools (3 animals/treatment group) for qRT-PCR confirmation of 4 pivotal genes which were: SOD1, calpain (CAPN2), Diablo and Cyclin D (CCND1). In addition, the relative expression of TPH2 was compared in the dorsal raphe block and in the laser captured pools to increase confidence in the captured material. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene, as the array hybridization data indicated that this gene was not regulated (data not shown).
Validation
To validate the laser capture pools, the relative expression of tryptophan hydroxylase 2 (TPH2) was examined in the laser capture pools versus the dorsal raphe blocks. TPH2 is the rate- limiting enzyme for serotonin synthesis in the brain. There was a significant enrichment of TPH2/GAPDH in the laser capture pools compared to the dorsal raphe blocks. TPH2/GAPDH was significantly increased by E and E+P treatment in the laser capture pools (ANOVA p < 0.02; SNK p < 0.05) and in the dorsal raphe block (ANOVA, p < 0.01; SNK p < 0.05). Figure 4 contains histograms of the relative expression in the laser capture pools (top) and of the relative expression in the dorsal raphe blocks (bottom) plotted on the same axis scales to illustrate the enrichment of TPH2 relative to GAPDH in the laser capture pools. The insert contains the relative expression of TPH2 in the dorsal raphe block on a smaller scale to better illustrate the regulation by E and E+P.
Using the KEGG analysis provided by GeneSifter, we compared the number of probe sets that changed 2-fold or greater with hormone therapy in the dorsal raphe blocks versus the laser captured serotonin neurons. Only 151 probe sets changed 2-fold or greater in the dorsal raphe blocks whereas 10,493 probe sets changed 2-fold or greater in the laser captured serotonin neurons. For example, in the laser captured serotonin neurons, hormone therapy altered 24 out of 70 probe sets in tryptophan metabolism 2-fold or greater, whereas no changes were detected in this pathway in the dorsal raphe block. This further indicates that the laser-captured pools were enriched in serotonin neurons. To further our hypothesis of neuroprotection, we examined the apoptosis and cell cycle pathways in the laser captured serotonin neurons.
Expression Changes Related to Neuroprotection in Laser Captured Serotonin Neurons
To further our hypothesis that hormone therapy protects serotonin neurons from cell death, we focused on 2-fold or greater expression changes in apoptosis and cell cycle pathways in the laser captured neurons. Hormone therapy induced 2-fold or greater changes in several pivotal genes in the caspase dependent and independent pathways, as well as other survival genes in the laser captured serotonin neurons (Figure 5). The expression of SOD1, calpain, Diablo and cyclin D were confirmed in laser capture pools from 3 animals in each treatment group with qRT-PCR (Figure 6).
Figure 5.
Diagrammatic illustration of the hormone therapy-induced gene changes related to apoptosis in laser-captured serotonin neurons (2-fold or greater compared to Ovx placebo control, n=2animals/treatment). Genes that exhibited a 2-fold or greater increase are shown in RED and genes that exhibited a 2-fold or greater decrease are shown in BLUE. Other information is shown in black. Arrows indicate downstream drive or activation, whereas t-bars indicate downstream blockade. SOD1 and FGFR2 increased in serotonin neurons in the same fashion as in the dorsal raphe block. In the caspase dependent pathway, RIP1, BID, Apaf1, Diablo and CARD8 were decreased. The expression of procaspase 3 increased but this may not translate to active protein. In the caspase independent pathway, AIF was decreased. There was a marked increase in IκBα, which binds NFκB in the cytoplasm. Other survival related genes that increased include NTRK2, PI3K (85Kd subunit), PKA (catalytic subunit) and calpain. (reprinted from [62])
Figure 6.
Histograms illustrating the relative expression of SOD1, calpain (CAPN2), Diablo and cyclin D (CCND1) in laser captured serotonin neurons (n=3 animals/treatment) as determined with qRT-PCR. There was a significant difference between the groups for all 4 genes. There was a significant increase in SOD1 and calpain with E and E+P treatment (posthoc comparison, SNK p < 0.05). There was a significant decrease in cyclin D with E and E+P treatment and a significant decrease in Diablo with E+P treatment (posthoc comparison, SNK p < 0.05). These changes in expression confirm the expression as reported by the microarray. (reprinted from [62])
As in the raphe block, SOD1 increased on the microarray and this was confirmed with qRT-PCR (Figure 6). FGFR2 also increased in the raphe block and in the laser captured serotonin neurons. This suggests that the effect of hormone therapy on SOD1 and FGFR2 may be global effects. The likely decrease in ROS that would ensue from elevated SOD1 protein and the increase in FGFR2 signaling may be an important part of hormone therapy-induced neuroprotection.
In the caspase dependent pathway, hormone therapy decreased RIP1, BID, Apaf1 and CARD8. The BID gene encodes a death agonist that heterodimerizes with bcl-2. The encoded protein is a member of the bcl-2 family of cell death regulators. It is a mediator of mitochondrial damage induced by caspase-8 (CASP8); CASP8 cleaves this encoded protein, and the COOH-terminal part translocates to mitochondria where it blocks bcl-2 and triggers cytochrome c release. Thus, decreasing expression of BID would promote cell survival. RIP1 encodes a TNF receptor (TNFRSF)-interacting serine-threonine kinase 1. It is an adaptor protein in the TNF signaling pathway that leads to apoptosis. Hence, a decrease in RIP1 would render the cell less susceptible to TNF signaling. Apaf1, or apoptotic peptidase activating factor 1, encodes a cytoplasmic protein that initiates apoptosis. Upon binding cytochrome c and dATP, this protein forms an oligomeric apoptosome. The apoptosome binds and cleaves caspase 9 preproprotein, releasing its mature, activated form. Activated caspase 9 stimulates the subsequent caspase cascade that commits the cell to apoptosis. Therefore, the hormone therapy induced decrease in Apaf1 in serotonin neurons is a pivotal means to decrease activity of the caspase dependent apoptosis pathway. Hormone therapy also decreased CARD8. Caspase recruitment domain (CARD)-containing proteins, such as CARD8, are involved in pathways leading to activation of caspases or nuclear factor kappa-B in the context of apoptosis or inflammation, respectively [72]. It is curious that we observed an increase in gene expression for procaspase 3. However, there appear to be mechanisms in place that would prevent its activation.
Hormone therapy decreased an extremely pivotal gene in the caspase independent pathway. AIF, or apoptosis inducing factor, also called PDCD8 or programmed cell death 8, was decreased 4-fold with E+P treatment. The AIF gene encodes a flavoprotein essential for nuclear disassembly in apoptotic cells that is found in the mitochondrial intermembrane space in healthy cells. Induction of apoptosis results in the translocation of this protein to the nucleus where it effects chromosome condensation and fragmentation. In addition, this gene product induces mitochondria to release the apoptogenic proteins cytochrome c and caspase-9 so it also ties into the caspase dependent pathway. Nonetheless in certain cell lines, AIF was sufficient to induce apoptosis when the caspases are inhibited [73].
Smac/DIABLO is a second mitochondria-derived activator of caspases. Expression of DIABLO was decreased 3-fold on the microarray and 5-fold in the qRT-PCR assay with E+P treatment (Figure 6). DIABLO encodes a protein that blocks the endogenous caspase inhibitors (IAPs) [74] and it plays a pivotal role in the mediation of apoptosis through TRAIL and the TNF pathway [75]. Hormone therapy increased several IAP genes in the raphe blocks and DIABLO would normally inhibit these IAPs, thereby removing a brake on the caspase dependent pathway. A decrease in DIABLO would allow activity of the IAPs. Moreover, we also observed a marked decrease in genes in the TNF/cytokine signaling pathway in the raphe block suggesting that hormone therapy may converge on multiple effectors of this pathway as a major mechanism of neuroprotection.
Hormone therapy induced a robust 23-fold increase in IκBα gene expression in serotonin neurons. The encoded protein binds to NFκB and prevents nuclear translocation and activation of stress related genes, which can lead to apoptosis depending on the cell context [76; 77], although in some paradigms, NFkB has neuroprotective actions [78]. We previously demonstrated that NFκB colocalizes in serotonin neurons and that hormone therapy decreased the nuclear location of NFκB, but there was no change in the expression of NFκB at gene or protein levels [55]. The microarray data suggests that an increase in IκBα may be an important mechanism by which hormone therapy reduced the translocation of NFκB to the nucleus. It is also worth noting that the 5HT1A autoreceptor gene contains NFκB response elements in the promoter and that hormone therapy decreases expression of the 5HT1A gene in the dorsal raphe. It follows that the hormone therapy-induced increase in IκBα and subsequent decrease in NFκB translocation may be mediating the effect of hormone therapy on 5HT1A gene expression.
Hormone therapy also increased several survival factors in serotonin neurons that are upstream of mitochondrial permeability. The expression of NTRK2 increased robustly with E treatment. This gene encodes a kinase that upon neurotrophin binding, phosphorylates itself and members of the MAPK pathway. Mutations in the gene have been associated with obesity and mood disorders [79; 80]. The expression of the gene coding the 85kD regulatory subunit of PI3K (phosphoinositide-3-kinase) increased 2-fold. This protein activates AKT, which in turn, phosphorylates and inactivates BAD [81]. BAD is a member of the bcl-2 family that opens mitochondrial pores by binding to bcl-2, thereby releasing cytochrome c [82; 83]. Hormone therapy also increased the gene expression of the catalytic subunit of PKA (cyclic AMP dependent protein kinase). PKA inhibits the activity of BAD, which would decrease mitochondrial permeability and the release of pro-apoptotic proteins. Previous work showed that estradiol prevented BAD phosphorylation in a model of ischemic neuronal death [84]. Of note, hormone therapy caused a robust 3-fold increase in calpain 2 that was confirmed with qRT-PCR (Figure 6). This is interesting because until recently the calpains were considered pro-apoptotic. However, new data suggests that they may be neuroprotective and prevent signaling through the TNF pathway [85; 86]. Thus, the ability of hormone therapy to block TNF signaling by decreasing gene expression in the intracellular signaling cascade and by increasing calpain further suggests that this is a pivotal site of action for hormone therapy induced neuroprotection.
Expression Changes in Cell Cycle Genes in Laser Captured Serotonin Neurons
As illustrated in Figure 7, hormone therapy-induced a 2-fold or greater decrease in Cyclins A, B, D1, and E. The cyclins play essential roles in the progression of the cell cycle. The most pivotal of the cyclins, cyclin D1, decreased 2-fold on the microarray and nearly 10-fold in the qRT-PCR assay with E and E+P treatment (Figure 6). Cyclin D1 is essential for cells to reenter the cell cycle and re-entry into the cell cycle is catastrophic for terminally differentiated cells, like serotonin neurons [25]. Therefore in neurons, cell cycle proteins are considered pro-apoptotic [87; 88].
Figure 7.
Diagrammatic illustration of the hormone therapy-induced changes in cell cycle regulatory genes in laser-captured serotonin neurons. Genes that exhibited a 2-fold or greater increase are shown in RED and genes that exhibited a 2-fold or greater decrease are shown in BLUE. Other information is shown in black. Arrows indicate downstream drive or activation, whereas t-bars indicate downstream blockade. There was a 2-fold or greater decrease in gene expression for Cyclins A, B, D1 and E. However, there was a 2-fold increase in gene expression for the checkpoint protein, ATM. Altogether, these changes would prevent serotonin neurons from re-entering the cell cycle which leads to catastrophic apoptosis in terminally differentiated neurons. (reprinted from [62])
In addition, hormone therapy increased the expression of ATM by 2-fold in serotonin neurons. ATM encodes a pivotal cell cycle checkpoint kinase that functions as a regulator of a wide variety of downstream proteins [89]. Activation of ATM plays a central role in shutting down cell cycle transitions through a series of effector molecules at each checkpoint [90]. Altogether, these data suggest that another mechanism by which hormone therapy is neuroprotective is by preventing re-entry of serotonin neurons into the cell cycle.
