Abstract
We studied the electrophysiological basis o object recognition by recording scalp EEG while participants played a virtual reality taxi driver game. Participants searched for passengers and stores during virtual navigation in simulated towns. We compared oscillatory brain activity for store views that were targets or non-targets (during store search) or neutral (during passenger search). Even though store category was solely defined by task context (rather than sensory cues), frontal electrophysiological activity in low frequency bands (primarily in the theta [4–8 Hz] band) reliably distinguished between target, non-target, and neutral store views. These results implicate low frequency oscillatory brain activity in frontal regions as an important variable in the study of cognitive processes involved in object recognition, categorization, and other forms of high-level perception.
Humans and other animals use locomotion to accomplish various goals such as finding shelter or food. For navigation in the environment to be successful, sensory input has to be mapped to high-level percepts that can range from very general (e.g., “shelter”) to very specific (e.g., “my home”). The goals at hand help to flexibly shape these percepts to allow us, for example, to view a bench, a tree stump, and a low wall as belonging to the goal category when searching for an object to sit on and to recognize only one of many chairs as the goal object when returning to a seat in a restaurant. In this paper we investigate the neurophysiological basis of this fast and effortless process of high-level perception in the context of spatial navigation.
Similar perception and decision processes are often studied in laboratory tasks that require no locomotion. In visual search, for example, the sensory input for a display is mapped to high-level “target” and “non-target” percepts on which the response is based (see e.g., Wolfe, 1998, for a review). Just as certain landmarks may be the goal of spatial search in some cases and not in others, the same stimuli can act as targets in some trials and non-targets in others during visual search. These studies have contributed significantly to our understanding of how visual features are integrated into percepts and of the role of attention in the parsing of visual scenes.
However, the simultaneous presentation of all stimuli typical in visual search studies makes it difficult, if not impossible, to investigate the processes that give rise to the perception of any particular stimulus within this display and its classification as target or non-target. Better suited for this purpose are studies that show one stimulus at a time in a rapid serial visual presentation (RSVP). In some RSVP studies (especially those studying “repetition blindness;” e.g., Kanwisher, 1987), effectively every stimulus is a target in the sense that each stimulus has to be kept in memory until all stimuli have been presented. In others (especially those studying the “attentional blink;” e.g., Raymond, Shapiro, & Arnell, 1992) only a small subset of the stimuli are targets, making these studies more conceptually similar to visual search studies (see Chun, 1997, for a review of these types of RSVP studies). RSVP studies as well as others presenting multiple stimuli nearby in time and space have consistently shown that stimuli that are presented close to a target stimulus profoundly affect its processing (e.g., Huber, Shiffrin, Lyle, & Ruys, 2001; Meyer & Schvaneveldt, 1971, 1976; Weidemann, Huber, & Shiffrin, 2005, 2008). Such studies provide important insights into the processes involved in integrating and differentiating sensory input into distinct percepts. This influence of nearby stimuli on the processing of the target, however, makes it difficult to study the formation of high-level percepts separately from the processes involved in parsing the sensory input.
Naturally, the issue of effects from nearby stimuli contaminating the study of target processing is not limited to the behavioral data discussed above, but applies at least as much to neurophysiological recordings (even when these provide high temporal resolution), such as the electroencephalogram (EEG). Oscillations in EEG activity have frequently been tied to task variables and cognitive processes. These effects manifest themselves in a wide range of oscillatory frequencies including low frequency bands in the delta (2–4 Hz) and especially the theta (4–8 Hz in humans, 4–12 Hz in rats) ranges (e.g., Hwang et al., 2005; Jacobs, Hwang, Curran, & Kahana, 2006; Sederberg et al., 2006; see Kahana, 2006 for a review). Indeed a common form of analysis is to average time-locked trials to study event related potentials (ERPs), which tend to emphasize low frequency fluctuations in the recorded EEG activity. Reliable ERP components have been identified several hundred ms after the offset of the event to which they correspond (e.g., a stimulus presentation). To avoid a superimposition of many similar effects, these components are usually studied by introducing temporal separations on the order of seconds between stimulus presentations. In RSVP tasks, stimuli are presented at a much higher rate which makes it very difficult to study the effects of any particular stimulus presentation in the RSVP sequence (cf. Vogel, Luck, & Shapiro, 1998).