In summary, it appears that hormone therapy is neuroprotective of serotonin neurons and probably other neurons in the midbrain at the level of gene expression by several major mechanisms. Hormone therapy induces a decrease in gene expression in the TNF/cytokine signaling pathway and in Smac/Diablo, a mediator of apoptosis by this pathway, and it increases calpain which blocks TNF signaling; hormone therapy decreases expression of genes that encode pivotal proteins in the caspase dependent and independent pathways; hormone therapy increases gene expression of pivotal survival factors; and hormone therapy alters gene expression governing cell cycle initiation and progression. Thus, hormone therapy provides trophic support and inhibits the expression of genes that increase vulnerability to cytokines and stress in serotonin neurons and the surrounding neuropile.
The changes observed in gene expression occurred in one month in otherwise normal animals in paired housing with environmental enrichment. Extrapolating from this data is straightforward. In the long-term absence of ovarian steroids, serotonin neurons would be less resilient and may die with normal life stress. However, it is necessary to demonstrate that the gene expression changes were manifested at the protein level, and that the proteins were active.
Apoptotic Protein Changes in the Dorsal Raphe with Hormone Therapy
Therefore, in the next study we examined the expression of pivotal proteins in the caspase dependent and caspase independent pathways. The expression of JNK1 and phosphorylated JNK1, the Bcl-2 family members (Bcl-2, Mcl-1, Bak and Bax), Apaf1, XIAP, caspase-3, cytochrome c and AIF were measured by western blot in subcellular fractions of the dorsal raphe region to determine the mechanism by which hormone therapy may promote neuroprotection in the absence of global injury [91].
In the microarray study, gene expression changes were examined in the dorsal raphe block, and also in laser captured serotonin neurons. To study protein changes we had to resort to analysis of the dorsal raphe block. We proposed that gene changes observed in the dorsal raphe block would be translated to protein changes that may be detectable by western blot. However, the changes in gene expression observed in the dorsal raphe block, although significant, were not robust fold changes. Thus, lack of translation to a detectable change in protein was also feasible. We expected that gene changes in the laser-captured neurons might be masked at the protein level. Nonetheless, we used the latter data set to provide hints of important regulatory events.
Twelve ovariectomized female rhesus macaques were treated with placebo, E and E+P as described above (n=4/group). Serum E concentrations (pg/ml) equaled 32±3, 123±29, and 122±16, respectively. Serum P concentrations (ng/ml) equaled <0.2, <0.2, and 4.6±0.3, respectively. The midbrain block was obtained without perfusion and fresh frozen in liquid nitrogen, stored frozen (−80C) for up to one week and then thawed, microdisected and the raphe region was processed for subcellular fractions. Subcellular fractionation and western blotting was performed followed by densitometric analysis of signal bands as previously described [91].
JNK1 expression and activity was decreased in hormone-treated animals
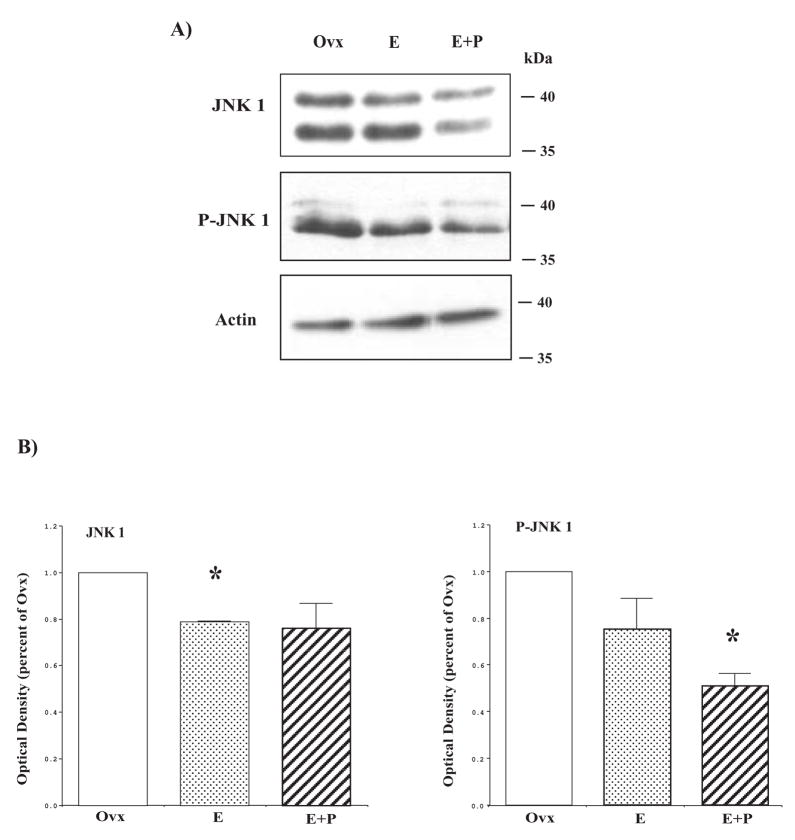
We first examined the pro-apoptosis gene, JNK1, which was down regulated at the gene level in the dorsal raphe block of hormone-treated animals [32]. Representative blots of JNK1 and P-JNK1 are shown in Figure 8, top and middle panels. The actin blot is shown in Figure 8, bottom panel, as a loading control. Consistent with our JNK1 gene expression data, JNK1 protein in E+P treated monkeys was down regulated compared to the control Ovx monkeys (Figure 8B, left panel). JNK1 is activated by phosphorylation by MAPK kinases MKK4/7. Thus, we immunoblotted to determine the phosphorylation status of JNK1 (P-JNK1) by which the activity of JNK1 can be inferred. P-JNK1 was reduced in E+P-treated animals compared to the Ovx animals (Figure 8B, right panel). Because the reduction of P-JNK1 could be a consequence of the reduction in JNK1 protein, we calculated the ratio of P-JNK1 to JNK1. There was no difference between treatment groups in the ratio of P-JNK1/JNK1 suggesting that the decrease in JNK1 protein was producing the decrease in detectable P-JNK1. Actin signals were not different across the gels so the JNK1 data were not normalized to actin.
Figure 8.
Hormone therapy decreases JNK1 expression and activity. (A) Cytoplasmic fraction from representative Ovx control, E and E+P-treated animals were immunoblotted for JNK1 and phospho-JNK1. JNK1 and phospho-JNK1 expression were reduced in E+P-treated animals. Actin was used as a loading control. (B) Histograms illustrating the optical density of JNK1 and phospho-JNK1 as the percentage of Ovx control (n=4/group). There was a significant difference between the groups for both JNK1 and phospho-JNK1 (ANOVA p < 0.05). JNK1 was significantly decreased in E treated animals and in E+P-treated animals, phospho-JNK1 was significantly reduced (posthoc comparison p<0.05). (reprinted from [91])
The JNK pathway is activated in response to environmental stress and largely, the activation leads to apoptosis. However, expression and activity of JNK under basal conditions and by non-noxious environmental stimuli also indicate a physiological role of JNKs in the nervous system [92]. JNKs appear to be involved in both neuronal regeneration and neuroplasticity, thus the activation of JNK pathways elicits very different types of cellular responses depending on the stimuli ranging from cell proliferation to cell death [93]. In this study, we showed that JNK1 and phospho-JNK1 were significantly decreased by E+P treatment. Similarly, E has been shown to attenuate hepatocellular injury following ischemia-reperfusion injury by significantly downregulating JNK activity [94].
JNK1 can activate either the caspase-dependent pathway or the caspase-independent pathway although this is somewhat of a misnomer since caspase 3 can be recruited in the so-called independent pathway. In either case, mitochondria pores are critical. Depending on the stress, mitochrondrial permeability can lead to the release of cytochrome c, which in turn activates the caspase dependent pathway. Alternatively, mitochrondrial permeability can result in the release of Smac/Diablo or AIF. Diablo can inhibit IAPs, but AIF translocates to the nucleus and precipitates chromatin condensation. This process is called the caspase independent pathway although AIF can recruit caspases. Co-occurance of both mechanisms is common in apoptosis, but the caspase independent pathway can lead to apoptosis even when the caspases are blocked [73]. Since JNK1 is a pro-apoptotic kinase that can activate both caspase-dependent and independent pathways, we examined both downstream pathways in our different treatment groups.
Bcl-2 family members were not altered by hormone therapy
The Bcl-2 family members consist of pro- and anti-apoptotic members, which play a crucial role in the regulation of apoptosis by regulating mitochondrial membrane permeability. To determine whether hormone therapy is neuroprotective as a result of an alteration in the expression ratio of the pro- or anti-apoptotic proteins, we examined the expression of the Bcl-2 family proteins. There were no significant changes in the anti-apoptotic proteins Bcl-2 and Mcl-1 or in the pro-apoptotic protein, Bak, in the mitochondrial fraction of hormone-treated animals although Mcl-1 exhibited an upward trend (Figure 9A and 9C, top panels). We speculate that Mcl-1 may increase in serotonin neurons but the change is masked by other neurons in the dorsal raphe block. In addition, we examined pro-apoptotic Bax expression both in the cytosolic and mitochondrial fractions (Figure 9A, 9B and 9C, bottom panels). There was no effect of hormone treatment on Bax expression in the cytosol or mitochondria. Representative western blots for each of the examined proteins are shown in Figure 9, parts A and B and the quantitation of each protein is shown in Figure 9, part C. Cox1 signal is shown as a loading control for the mitochondrial proteins, and actin signal is shown as a loading control for the cytoplasmic protein. There was no difference in Cox1 or actin signals across the gels, so the Cox1 and actin signals were not used to normalize the data. In summary, hormone therapy did not exert a significant effect on the Bcl-2 family members.
Figure 9.
Hormone therapy does not alter the expression of Bcl-2 family members. (A) Mitochondrial fractions from representative Ovx control, E and E+P treated animals were immunoblotted for Bcl-2, Mcl-1, Bak and Bax. COX I was used as loading control. (B) Cytoplasmic fractions from representative animals of each treatment group were immunoblotted for Bax. Actin was used as loading control. (C) Histograms illustrating the optical density of Bcl-2 family members (Bcl-2, Mcl-1, Bak, and Bax) as the percentage of Ovx control (n=4/group). There was no significant difference between the groups for any of the Bcl-2 family members in either the mitochondrial or cytoplasmic fractions (ANOVA p > 0.1). (reprinted from [91])
Mitochondrial Bcl-2 family members play an important role in mitochondrial permeability. Anti-apoptotic members maintain pore integrity and prevent the release of cytochrome c, which leads to caspase activation. Pro-apoptotic members bind anti-apoptotic members and thereby open pores through which cytochrome c is released. Several studies have suggested that E effects the expression of the Bcl family members [95; 96; 97; 98; 99]. These studies involve gross insults, such as glutamate toxicity, TNFα or β-amyloid peptide-induced apoptosis, and ischemic brain injury, which elevate pro-apoptotic Bcl-2 family members. E treatment decreased pro-apoptotic Bcl-2 family members and increased anti-apoptotic Bcl-2 family members and ameliorated the damage. However, in our non-insult model, there was no difference in anti-apoptotic Bcl-2 protein with treatment, which is consistent with the lack of difference observed at the level of gene expression [100]. Anti-apoptotic Mcl-1 gene expression was previously found to increase with hormone therapy and it exhibits a similar, though nonsignificant, trend at the protein level. However, pro-apoptotic Bak and Bax were unchanged with hormone therapy at both gene and protein levels. Thus, there was little or no effect of hormone therapy on these members of the Bcl family in the raphe block. However, this does not rule out a potential regulation in serotonin neurons per se.
Our data differ from previous reports using non-injured ovariectomized rats and primary cultures of hippocampal neurons. E treatment of ovariectomized rats for 4 weeks increased the expression of Bcl-2 and decreased the expression of Bax in the hippocampus [101]. Likewise, E treatment of cultured rat hippocampal neurons increased Bcl-2 [65]. E treatment universally increases Bcl-2 in cultured neurons with cytotoxic insults [102; 103; 104]. However, in a study of apoptotic versus necrotic cell death, it was reported that E was protective of induced apoptotic death in cultures of hippocampus and septum, but not cortex, and this was linked to the density of ERα [105]. In contrast, another study showed E protection of glutamate-treated cortical cultures that included an increase in Bcl-2 [106]. Thus, our results may be due to a species difference, the area of the brain or to the paucity of ERα in the raphe region.
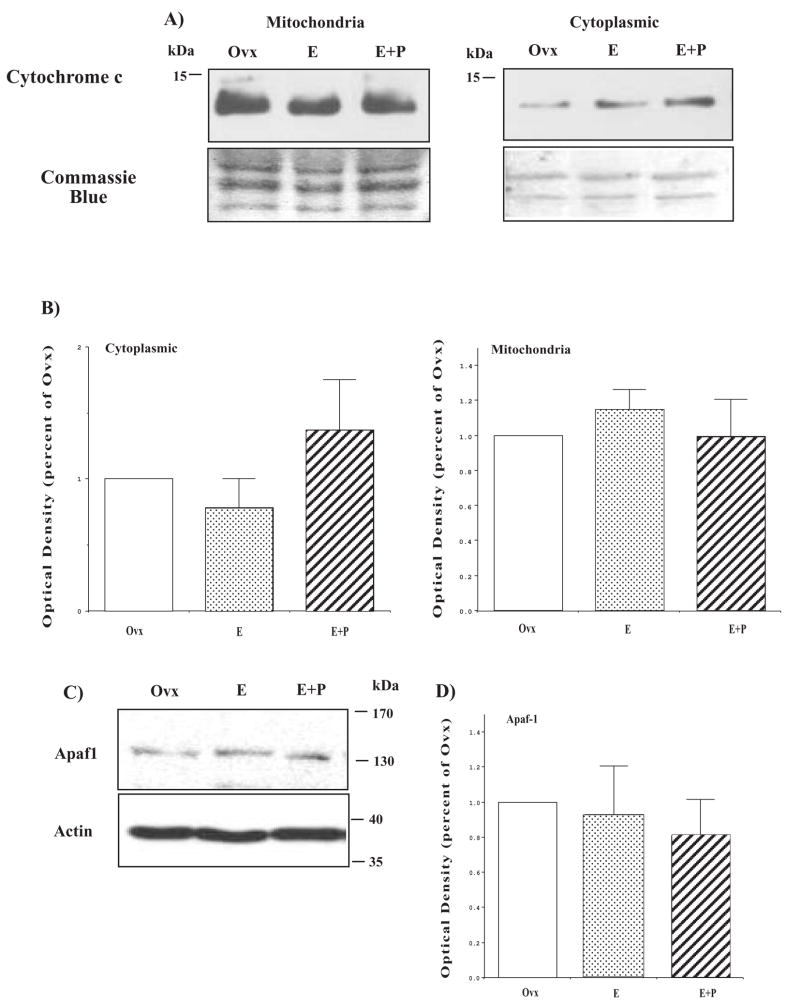
No effect of hormone treatment on cytochrome c and Apaf1
The release of different pro-apoptotic proteins present in the mitochondria, such as cytochrome c or AIF, is observed in both caspase-dependent or -independent apoptotic cell death. Thus, we examined the expression of cytochrome c in mitochondria and cytosolic fractions. There was no difference in total cytochrome c (data not shown) and cytochrome c was present in the cytoplasm in all treatment groups. However, the amount of cytochrome c present in the cytoplasm was independent of treatment (Figure 10). The blot was stained with Coomassie blue and demonstrated consistent loading with equal band density of various visible proteins. Cytochrome c activates the adaptor molecule apoptotic peptidase-activating factor 1 (Apaf1), generating the complex known as the apoptosome. Each apoptosome recruits procaspase-9, which leads to cell death. Therefore, we determined whether Apaf1 was influenced by hormone therapy. As shown in Figure 10, there was no significant effect of hormone treatment on Apaf1 expression. The Apaf1 blot was reprobed for actin as a loading control and there was no difference in actin signals across the gels. Therefore, the Apaf1 data was not normalized by actin.
Figure 10.
Hormone therapy did not affect apoptosome components. (A) Immunoblots showing cytochrome c in mitochondria and cytoplasm from representative Ovx control, E and E+P-treated animals (B) Histograms illustrating the optical density of cytochrome c as the percentage of Ovx control in cytoplasm and mitochondria (n=4/group). There was no significant difference between the groups for either cytoplasmic or mitochondrial cytochrome c expression (ANOVA p > 0.1). (C) Immunoblots showing Apaf1 expression in representative Ovx control, E and E+P treated animals. (D) Histogram illustrating the optical density of Apaf1 as the percentage of Ovx control (n=4/group). There was no significant difference between the groups for Apaf1 (ANOVA p > 0.1). (reprinted from [91])
Cytochrome c is a peripheral protein of the mitochondrial inner membrane and functions as an electron shuttle between complex III and complex IV of the respiratory chain and its activity is necessary for life. Upon apoptotic stimuli, cytochrome c is released from mitochondria into the cytoplasm where it oligomerizes with Apaf1 forming a complex known as apoptosome. Each apoptosome then recruits caspase 9, which leads to activation by self-proteolysis. Recent data suggests that cytochrome c release does not act in an all-or nothing manner, but rather acts in a biphasic fashion [107; 108; 109]. Although cytochrome c plays a pivotal role in glutamate-induced neuron death, which is ameliorated by pretreatment with E [110], we observed no change in cytochrome c with hormone treatment in the dorsal raphe region.
The effect of hormone treatment on caspases and IAP
Active caspase 9 cleaves the effector caspases (caspases 3, 7, and 10), and effector caspases then cleave and activate many substrates that commit the cell to death [111]. Caspase 3 is considered the central and final apoptotic effector caspase responsible for much of biological apoptosis. There are several studies showing that E prevents the up-regulation of caspase 3 induced by glutamate, H2O2, or β-amyloid apoptosis [95; 112; 113; 114].
In our study with Affymetrix Rhesus Macaque Gene Chips, there was no effect of E or E+P on caspase 3 in the dorsal raphe block [100]. This seemed inconsistent with a neuroprotective action of ovarian hormones. Thus, we examined the expression level of pro- and cleaved (active) caspase 3 proteins in the dorsal raphe block. A representative blot is shown in Figure 11A. We saw little to no detectable cleaved caspase 3 in any treatment group (Figure 11A). Unlike the gene expression detected on the microarray, the total protein level of pro-caspase 3 was decreased in E+P-treated animals compared to Ovx animals (Figure 11B). However, there was no active caspase-3 expression in any of the treatment groups. The discrepancy between gene and protein expression in the dorsal raphe block suggests that mechanisms involving procaspase degradation may be in effect. However, future study of procaspase in serotonin neurons by immunohistochemistry is needed. In addition, caspase cleavage can be regulated independently of the apoptosome.
Figure 11.
Effect of hormone therapy on effector caspase-3 and caspase inhibitor. (A) Immunoblot examining pro-caspase 3 and cleaved caspase 3 in representative Ovx control, E and E+P treated animals. (B) Histograms illustrating the optical density of pro-caspase 3 as the percentage of Ovx control (n=4/group). There was a significant difference between the groups for pro-caspase 3 (ANOVA p < 0.05). Pro-caspase 3 was decreased in the E+P-treated animals (posthoc comparison p<0.05). (C) XIAP expression examined by immunoblot in representative Ovx, E and E+P treated animals. Actin was used as loading control. D) Histograms illustrating the optical density of XIAP as the percentage of Ovx control (n=4/group). There was no difference in XIAP with hormone treatment. (reprinted from [91])
Another class of proteins called inhibitors of apoptosis (IAPs) suppresses apoptosis by interacting with and inhibiting the enzymatic activity of caspases [68; 115]. Hormone therapy increased gene expression of the most potent and well-known member, X-chromosome-linked IAP (XIAP or BIRC4), in the dorsal raphe block [100]. However, we did not observe any effect of hormone treatment on XIAP by western blot analysis (Figure 11C, 11D). Each blot was reprobed for actin to demonstrate consistent loading of samples, but the data was not normalized by actin.
In summary, the data suggest that hormone therapy may act globally in the caspase-dependent pathway by decreasing the synthesis of pro-caspase and perhaps by increasing the expression of Mcl-1. However, many other effectors of caspase-dependent cell death were not altered, especially the pivotal proteins cytochrome c and Apaf1. Altogether, these results did not provide compelling evidence for a global action of hormone therapy on the caspase dependent pathway. In addition, the data reinforce the importance of determining whether changes in gene expression are truly manifested at the protein level.
Hormone therapy acts through the caspase-independent pathway
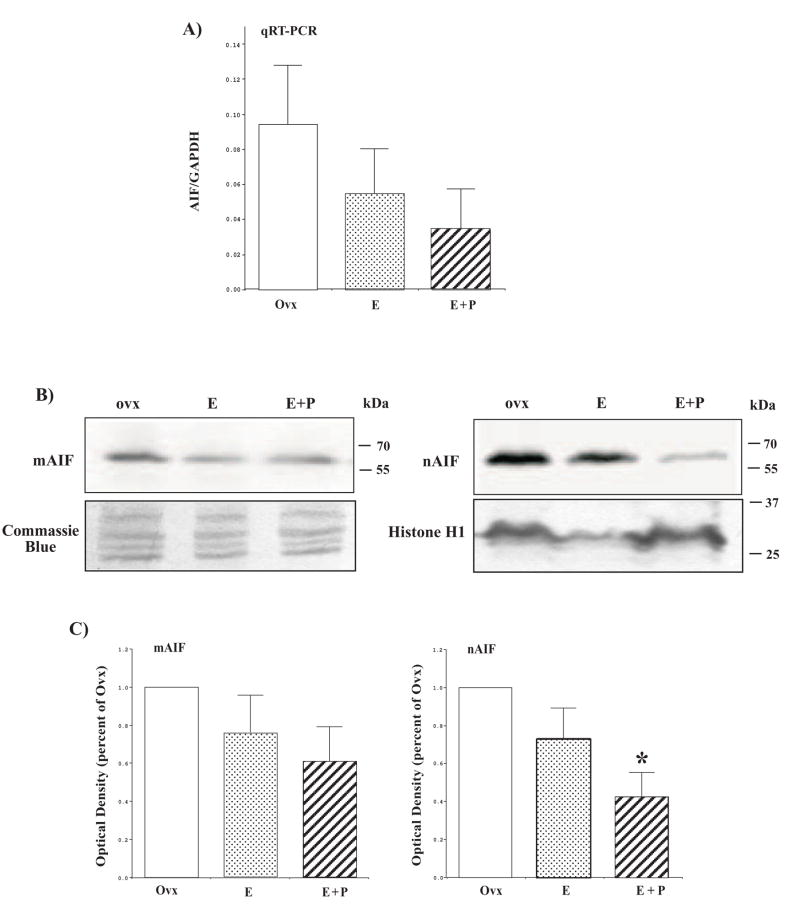
In our recent study with Affymetrix Rhesus Macaque Gene Chips, AIF gene expression was reduced in laser captured serotonin neurons from E and E+P- treated animals [100]. Examination of AIF in mRNA extracted from the dorsal raphe block with qRT-PCR reflected the microarray results with the laser captured serotonin neurons, but the decrease did not reach statistical significance due to the variance, which is potentially due to the presence of other cell types. (Figure 12). Nonetheless, it was important to examine AIF in the nuclear and mitochondrial fractions from the raphe blocks. There was a declining trend in AIF in the mitochondria but significantly less AIF had translocated to the nucleus in animals treated with E+P (Figure 12). The lower expression of AIF in the mitochondria and nuclei suggest that total AIF may be reduced by hormone treatment, reflecting the mRNA expression. Thus, although cytochrome c release was not altered, hormone therapy significantly decreased AIF release from the mitochondria. The mitochondrial blot was stained with Coomassie blue and demonstrated consistent loading with equal density of multiple visible protein bands. The nuclear blot was reprobed for Histone H1 and showed that the decrease in nuclear AIF was not due to a decrease in protein loaded. Hence, the data was not normalized by Histone H1.