When navigating through the world, salient landmarks can be separated arbitrarily which facilitates the study of their recognition. Moreover, sensory input during navigation in natural environments provides other important advantages: As in visual search tasks, the target object is presented within a context of irrelevant stimuli, but this context often shares few features with the target, thus making it easier to identify. Furthermore, spatial search in natural settings often imposes strong constraints on possible target locations, which can severely reduce the need to engage in visual search (e.g., a quick glance at an intersection can be enough to determine whether or not a traffic light is present). Studying neurophysiological correlates of spatial search before and after onset of target and similar non-target stimuli can therefore provide important insights into the neural basis of high-level perception that are difficult to obtain from more traditional experimental paradigms.
High level perception during spatial navigation
In order to present target stimuli sufficiently far apart to allow for reliable estimates of low frequency fluctuations in the EEG signal while keeping participants engaged, we asked participants to play the role of a taxi driver in an immersive virtual reality computer game. In this game, participants navigated in a virtual town with specific goals that varied from trial to trial such that a particular store could have been the goal location on one trial and irrelevant on the next. Whereas this dynamic and interactive display affords less control over the timing of stimulus presentations than more traditional experimental paradigms, it allowed us to separate target and non-target objects in (virtual) space such that the separation between subsequent objects was on the order of seconds. The interactive nature of the task has the advantage that it keeps participants engaged continuously, despite these large temporal separations between presentations of potentially relevant objects.
Methods
Participants
Twenty adults (ages 19–29, M = 22; 11 male, 9 female) participated in three experimental sessions for monetary compensation ($15 per hour plus a performance-based bonus of up to $10 per session). All participants were right-handed native-English speakers and had normal or corrected-to-normal vision. Each session, including application of the electrode net and running in the task, lasted approximately ninety minutes.
EEG recordings
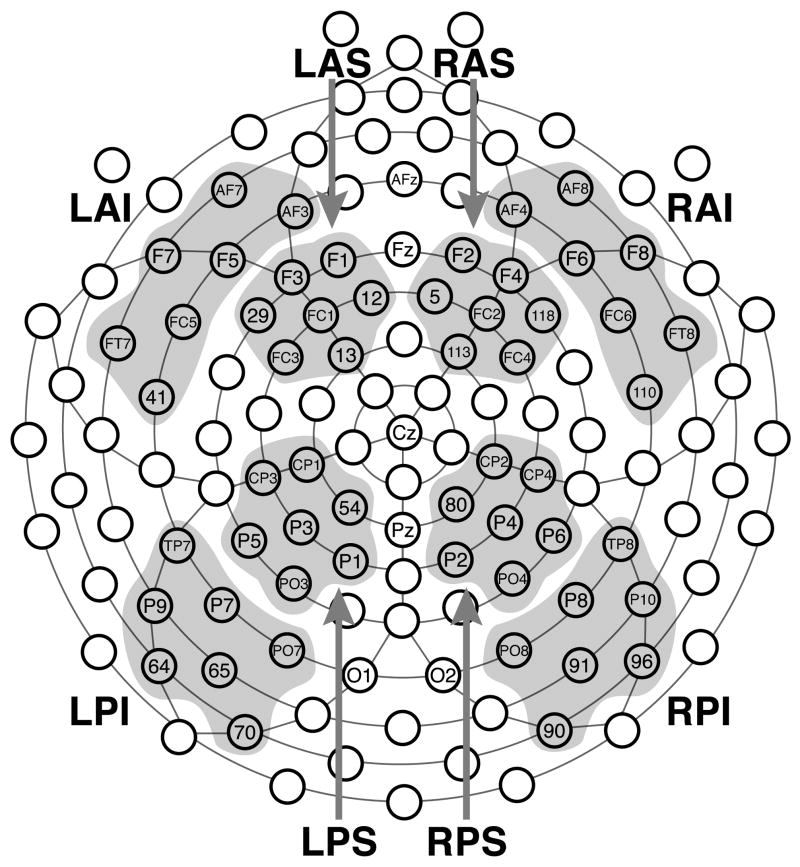
A 128-channel Geodesic Sensor Net™ was used to measure the EEG at the scalp with a sampling rate of 500 Hz (cf. Figure 1). The net was connected to an AC-coupled, high-input impedance amplifier (200 MΩ, Net Amps™, Electrical Geodesics Inc., Eugene, OR). The electrodes were adjusted until impedances were less than 50 kΩ.