Figure 12.
Effect of hormone therapy on AIF protein expression and nuclear translocation from the mitochondria. (A) Histogram illustrating the relative expression of AIF in the dorsal raphe block as determined with qRT-PCR (n=3/group). Although the difference was not statistically significant, there was a downward trend in AIF gene expression in E and E+P-treated animals. (B) Immunoblot examining AIF expression in the mitochondria (mAIF) and in the nucleus (nAIF) of representative Ovx control, E and E+P treated animals. There appears to be more AIF in the mitochondrial fraction and less AIF in the nuclear fraction with E+P treatment. (C) Histograms illustrating the optical density of mitochondrial AIF and nuclear AIF as the percentage of Ovx control (n=4/group). There was a significant difference between the groups for nuclear AIF (ANOVA p < 0.05). Nuclear translocation of AIF from mitochondria was significantly decreased in E+P-treated animals (posthoc comparison p<0.05). (reprinted from [91])
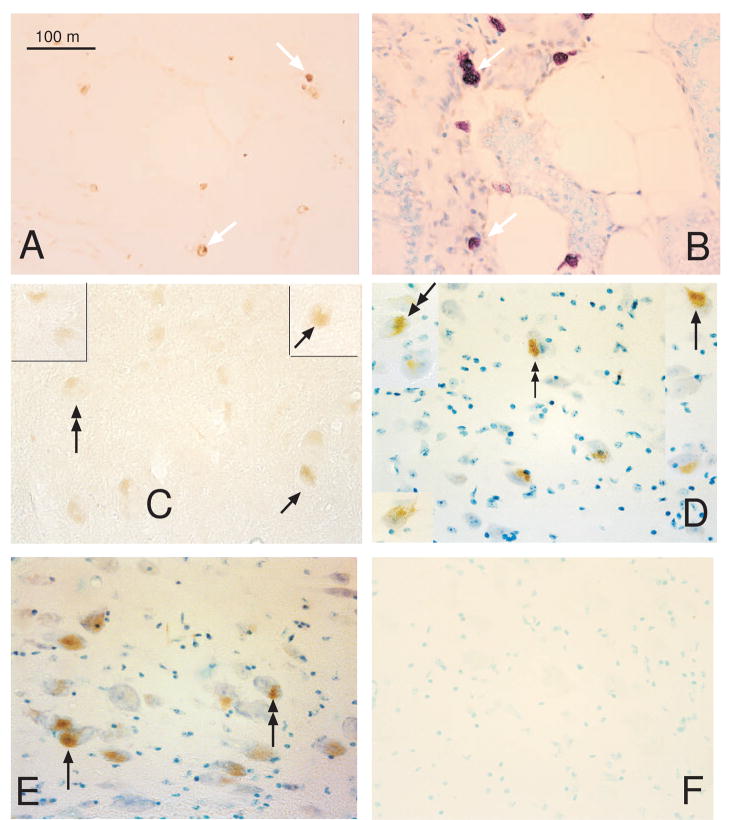
AIF was then examined in intact sections of the midbrain with immunocytochemistry. AIF was detected in the large serotonin-like neurons of the dorsal raphe and median raphe, but not outside of these areas. Figure 13 illustrates the immunostaining for AIF in the dorsal raphe. The median raphe was largely excluded in the dissection of the dorsal raphe block. These results suggest that the majority of the AIF protein detected in the dorsal raphe block was derived from serotonin neurons and this is consistent with AIF gene expression in laser captured serotonin neurons. It also supports the notion that the qRT-PCR analysis of AIF in the raphe block was diluted with other cell types.
Figure 13.
Photomicrographs of AIF immunostaining in the midbrain region containing the dorsal and median raphe nuclei. AIF immunostaining was detected only in the large serotonin-like neurons of the raphe nuclei and not in other areas. (A) Low power photomicrograph of the AIF positive neurons in the dorsal raphe nucleus. (B) High power photomicrograph of the AIF positive neurons in the median raphe region, which was not part of the dorsal raphe block used for protein analysis. (C) High power photomicrograph of the AIF positive neurons in the dorsal raphe nucleus. (reprinted from [91])
Although caspase activation is considered a hallmark of apoptotic cell death, other pathways have been described that do not require caspase activation. In addition to cytochrome c release, mitochondria can also release factors involved in caspase-independent cell death such as AIF. AIF is believed to play a central role in the regulation of caspase-independent cell death [73]. Upon apoptotic stimuli, AIF is released from the mitochondria and translocates to the nucleus where it triggers chromatin condensation and large DNA fragmentation. Under nonstressed conditions, AIF is essential for mitochondrial health and function. In healthy cells, AIF is retained in the mitochondria where it is believed to have an oxidoreductase function [116]. However, in response to apoptotic stimuli, AIF becomes an active executioner of the cell. AIF nuclear translocation is thought to be a commitment point to neuronal cell death, which occurs prior to caspase activation and thus may account for the limited effects of caspase inhibitors on AIF. AIF gene expression was significantly decreased by hormone therapy in laser captured serotonin neurons [100] and there was a similar trend in the dorsal raphe block. At the protein level, AIF exhibited a downward trend in the mitochondria and significantly less AIF was translocated to the nucleus with hormone treatment. The lower expression of AIF in the mitochondria and nuclei suggest that total AIF may be reduced by hormone treatment in a manner similar to the mRNA. Thus, under normal ovarian conditions, E and P act to maintain mitochrondrial integrity, which retains AIF in the mitochondrial membrane, and hormone therapy may decrease total AIF synthesis.
Although the serotonin neural population is prevalent in the dorsal raphe block that was used for this protein analysis, it remained a possibility that there could be changes in the caspase cascade within serotonin neurons that are masked by the presence of other cells and glia. It was also possible that the changes in JNK1 and AIF occurred in most or all of the cells in the dorsal raphe block and not only in serotonin neurons. Indeed, JNK1 was immunostained in neurons both within the dorsal raphe and elsewhere (data not shown). However, AIF was immunostained only in large, serotonin-like neurons of the dorsal and median raphe nuclei. Our dorsal raphe block excluded the median raphe nucleus, suggesting that the observed changes in AIF at the protein level were contributed by the large serotonin-like neurons of the dorsal raphe. Double immunocytochemistry is anticipated to phenotypically define the neurons. Certainly, further studies on protein expression changes specifically in serotonin neurons are needed and, as technology advances, the ability to perform a proteonomic analysis of laser captured serotonin neurons may be the next step. Nonetheless, the data highlight an important difference in the mechanism of action of ovarian hormones on neuroprotection in the presence or absence of gross injury. That is, the caspase-independent pathway was inhibited by ovarian steroids in the absence of gross injury.
Neurotoxic Protein Changes in the Dorsal Raphe with Hormone Therapy
E-induced neuroprotection may encompass different processes and is likely to proceed through several distinct but interrelated mechanisms. Neurotoxicity can induce cell damage and excessive glutamate excitation is a well-known mediator of neurotoxic cell death. The quinolones are another potent neurotoxic family produced through metabolism of tryptophan. KMO is an enzyme involved in tryptophan metabolism, which regulates the balance of neuroprotective to neurotoxic kynurenine metabolites. As described above, we found that gene expression for the enzyme kynurenin mono-oxygenase (KMO) decreased on the human microarray in the raphe block with E and E+P treatment and this was confirmed with qRT-PCR (Figure 1). There was a significant reduction in KMO mRNA in ovariectomized animals treated with E and E+P (ANOVA p< 0.01). Therefore, we hypothesized that ovarian hormones may improve serotonin neuronal survival by promoting cellular resilience through the KMO pathway. We further hypothesized that E decreased KMO and shifted tryptophan metabolism towards the pathways producing serotonin and/or neuroprotective kynurenic acid and away from the pathway producing several neurotoxic kynurenines. We speculated that the decrease in KMO could be significant in serotonin neurons, which have a robust tryptophan metabolism. KMO activation could lead to the overproduction of several neurotoxic quinolones and thereby adversely affect the health of serotonin neurons. However, KMO has been detected in neurons and glia. Therefore, we sought to determine whether the serotonin neurons of the dorsal raphe express KMO protein and whether administration of ovarian steroids alters KMO protein expression. For this work, Pacific Immunology, San Diego, CA was contracted to produce a rabbit anti-human antibody to KMO based upon a peptide consisting of amino acids 28–44.
KMO is located in the mitochondrial membrane. Therefore, KMO was initially examined in a crude membrane pellet from dorsal raphe blocks from ovariectomized monkeys treated with placebo, E and E+P for 28 days. Serum E concentrations (pg/ml) equaled 14±0.9, 57±12 and 57±8, respectively. Serum P concentrations (ng/ml) equaled 0.2±0.02, 0.15±0.04 and 2.4±0.4, respectively. Western blots of KMO and actin were performed followed by densitometric analysis of signal bands. As shown in Figure 14, there was a significant difference in the KMO optical density and in the KMO/actin ratio in animals treated with E or E+P compared to ovariectomized controls (ANOVA p =0.01 and 0.04, respectively). Posthoc tests indicated that KMO protein was lower in E and E+P groups (p <0.05) and that the KMO/actin ratio was lower in the E+P group compared to the placebo groups (Ovx).
Figure 14.
Optical density analysis of KMO protein on western blots. KMO was measured in a crude membrane pellet after homogenization of the dorsal raphe block from ovariectomized monkeys treated with placebo (Ovx, n=6), or treated with estradiol (E, n=4), or treated with estradiol plus progesterone (EP, n=4). Representative bands are illustrated.
Top histogram: Optical density of KMO signal bands on western blots. There was a significant difference between the groups (ANOVA, p = 0.01). Posthoc analysis indicated that both E-treated and E+P-treated groups were significantly different from the Ovx group (Student Newman Keul’s p < 0.05).
Bottom histogram: Ratio of the KMO optical density to the actin optical density in the same animals as above. There was a significant difference between the groups (ANOVA, p=0.04). Posthoc analysis indicated there was a significant difference between the EP-treated group and the Ovx group (Student Newman Keul’s p < 0.05). (presented at the Annual Meeting of the Society for Neuroscience, Washington, DC, 2008; Henderson JA and Bethea CL, poster 279.19)
Next, KMO was examined in the dorsal raphe with immunocytochemistry. Twenty ovariectomized female rhesus macaques were treated with placebo, E, P and E+P as described above (n=5/group). Serum E concentrations (pg/ml±SEM) equaled 8.4±1.7, 78.6±7.1, 14.0±6.1 and 89.0±12.7, respectively. Serum P concentrations (ng/ml±SEM) equaled 0.09±0.02, 0.14±0.02, 6.1±0.6 and 3.8±0.7, respectively. Following euthanasia, the left ventricle of the heart was cannulated and the head of each animal was perfused with 1 liter of saline followed by 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5 (both solutions made with DEPC-treated water [0.1% diethyl pyrocarbonate] to minimize RNase contamination). The brains were removed and dissected. Tissue blocks were post-fixed in 4% paraformaldehyde for 3 h, then transferred to 0.02M potassium phosphate-buffered saline (KPBS) containing 10%, followed by 20% glycerol and 2% dimethyl sulfoxide at 4°C for 3 days to cryoprotect the tissue. After infiltration, the block was frozen in isopentene cooled to −55°C, and stored at −80°C until sectioning. Sections (25 μm) were cut on a sliding microtome, mounted on Super Frost plus slides (Fisher Scientific, Pittsburgh, PA), dehydrated under vacuum and then frozen at −80°C until assay.
The antibody to KMO demonstrated robust staining of the large serotonergic-like neurons of the dorsal raphe as shown in Figure 15. The punctate staining pattern is consistant with the mitochondrial location of KMO. Stereological analysis with segmentation of the KMO-positive areas was conducted on 7 levels through the dorsal raphe at 250 μm intervals and the average positive pixel area at each level is shown in Figure 16. The average of the levels was obtained for each group and compared between groups using a 2 (E, no E) × 2 (P, no P) ANOVA as shown in Figure 17. The analysis revealed animals with E treatment (E and EP groups) had lower KMO positive pixel area (p=0.01) compared to animals without E treatment (OVX and P groups). The total area analyzed was not different. These results suggest that E is playing a predominant role in the reduction of KMO gene and protein expression. Decreased concentrations of KMO may be neuroprotective through increased accumulation of the neuroprotective metabolite kynurenic acid and/or decreased levels of the neurotoxic quinolone metabolites.
Figure 15.
Photomicrographs of KMO immunostaining in the dorsal raphe nucleus. KMO is widespread in neurons and glia, but the serotonin neurons appear to have higher concentrations that neighboring cells. Of the KMO-positive cells in the dorsal raphe, some cells along the dorsal edges were extremely darkly stained, and other cells in the body of the nucleus were lighter.
A. Photomicrograph of cells from the body of the dorsal raphe that are stained for KMO.
B. Photomicrograph of cells along the dorsal edge of the dorsal raphe that are stained for KMO.
C. Higher magnification photomicrograph of cells from the body of the dorsal raphe that are stained for KMO. The densely labeled mitochondria that contain KMO are represented by the punctuate staining pattern.
D. Higher magnification photomicrograph of cells along the dorsal edge of the dorsal raphe that are stained for KMO. The densely labeled mitochondria that contain KMO are represented by the punctate staining pattern.
E. Lower power montage of the entire dorsal raphe showing the organization of the cells in the body (top) and the intensely labeled cells along the dorsal edge.
F. Very high magnification photomicrograph of cells from the body of the dorsal raphe showing the punctate mitochondrial-staining pattern consistent with the localization of KMO. (presented at the Annual Meeting of the Society for Neuroscience, Washington, DC, 2008; Henderson JA and Bethea CL, poster 279.19)
Figure 16.
Mean of the KMO positive pixel area at each of 7 levels of the dorsal raphe nucleus in ovariectomized monkeys treated with placebo (Ovx), E, P or E+P (=5/treatment). Except for level 7, there was an apparent decrease in KMO positive pixels with E and EP treatment. After average of the levels, there was a significant difference between the groups (1 way ANOVA, p=0.003). Posthoc analysis indicated that E- and EP-treated groups were significantly different from the Ovx-placebo and P-treated groups (p < 0.05). (unpublished)
Figure 17.
Overall mean±SEM of KMO positive pixel area across the 7 levels for each group. Two-way ANOVA was performed in which groups administered E (E and EP) were compared to groups without E (OVX, P). There was a significant difference between the treatment groups (p<0.001) and between the levels (p=0.009) but there was no interaction between treatment and level. (presented at the Annual Meeting of the Society for Neuroscience, Washington, DC, 2008; Henderson JA and Bethea CL, poster 279.19)
Chromosome Fragmentation with Hormone Therapy
Based upon the observations described above, we predicted that ovarian hormone administration would decrease DNA fragmentation in serotonin neurons, whether it was a product of apoptosis or neurotoxic insult. However, it was unclear whether we could detect DNA fragmentation in non-injured brain tissue. Nonetheless, apoptosis is a homeostatic mechanism so it was possible that it would be ongoing at some level even in non-injured brain.
Several methods are available to detect apoptosis. The most labor intensive is electron microscopy, which can detect nuclear fragmentation. This procedure is difficult to apply to large areas and suffers from low numbers of total recorded events although it is used to validate other methods. DNA laddering can be detected in cell homogenates but morphological features are lost. Another well accepted method is the TUNEL assay. TUNEL (terminal deoxynucleotidyl transferase nick end labeling) is commonly employed to detect DNA condensation and fragmentation although it cannot distinguish between different kinds of cell death such as apoptosis or necrosis [117]. Nonetheless, one is able to screen large areas of neurons for early and late stages of DNA fragmentation.
Therefore, we used the TUNEL assay to determine the effect of hormone therapy on DNA fragmentation in the dorsal raphe nucleus and to determine if the fragmentation was occurring in serotonin neurons [118]. Sections were used from twenty ovariectomized female rhesus macaques that were treated with placebo, E, P and E+P (n=5/group) and perfused with 4% paraformaldehyde (described in previous section).
TUNEL assay
For the in situ analysis of DNA fragmentation, the TUNEL staining was performed on sections from perfused brains that were mounted, dried and stored at −80C using a commercial kit (Apopka Kit-S7100, Chemicon, Temecula, CA) according to the directions of the manufacturer. Some of the slides were counterstained with 0.5% methyl green and coverslipped. For positive controls, specimens of mammary gland tissue from rats were provided with the ApopTag Kit. Extensive apoptosis occurs 3–5 days after weaning of rat pups and post-weaning mammary tissue presents typical chromatin fragmentation labeled with TUNEL. Eight levels of the dorsal raphe were analyzed at a magnification of 1,000X with careful registration of the morphological features from ovariectomized macaques treated with placebo, E, P or E+P (n=5/treatment) as described above.
Quantitative Tissue Analysis
Sections were anatomically matched between animals using anatomical reference points. The Marianas stereological workstation with Slidebook 4.2 was used for analysis. Each section was examined through the rostro-caudal extent of the dorsal raphe (eight levels, 250 μm interval between sections). A montage of the entire area containing apoptotic neurons was built by the workstation and that area was defined and measured (μ2 total area).
For cell counting, the area of interest was outlined on the montage and detectable apoptotic cells were identified and counted by hand. All cell counts refer to detectable neurons only. The number of apoptotic neurons in the designated area of the eight sections was summed, generating one value for each animal. The individual sums were averaged to obtain the group mean. In addition, Slidebook 4.2 provides the square microns of the outlined area. The thickness of each section (25 μm × 8 sections = 200 μm) in microns was used as the length of the region. The average area of the region for each animal (μ2 outlined by Slidebook 4.2) was multiplied by the length to obtain the volume of the region (μ3 109 = mm3). The total number of TUNEL-positive neurons in each animal was divided by the volume of the area examined to obtain the number of TUNEL-positive neurons per cubic millimeter for each animal. The individual numbers of TUNEL positive neurons mm3 were then averaged to obtain the group means. Differences in cell numbers between the groups were determined with a Mann-Whitney nonparametric test
TUNEL staining in the dorsal raphe nucleus
Figure 18 illustrates TUNEL staining in the rat mammary gland (positive control tissue) and in the dorsal raphe nucleus. Panels A and B contain photomicrographs of the rat mammary gland in the absence (A) and presence (B) of methyl green counterstain. White arrows indicate TUNEL-positive cells in the rat mammary tissue. Panels C and D contain photomicrographs of the dorsal raphe nucleus in the absence (C) and presence (D) of methyl green counterstain. The TUNEL positive cells are scattered across the dorsal raphe so distant cells were placed in inserts to panels C and D. The DNA fragmentation detected in the dorsal raphe by the TUNEL assay occurs in two forms referred to as type I and type II [119; 120; 121]. Complete dark staining of the nucleus, called type I, and peripheral staining in the perinuclear area, called type II, may reflect different stages of the DNA fragmentation process that starts in the periphery and moves inward [122]. Alternatively, the perinuclear, type II staining could indicate DNA leakage from the nucleus [123]. Single arrows indicate type I staining and double arrows indicate type II staining. Panel E contains a photomicrograph of a section that was pretreated with DNAse for 10 minutes prior to TUNEL staining and methyl green counterstain. This treatment digested the DNA and more nicks are available for labeling. Thus, there are more TUNEL-positive cells apparent in this small area. Panel F contains a photomicrograph of a counterstained section of the dorsal raphe in which the Tdt enzyme was omitted during the TUNEL assay. The TUNEL staining was abolished by omission of the Tdt enzyme in this negative control section.
Figure 18.
Illustration of DNA fragmentation and TUNEL staining in the post-weaning rat- mammary gland and in the dorsal raphe nucleus of rhesus monkeys. There are 2 staining patterns observed which have been previously reported in other models and referred to as type I and type II. Complete dark staining of the nucleus, type I, and peripheral staining in the perinuclear area, type II, may reflect different stages of the DNA fragmentation process that starts in the periphery and moves inward. Alternatively, the perinuclear, type II staining could indicate DNA leakage from the nucleus. Double-head arrows indicate Type 1 staining and single-head arrows indicate Type 2 staining.
Panel A - TUNEL staining of the mammary gland without counterstain.
Panel B - TUNEL staining of the mammary gland with methyl green counterstain.
Panel C - TUNEL staining in the dorsal raphe nucleus without counterstain.
Panel D – TUNEL staining of the dorsal raphe nucleus with methyl green counterstain.
Panel E- TUNEL staining of the dorsal raphe nucleus after exposure of the section to DNAse for 10 minutes prior to TUNEL assay. There is an increase in the number of cells exhibiting different degrees of DNA fragmentation.
Panel F – Negative control section of the dorsal raphe generated by omission of the Tdt reagent in the TUNEL assay. There was no apparent DNA fragmentation detected. (reprinted from [118])
In order to perform a stereological analysis of the dorsal raphe nucleus in which positive and negative staining could be segregated, it was necessary to omit the methyl green counterstain. Eight levels of the dorsal raphe nucleus, which were 250μ apart in a rostral to caudal direction from all animals were processed for TUNEL staining (n=5 animals/group).
Figure 19 illustrates the TUNEL staining at level 5 from one representative animal of each treatment group. Visually, there was a robust decrease in the number of TUNEL-positive cells in the dorsal raphe nucleus from animals treated with E alone, P alone and E+P compared to the placebo treated group (OVX). However, there was individual variation between monkeys hindering analysis of variance across all treatment groups.
Figure 19.
Comparison of TUNEL staining at level 5 of the dorsal raphe nucleus from a representative animal in each treatment group: The animals were ovariectomized and treated with placebo (OVX), estradiol (E), progesterone (P) or estradiol plus progesterone (EP) for 28 days. Arrows indicate TUNEL positive cells. (reprinted from [118])
Nonetheless as illustrated in Figure 20, top panel, comparison of individual groups with the OVX-placebo group using a Mann-Whitney nonparametric test confirmed that both P treatment and E+P treatment caused a significant decrease in the total number of TUNEL-positive cells in the 8 levels of dorsal raphe nucleus that were measured (p =0.04, both treatments). When the number of TUNEL-positive cells/cubic millimeter (mm3) was computed, there was a significant reduction of TUNEL-positive cells after treatment with E+P (p=0.04), and a nearly significant reduction occurred with P-alone (p=0.1) as illustrated in Figure 20, middle panel. The total area that was measured did not differ between groups (Figure 20, bottom panel).
Figure 20.
Histograms illustrating the results of stereological analysis of the number of TUNEL-positive neurons in the dorsal raphe nucleus of rhesus macaques treated for 28 days with placebo (OVX), estradiol (E), progesterone (P) or estradiol plus progesterone (EP). TUNEL stained neurons were counted on the montage within a defined area (μ2).
(A) Average (±SEM) of the total number of TUNEL-positive cells in 8 levels of the dorsal raphe nucleus in each treatment group (n=5 animals/group). There was a significant decrease in the total number of TUNEL positive neurons in the P and EP- treated groups compared with the OVX group (p=0.04 for P versus Ovx and p=0.04 for EP versus Ovx, Mann-Whitney nonparametric test).
(B) Average (±SEM) of the TUNEL-positive neurons per cubic millimeter (mm3) of eight levels of the dorsal raphe nucleus in each treatment group (n=5 animals/group). There was a significant decrease in TUNEL-positive cells/mm3 in the E+P- treated group (p=0.04, Mann-Whitney nonparametric test), while the P group was nearly significantly different (p=0.1, Mann-Whitney nonparametric test).
(C) Comparison of the total volume of the area measured in each treatment group showed no difference. (reprinted from [118])
Double immunostaining TUNEL + tryptophan hydroxylase (TPH)
To determine if serotonin neurons exhibited DNA fragmentation, sections from an OVX placebo-treated animal were double immunolabeled for TUNEL plus TPH. The TUNEL staining was executed as described above, using the ApopTag Kit-S7100 commercial kit. After development of the TUNEL staining in DAB, the sections were processed for TPH immunocytochemistry using an anti-human TPH antibody (1/3000; Novus Biologicals, Littleton, CO) and developed with alkaline phosphatase and blue substrate (Vector Laboratories, Burlingame, CA). Photomicrographs of the sections were obtained on a Leica DMLB microscope with a Leica DFC290 digital camera.
Figure 21 contains photomicrographs of serotonin neurons that exhibit DNA fragmentation as well as, TUNEL-negative serotonin neurons. The animal chosen for the double-labeling experiment was an Ovx-placebo animal, which exhibited a high level of TUNEL staining in the single-label experiment. Type I and type II TUNEL staining was observed in serotonin neurons. The double-labeled neurons with brown TUNEL staining and blue TPH staining are indicated with 2 different black arrows. Serotonin neurons with type I staining are indicated with black single arrows and serotonin neurons with type II staining are indicated with black double-headed arrows. Serotonin neurons, which lack detectable TUNEL staining, are indicated with green arrows. In addition, the TUNEL-positive serotonin neurons exhibit different degrees of cellular degradation. That is, some neurons are still relatively intact with dense TPH cytoplasmic staining whereas other neurons are degraded with little visible cytoplasmic TPH. TUNEL-positive neurons that appear severely degraded and disintegrated are indicated with red arrows. This leads to the speculation that a significant number of the TUNEL-positive cells with little or no visible TPH staining may have been serotonergic, but degradation and disintegration were advanced and/or phagocytosis was underway at the time of euthanasia. Little or no TUNEL staining was observed in the nearby periaquaductal gray region (Figure 21G)
Figure 21.
Illustration of colocalization of TUNEL staining and tryptophan hydroxylase (TPH) in neurons of the dorsal raphe nucleus. Photomicrographs are from the dorsal raphe nucleus of an OVX monkey following double-immunohistochemistry for TPH (blue, cytoplasmic) and TUNEL (brown, nuclear, perinuclear). Single arrows indicate double-labeled neurons with type I TUNEL staining. Double-headed arrows indicate double-labeled neurons with type II TUNEL staining. Green arrows indicate neurons that are stained only for TPH. Red arrows indicate TUNEL-positive neurons that are in advanced stages of disintegration.
A. Photomicrograph of a region of the dorsal raphe containing 2, or possibly 3, serotonin neurons with no TUNEL staining (green arrows and adjacent cell). One cell is present with TUNEL staining in the perinuclear area and TPH staining in the cytoplasm (double arrow). Other TUNEL positive cells are apparent that are either not serotonergic, or degradation has proceeded to the point that TPH is no longer present. The scale bar also applies to panels B, C and the upper left picture in panel E.
B. Photomicrograph of a region of the dorsal raphe containing TUNEL-positive cells at different stages of disintegration. The double-headed arrow highlights a TUNEL-positive cell with TPH-positive cytoplasm remaining. The red arrow highlights a TUNEL-positive cell in an advanced stage of degradation with little TPH-positive cytoplasm remaining. Other TUNEL positive cells are evident that are either not serotonergic, or degradation has proceeded to the point that TPH is no longer present.
C. Photomicrograph of a region of the dorsal raphe from an ovariectomized macaque treated with placebo that exhibited very high numbers of TUNEL positive cells in the stereological analysis. There are few remnants of TPH-positive cytoplasm remaining in this cluster of dying cells.
D. Photomicrograph of two neurons double-labeled for TUNEL and TPH. The left neuron exhibits type I TUNEL staining (single arrow), which is predominantly nuclear, whereas the right neuron exhibits type II staining (double arrow) in which the DNA fragmentation is perinuclear.
E. Multi-panel with photomicrographs of TPH-positive neurons with different degrees of DNA fragmentation, and one TPH-positive neuron with no apparent TUNEL staining (bottom right, green arrow). Single arrows indicate serotonin neurons with type I TUNEL staining and double-headed arrows indicate serotonin neurons with type II TUNEL staining. The red arrows indicate serotonin neurons with TUNEL staining that is in an advanced stage of degradation as indicated by the sparse TPH staining.
F. Photomicrograph of a region of the dorsal raphe that contains a neuron that is double labeled for TUNEL and TPH (double arrow), a serotonin neuron with no apparent TUNEL stain (green arrow), and a TUNEL–positive neuron in an advanced stage of degradation (red arrow).
G. Photomicrograph of the periaquaductal gray region of the same section as panel C. There was no apparent TUNEL staining in this region. TUNEL staining was absent or rare in other adjacent neural structures of the midbrain suggesting that serotonin neurons are particularly vulnerable to steroid withdrawal. (reprinted from [118]).
Therefore, this study showed that P administered alone or in conjunction with E, decreased the number of TUNEL-positive cells in the dorsal raphe nucleus, and that TUNEL staining co-localized in serotonin neurons in the dorsal raphe nucleus. These data confirm the hypothesis that ovarian steroids protect serotonin neurons from DNA fragmentation, which may be an indication of impending cell death. Thus, ovarian steroids protect serotonergic neurons in nonhuman primates that did not suffer any kind of experimental brain injury. This would encourage serotonin neuronal resilience and decrease cellular vulnerability to stresses associated with normal life such as disease-generated cytokines, free radicals or psychological trauma.
Although there was a downward trend with E treatment, P appears to play a more important role than previously thought, in that P alone and E plus P treatment significantly reduced TUNEL-positive cells. It should be noted that TUNEL staining could not distinguish between DNA fragmentation due to apoptosis or necrosis. However, the lack of prevalent vacuoles and neutophil infiltration suggests that the majority of the DNA fragmentation results from apoptosis.
There was variance within the treatment groups, which may have decreased with more animals although 5 monkeys per treatment group are considered adequate by power analysis. Also, we treated with ovarian steroids for only one month and this may be a contributing factor. That is, longer treatment periods may have resulted in more consistent reduction of TUNEL staining. Likewise, these were Chinese rhesus macaques and they were not genotyped. Hence as with human populations, there could be genetic predisposition and/or other developmental variables that render a primate more or less sensitive to the actions of ovarian steroids on DNA fragmentation.
It may be questioned how E and P decrease DNA fragmentation after one month of treatment. That is, are they reversing a process that is underway? We speculate that DNA repair is possible if the fragmentation process has not proceeded to an advanced stage. Indeed, some of the milder affected cells with minor type II staining could be rescued with hormone therapy. This speculation means that not all of the TUNEL-positive cells are actively dieing, but they are endangered and vulnerable. Future studies on DNA repair mechanisms are certainly indicated. Moreover, this question redirects attention to the issue of the length of time after ovariectomy before hormone therapy is initiated. Our animals were treated between 3 and 8 months after ovariectomy. After longer time periods, DNA fragmentation may be more advanced, neurons may die and rescue may become impossible. Moreover, there may be a difference between 3 and 8 months of ovariectomy, which is more profound than previously anticipated. Unfortunately, it is extremely unlikely to have 20 animals available for assignment that were ovariectomized at the same time, so we have to use a practical time frame.
Recent clinical studies have shown that hormone therapy administered during the perimenopausal period is beneficial for treatment of depressive and anxious symptoms associated with the onset of menopause [124]. However, the Women’s Health Initiative found that hormone therapy initiated long after menopause was harmful in neural systems that have a serotonergic component, such as integrative cognition [125]. Moreover, Brinton and colleagues demonstrated that E treatment of unhealthy cells is disastrous for the cell, whereas E treatment of healthy cells is neuroprotective [126; 127]. In light of the data from this study, it is attractive to speculate that hormone therapy administered long after menopause will act deleteriously on the advanced TUNEL-positive cells of the dorsal raphe nucleus and promote the death of serotonin neurons. Conversely, hormone therapy at perimenopause will act in the raphe with few TUNEL- positive cells and thus promote cellular resilience amongst the serotonin neuronal population. Long-term survival of serotonin neurons will have a positive effect on many aspects of mood and higher brain function during aging.
TUNEL staining was restricted to the dorsal and median raphe nuclei in that it was not observed in other regions of the midbrain. In addition, AIF immunostaining was confined to the serotonin-like neurons of the raphe and was not observed in other midbrain structures [91]. Thus, TUNEL staining and AIF staining were largely confined to serotonin neurons in the midbrain, which also express robust concentrations of NFkB [55]. Altogether the data from the microarray studies, the protein studies and the TUNEL studies point to ovarian steroids decreasing activity of the caspase-independent pathway and preventing re-entry into the cell cycle. Upon steroid withdrawal, there is an increase in cyclins, and an increase in JNK1 and AIF translocation to the nucleus, which lead to DNA fragmentation. In addition, there is a robust pool of NFkB in serotonin neurons, and NFkB translocation to the nucleus increases upon steroid withdrawal [55]. Each of these proteins transduces stress signals such as cytokine and toll receptor activation.
It is curious that the serotonin neurons appear to greatly exceed neighboring neurons in the expression of AIF and TUNEL. It is as though evolution decided that these large, critically important neurons should be extremely vulnerable to internal and external perturbations rather than being tough and resilient. In women of course, evolution did not factor in a greatly extended life span past childbearing years.
In conclusion, the ovarian steroids, E and P increase the expression of anti-apoptotic genes and/or down-regulate the pro-apoptotic ones [62; 97]. In animal models, administration of E prior to, or coincident with, trauma or global ischemia decreased tissue damage [10; 11]. Moreover, caspases were activated in the damaged tissue suggesting that the caspase-dependent pathway is involved in apoptosis resulting from ischemia [12; 13]. In addition, E treatment decreases caspase activation in the damaged area of the brain [14]. In the neocortex of rats injected with gp120, a human glycoprotein, which may be responsible for the neuronal loss observed post-mortem in the brain of AIDS patients, E reduced apoptosis by a mechanism involving activation of ER [128]. However, in our model of surgical menopause with hormone therapy and no inflicted neural injury, it appears that the caspase-independent pathway plays a prominent role in the midbrain serotonin neurons. To definitively draw this conclusion, we need a better understanding of protein changes specifically within serotonin neurons. Hopefully, future technological advances will enable proteonomic analysis of laser-captured neurons. In the meantime, ICC and stereological analysis of caspase-dependent and –independent execution proteins is underway.
The neuroprotective role of P has been studied in different experimental models such as traumatic brain injury, induced ischemia and spinal cord lesions [129]. In rats, after traumatic brain injury, P treatment reduces edema and increases the number of surviving neurons [130; 131]. In mice, P has a beneficial effect on motor ability and spatial memory performance after cerebral ischemia, and in a model of brain injury in rats, P decreased apoptotic cell death by decreasing expression of the pro-apoptotic protein, caspase-3 [132; 133]. Progesterone significantly reduced the neuronal loss in hippocampus and in the caudate nucleus after global ischemia [134; 135]. Moreover P exhibits neuroprotective effects in several neurodegenerative conditions such as stroke, Alzheimer’s disease and schizophrenia [129; 136] Notably, allopregnanolone, a P metabolite that acts at GABA-A receptors, also exhibits anti-apoptotic effects and reduces functional deficits in rats after traumatic brain injury [133], suggesting that allopregnanolone could be the molecule through which P induces its neuroprotective effects. In the absence of E treatment, the expression of nuclear progestin receptors (PR) would be reduced [137]. Therefore, in this study the decrease in TUNEL staining observed in the P-only treatment group is consistent with an action through allopregnanolone. In the E+P treatment group, E should induce nuclear PR, and therefore P could additionally work through PR to promote cellular resilience. Likewise, the decrease in TUNEL staining with E+P could be solely due to an action of allopregnanolone. The intracellular crosstalk between allopregnanolone, the GABA-A receptor and the apoptosis-related pathways needs further investigation.
Altogether, ovarian steroids, by affecting the balance between apoptotic and anti-apoptotic genes, may protect serotonin neurons from cell death induced by several stimuli such as cytokines, free oxygen radicals, or neurotoxic agents [138]. However, different mechanisms may be involved in normal and injured tissues. Comparison of our data with studies in injured brain requires consideration that DNA degradation depends on several interacting pathways, and different insults (or their absence) can activate different signaling events. Consequently this may lead to different morphology for chromatin condensation and DNA fragmentation [139].
Although the responses to E and E+P are usually quite similar, E+P augmented the translocation of AIF and caused the greatest reduction in TUNEL staining. This may raise the question of whether E alone was as effective as E+P? Our results are very likely due to the length of time that we treat (one month) and the doses of E and P that we employ. In earlier studies of serotonin–related protein expression in the dorsal raphe block, we noted that with 5 months of E alone, there was a tendency for 5HT1A [140] and MAO-A [141] to escape the suppression observed with 1 month of E alone. However, with E+P treatment for 5 months, the downregulation was maintained. Also, 5 months of E alone increased SERT [141] and 5HT2C [140] proteins, wherease 1 month did not. Yet, 5 months of E+P maintained SERT and 5HT2C levels near the ovx-placebo range. In addition, the ratio of E to P is absolutely critical. With a ratio of E to P in serum around 1:50, P does not block the effect of E on most of our endpoints. Thus, a range of E from 60 to 150 pg/ml works well as long as the P concentrations are adjusted accordingly. However, significantly different results may ensue if serum P greatly exceeds a 50-fold higher concentration than serum E. Altogether, we speculate that a little P added to E may be helpful with long-term hormone therapy, but only if natural formulations are used.
In summary, ovarian steroids were protective of serotonin neurons in an animal model of surgical menopause by decreasing gene and protein expression in the caspase-independent pathway and by decreasing genes involved in the cell cycle. In turn, these actions decrease DNA fragmentation in serotonin neurons of the dorsal raphe nucleus. This action would increase the cellular resilience of serotonin neurons to normal life stresses and may prevent serotonin neuron death in women facing decades of life after menopause. Moreover, these data suggest that ovarian steroids may protect serotonin neurons from signals that arise from normal life stresses such as cytokines from illness, glutamate excitotoxicity or psychological stress and trauma.
Acknowledgments
We are deeply grateful to the dedicated staff of the Division of Animal Resources including the staff of the Departments of Surgery and Pathology for their expertise and helpfulness in all aspects of monkey management. We thank the staff of the OHSU Gene Microarray Shared Resource, who were essential for this study. We are indebted to the Endocrine Services Laboratory for radioimmunoassay of steroid hormones.
Supported by NIH grants: MH62677 to CLB, U54 contraceptive Center Grant HD 18185, and RR00163 for the operation of ONPRC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bethea CL. Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology. 1993;57:1–6. doi: 10.1159/000126334. [DOI] [PubMed] [Google Scholar]
- 2.Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERβ) mRNA and protein in serotonin neurons of macaques. Molecular Brain Research. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 5.Lu NZ, Bethea CL. Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 6.Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution and function in female macaques. Molecular Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- 7.Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–9. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–15. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 9.Simpkins JW, Yang SH, Wen Y, Singh M. Estrogens, progestins, menopause and neurodegeneration: basic and clinical studies. Cell Mol Life Sci. 2005;62:271–80. doi: 10.1007/s00018-004-4382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–31. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 11.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 12.Springer JE. Apoptotic cell death following traumatic injury to the central nervous system. J Biochem Mol Biol. 2002;35:94–105. doi: 10.5483/bmbrep.2002.35.1.094. [DOI] [PubMed] [Google Scholar]
- 13.Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:269–87. doi: 10.2174/1568008054863754. [DOI] [PubMed] [Google Scholar]
- 14.Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–43. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–9. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: an exploratory case-control study. Mov Disord. 2001;16:830–7. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 17.Ragonese P, D’Amelio M, Savettieri G. Implications for estrogens in Parkinson’s disease: an epidemiological approach. Ann N Y Acad Sci. 2006;1089:373–82. doi: 10.1196/annals.1386.004. [DOI] [PubMed] [Google Scholar]
- 18.Tejani-Butt SM, Yang J, Pawlyk AC. Altered serotonin transporter sites in Alzheimer’s disease raphe and hippocampus. NeuroReport. 1995;6:1207–1210. doi: 10.1097/00001756-199505300-00033. [DOI] [PubMed] [Google Scholar]
- 19.Benninghoff J, Schmitt A, Mossner R, Kesch KP. When cells become depressed: focus on neural stems cells in novel treatment strategies against depression. J Neural Transm. 2002;109:947–962. doi: 10.1007/s007020200078. [DOI] [PubMed] [Google Scholar]
- 20.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–96. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 21.Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 22.Oo TF, Blazeski R, Harrison SM, Henchcliffe C, Mason CA, Roffler-Tarlov SK, Burke RE. Neuron death in the substantia nigra of weaver mouse occurs late in development and is not apoptotic. J Neurosci. 1996;16:6134–45. doi: 10.1523/JNEUROSCI.16-19-06134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 24.Krantic S, Mechawar N, Reix S, Quirion R. Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci. 2005;28:670–6. doi: 10.1016/j.tins.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isacson O. On neuronal health. Trends Neurosci. 1993;16:306–8. doi: 10.1016/0166-2236(93)90104-t. [DOI] [PubMed] [Google Scholar]
- 27.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- 28.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S481–90. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy AP, Bethea CL. Preliminary array analysis reveals novel genes regulated by ovarian steroids in the monkey raphe region. Psychopharmacology (Berl) 2005;180:125–40. doi: 10.1007/s00213-005-2154-1. [DOI] [PubMed] [Google Scholar]
- 33.Kumagae Y, Zhang Y, Kim OJ, Miller CA. Human c-Jun N-terminal kinase expression and activation in the nervous system. Brain Res Mol Brain Res. 1999;67:10–7. doi: 10.1016/s0169-328x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. Embo J. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 35.Park DS, Stefanis L, Yan CY, Farinelli SE, Greene LA. Ordering the cell death pathway. Differential effects of BCL2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J Biol Chem. 1996;271:21898–905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 36.Schauwecker PE. Seizure-induced neuronal death is associated with induction of c-Jun N-terminal kinase and is dependent on genetic background. Brain Res. 2000;884:116–28. doi: 10.1016/s0006-8993(00)02888-2. [DOI] [PubMed] [Google Scholar]
- 37.Erickson JB, Flanagan EM, Russo S, Reinhard JF., Jr A radiometric assay for kynurenine 3-hydroxylase based on the release of 3H2O during hydroxylation of L-[3,5-3H]kynurenine. Anal Biochem. 1992;205:257–62. doi: 10.1016/0003-2697(92)90432-7. [DOI] [PubMed] [Google Scholar]
- 38.Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem. 1995;65:2621–32. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- 39.Saito K, Heyes MP. Kynurenine pathway enzymes in brain. Properties of enzymes and regulation of quinolinic acid synthesis. Adv Exp Med Biol. 1996;398:485–92. [PubMed] [Google Scholar]
- 40.Ito Y, Saito K, Maruta K, Nakagami Y, Koike T, Oguri Y, Nagamura Y. Kynurenine concentration of serum was increased by exercise. Adv Exp Med Biol. 1999;467:717–22. doi: 10.1007/978-1-4615-4709-9_93. [DOI] [PubMed] [Google Scholar]
- 41.Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–31. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 42.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiarugi A, Cozzi A, Ballerini C, Massacesi L, Moroni F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience. 2001;102:687–95. doi: 10.1016/s0306-4522(00)00504-2. [DOI] [PubMed] [Google Scholar]
- 44.Morita T, Saito K, Takemura M, Maekawa N, Fujigaki S, Fujii H, Wada H, Takeuchi S, Noma A, Seishima M. L-tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid induces apoptosis in macrophage-derived cells under pathophysiological conditions. Adv Exp Med Biol. 1999;467:559–63. doi: 10.1007/978-1-4615-4709-9_69. [DOI] [PubMed] [Google Scholar]
- 45.Chiarugi A, Meli E, Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J Neurochem. 2001;77:1310–8. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 46.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–10. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H, Leeds P, Chen RW, Wei W, Leng Y, Bredesen DE, Chuang DM. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J Neurochem. 2000;75:81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- 48.Stone TW. Endogenous neurotoxins from tryptophan. Toxicon. 2001;39:61–73. doi: 10.1016/s0041-0101(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 49.Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 2003;41:852–9. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 50.Malina HZ, Martin XD. Deamination of 3-hydroxykynurenine in bovine lenses: a possible mechanism of cataract formation in general. Graefes Arch Clin Exp Ophthalmol. 1995;233:38–44. doi: 10.1007/BF00177784. [DOI] [PubMed] [Google Scholar]
- 51.Malina HZ, Richter C, Mehl M, Hess OM. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001;1:7. doi: 10.1186/1472-6793-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou ST, Callaghan D, Fournier MC, Hill I, Kang L, Massie B, Morley P, Murray C, Rasquinha I, Slack R, MacManus JP. The transcription factor E2F1 modulates apoptosis of neurons. J Neurochem. 2000;75:91–100. doi: 10.1046/j.1471-4159.2000.0750091.x. [DOI] [PubMed] [Google Scholar]
- 53.Konishi Y, Bonni A. The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J Neurosci. 2003;23:1649–58. doi: 10.1523/JNEUROSCI.23-05-01649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou ST, Cowan E, Walker T, Ohan N, Dove M, Rasqinha I, MacManus JP. The transcription factor E2F1 promotes dopamine-evoked neuronal apoptosis by a mechanism independent of transcriptional activation. J Neurochem. 2001;78:287–97. doi: 10.1046/j.1471-4159.2001.00402.x. [DOI] [PubMed] [Google Scholar]
- 55.Bethea CL, Reddy AP, Smith LJ. Nuclear factor kappa B in the dorsal raphe of macaques: an anatomical link for steroids, cytokines and serotonin. J Psychiatry Neurosci. 2006;31:105–14. doi: 10.1016/j.yfrne.2006.03.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wafford KA, Macaulay AJ, Fradley R, O’Meara GF, Reynolds DS, Rosahl TW. Differentiating the role of gamma-aminobutyric acid type A (GABAA) receptor subtypes. Biochem Soc Trans. 2004;32:553–6. doi: 10.1042/BST0320553. [DOI] [PubMed] [Google Scholar]
- 58.Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABAA-receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–41. doi: 10.1016/s0074-7742(01)46064-8. [DOI] [PubMed] [Google Scholar]
- 59.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–68. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 61.Henkel V, Baghai TC, Eser D, Zill P, Mergl R, Zwanzger P, Schule C, Bottlender R, Jager M, Rupprecht R, Hegerl U, Moller HJ, Bondy B. The gamma amino butyric acid (GABA) receptor alpha-3 subunit gene polymorphism in unipolar depressive disorder: a genetic association study. Am J Med Genet. 2004;126B:82–7. doi: 10.1002/ajmg.b.20137. [DOI] [PubMed] [Google Scholar]
- 62.Bethea C, Reddy A. Effect of ovarian hormones on survival genes in laser captured serotonin neurons from macaques. J Neurochem. 2008;105:1129–1143. doi: 10.1111/j.1471-4159.2008.05213.x. [DOI] [PubMed] [Google Scholar]
- 63.Davis AS, Zhao H, Sun GH, Sapolsky RM, Steinberg GK. Gene therapy using SOD1 protects striatal neurons from experimental stroke. Neurosci Lett. 2007;411:32–6. doi: 10.1016/j.neulet.2006.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–9. [PubMed] [Google Scholar]
- 65.Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 66.Toborek M, Son KW, Pudelko A, King-Pospisil K, Wylegala E, Malecki A. ERK 1/2 signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. J Cell Biochem. 2007;100:279–92. doi: 10.1002/jcb.21013. [DOI] [PubMed] [Google Scholar]
- 67.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–43. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 68.Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A. 2003;100:8921–6. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293:263–9. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 70.Marcelain K, De La Torre C, Gonzalez P, Pincheira J. Roles of nibrin and AtM/ATR kinases on the G2 checkpoint under endogenous or radio-induced DNA damage. Biol Res. 2005;38:179–85. doi: 10.4067/s0716-97602005000200007. [DOI] [PubMed] [Google Scholar]
- 71.Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, Colburn NH. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–76. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- 72.Bouchier-Hayes L, Conroy H, Egan H, Adrain C, Creagh EM, MacFarlane M, Martin SJ. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J Biol Chem. 2001;276:44069–77. doi: 10.1074/jbc.M107373200. [DOI] [PubMed] [Google Scholar]
- 73.Krantic S, Mechawar N, Reix S, Quirion R. Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol. 2007;81:179–96. doi: 10.1016/j.pneurobio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 75.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang ZQ, Wang X, Li LY, Wang Y, Chen RW, Chuang DM, Chase TN, Qin ZH. Nuclear factor-kappaB-dependent cyclin D1 induction and DNA replication associated with N-methyl-D-aspartate receptor-mediated apoptosis in rat striatum. J Neurosci Res. 2007;85:1295–309. doi: 10.1002/jnr.21248. [DOI] [PubMed] [Google Scholar]
- 77.Post A, Crochemore C, Uhr M, Holsboer F, Behl C. Differential induction of NF-kappaB activity and neural cell death by antidepressants in vitro. Eur J Neurosci. 2000;12:4331–7. doi: 10.1046/j.0953-816x.2000.01352.x. [DOI] [PubMed] [Google Scholar]
- 78.Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J Neurochem. 2005;95:9–19. doi: 10.1111/j.1471-4159.2005.03319.x. [DOI] [PubMed] [Google Scholar]
- 79.Adams JH, Wigg KG, King N, Burcescu I, Vetro A, Kiss E, Baji I, George CJ, Kennedy JL, Kovacs M, Barr CL. Association study of neurotrophic tyrosine kinase receptor type 2 (NTRK2) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;132:90–5. doi: 10.1002/ajmg.b.30084. [DOI] [PubMed] [Google Scholar]
- 80.Gray J, Yeo G, Hung C, Keogh J, Clayton P, Banerjee K, McAulay A, O’Rahilly S, Farooqi IS. Functional characterization of human NTRK2 mutations identified in patients with severe early-onset obesity. Int J Obes (Lond) 2007;31:359–64. doi: 10.1038/sj.ijo.0803390. [DOI] [PubMed] [Google Scholar]
- 81.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 82.Zhu ZH, Yang R, Fu X, Wang YQ, Wu GC. Astrocyte-conditioned medium protecting hippocampal neurons in primary cultures against corticosterone-induced damages via PI3-K/Akt signal pathway. Brain Res. 2006;1114:1–10. doi: 10.1016/j.brainres.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 83.Koh PO. Estradiol prevents the injury-induced decrease of 90 ribosomal S6 kinase (p90RSK) and Bad phosphorylation. Neurosci Lett. 2007;412:68–72. doi: 10.1016/j.neulet.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 84.Won CK, Ha SJ, Noh HS, Kang SS, Cho GJ, Choi WS, Koh PO. Estradiol prevents the injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett. 2005;387:115–9. doi: 10.1016/j.neulet.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 85.Lu T, Xu Y, Mericle MT, Mellgren RL. Participation of the conventional calpains in apoptosis. Biochim Biophys Acta. 2002;1590:16–26. doi: 10.1016/s0167-4889(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 86.Tan Y, Wu C, De Veyra T, Greer PA. Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem. 2006;281:17689–98. doi: 10.1074/jbc.M601978200. [DOI] [PubMed] [Google Scholar]
- 87.Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 88.Becker EB, Bonni A. Beyond proliferation--cell cycle control of neuronal survival and differentiation in the developing mammalian brain. Semin Cell Dev Biol. 2005;16:439–48. doi: 10.1016/j.semcdb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–22. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 90.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–42. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 91.Tokuyama Y, Reddy A, Bethea C. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–31. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu X, Raber J, Yang D, Su B, Mucke L. Dynamic regulation of c-Jun N-terminal kinase activity in mouse brain by environmental stimuli. Proc Natl Acad Sci U S A. 1997;94:12655–60. doi: 10.1073/pnas.94.23.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herdegen T, Skene P, Bahr M. The c-Jun transcription factor--bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–31. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 94.Vilatoba M, Eckstein C, Bilbao G, Frennete L, Eckhoff DE, Contreras JL. 17beta-estradiol differentially activates mitogen-activated protein-kinases and improves survival following reperfusion injury of reduced-size liver in mice. Transplant Proc. 2005;37:399–403. doi: 10.1016/j.transproceed.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Lu X, Bhavnani BR. Equine estrogens differentially inhibit DNA fragmentation induced by glutamate in neuronal cells by modulation of regulatory proteins involved in programmed cell death. BMC Neurosci. 2003;4:32. doi: 10.1186/1471-2202-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koski CL, Hila S, Hoffman GE. Regulation of cytokine-induced neuron death by ovarian hormones: involvement of antiapoptotic protein expression and c-JUN N-terminal kinase-mediated proapoptotic signaling. Endocrinology. 2004;145:95–103. doi: 10.1210/en.2003-0803. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport. 1998;9:593–7. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 98.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–93. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27:1422–33. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-releasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2008;33:546–56. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- 101.Sharma K, Mehra RD. Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res. 2008;1204:1–15. doi: 10.1016/j.brainres.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 102.Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–8. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 103.Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–58. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 104.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 105.Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21:2600–9. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–75. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- 107.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 108.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 109.Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–7. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release. BMC Neurosci. 2005;6:13. doi: 10.1186/1471-2202-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 112.Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J Neurotrauma. 2005;22:345–52. doi: 10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- 113.Lu A, Frink M, Choudhry MA, Schwacha MG, Hubbard WJ, Rue LW, 3rd, Bland KI, Chaudry IH. Mitochondria play an important role in 17beta-estradiol attenuation of H(2)O(2)-induced rat endothelial cell apoptosis. Am J Physiol Endocrinol Metab. 2007;292:E585–93. doi: 10.1152/ajpendo.00413.2006. [DOI] [PubMed] [Google Scholar]
- 114.Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007;144:119–27. doi: 10.1016/j.neuroscience.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Oxidative stress is associated with XIAP and Smac/DIABLO signaling pathways in mouse brains after transient focal cerebral ischemia. Stroke. 2004;35:1443–8. doi: 10.1161/01.STR.0000128416.28778.7a. [DOI] [PubMed] [Google Scholar]
- 116.Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G, Penninger JM, Peleato ML, Kroemer G, Susin SA. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem. 2001;276:16391–8. doi: 10.1074/jbc.M010498200. [DOI] [PubMed] [Google Scholar]
- 117.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in serotonin neurons of non-injured rhesus macaques. Molecular Psychiatry. 2009 doi: 10.1038/mp.2009.97. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piantadosi CA, Zhang J, Levin ED, Folz RJ, Schmechel DE. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol. 1997;147:103–14. doi: 10.1006/exnr.1997.6584. [DOI] [PubMed] [Google Scholar]
- 120.Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–83. [PMC free article] [PubMed] [Google Scholar]
- 121.Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res. 2004;1022:54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 122.Chang GQ, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 123.Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci. 1998;18:5663–72. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 125.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 126.Zhao L, O’Neill K, Diaz Brinton R. Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev. 2005;49:472–93. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 127.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 128.Corasaniti MT, Amantea D, Russo R, Piccirilli S, Leta A, Corazzari M, Nappi G, Bagetta G. 17beta-estradiol reduces neuronal apoptosis induced by HIV-1 gp120 in the neocortex of rat. Neurotoxicology. 2005;26:893–903. doi: 10.1016/j.neuro.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 129.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18:161–6. [PubMed] [Google Scholar]
- 131.Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006;84:420–8. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 132.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–13. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 133.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–18. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez-Vidal MD, Cervera-Gaviria M, Ruelas R, Escobar A, Morali G, Cervantes M. Progesterone: protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch Med Res. 1998;29:117–24. [PubMed] [Google Scholar]
- 135.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 137.Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- 138.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 139.Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol. 2003;53(Suppl 3):S61–70. doi: 10.1002/ana.10489. discussion S70–2. [DOI] [PubMed] [Google Scholar]
- 140.Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33:285–93. doi: 10.1007/s12020-008-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]