Figure 1.
The 128-channel Geodesic Sensor Net™ used to measure the EEG and the regions of interest (ROI) on which the analyses were based. Each ROI is labeled with a 3 letter name that describes its position on the skull: R = right, L = left, S = superior, I = inferior, A = anterior, P = posterior
A central vertex reference (Cz) was used during recording, and all analyses were based on referencing to the average of all electrodes (cf. Dien, 1998). Channels deemed too noisy by visual inspection were excluded from the average in the referencing process and from all subsequent analyses. Furthermore, events with eye-movement or eye-blink artifacts (i.e., those with an electrooculogram exceeding ±100 μV), as well as those where the measured voltage fell outside the range of ±75 μV, were excluded from the analyses. In order to simplify the analyses and increase power to detect regional differences we grouped the electrodes into 8 regions of interests (ROIs) inspired by those used in other studies (e.g., Curran, 2004; Curran, DeBuse, Woroch, & Hishrman, 2006; Curran, DeBuse, & Lenynes, 2007; Curran & Hancock, 2007; Curran & Friedman, 2004). The shaded regions in Figure 1 illustrate these ROIs, and only data from electrodes that fell into these ROIs were analyzed.
Procedure
Participants played the role of a taxi driver within a virtual, three-dimensional town. Each trial first required participants to navigate trough the town looking for passengers (passenger search). Once a passenger was picked up, the participant was instructed to drive the passenger to a particular store within the town (store search). Participants were urged to find the target locations as quickly and accurately as possible by using the fastest route. Each experimental session consisted of three test towns with fifteen passenger deliveries per town. The first and second towns in each session were novel, and the third town was identical to the first.1 Each town was laid out on a 6 × 6 grid of blocks (see Figure 2a), with a single store or background building on each block. Five stores and thirty-one background buildings were randomly placed in each town, subject to the requirement that they were not placed in adjacent locations or in all four corners.
Figure 2.
a. Top-down view of a possible town layout. Squares with s inside indicate store locations; dark squares surrounded by a lighter area indicate building and sidewalk locations. b. A passenger, store, and buildings within the virtual town. c. The store in this figure occupies approximately 0.35% of the display (arrow added for illustrative purposes).
At the beginning of the experiment, participants first delivered passengers to stores in a small (3 × 3 blocks) practice town to familiarize them with the navigation in the virtual environment (these stores were not reused in the larger test towns). Before entering each of the three test towns, participants viewed static 2-D images of all 10 storefronts along with the store names to ensure that participants were highly familiar with the appearance of the stores (this list was presented five times, each time in a new random order and participants were asked to passively view it to familiarize themselves with the stores).2
Participants navigated from a first-person perspective using a computer-game controller, which was held with both hands resting in the lap, thereby reducing arm movements that could cause muscle artifacts in the electrophysiological recordings.3 For the first delivery of every town, a passenger was placed directly in front of the virtual taxi. During each of the remaining passenger-search phases, six passengers were distributed at different random locations within the town such that there was at most one passenger on any given block and passengers were usually not in the line of sight of the upcoming target store. Figure 2b shows a typical view during a passenger-search phase. Participants were asked to visit all five stores before a store was requested again. To motivate participants to learn the layout of each town the monetary bonus was based on how fast these deliveries were made.
Data analyses
The dynamic and interactive nature of the display presents a challenge when trying to time-lock the analyses to the presentation of (potential) target stimuli. When stimuli first appeared on the screen they were typically viewed from a distance and only became discernible when they were approached. All of our analyses concern the processing of store stimuli and we time-locked the analyses to the moment where a given store presentation exceeded a minimal size threshold (0.35% of the display). We chose this threshold based on preliminary analyses that indicated that target stores were directly approached on 95% of those trials in which they exceeded it. Figure 2c illustrates a typical view of a store at that threshold.
We classified stores as targets and non-targets during store search and as neutral during passenger search. We limited the analyses to events where only a single store was viewed and stayed in view for at least 1.5 s after the store surpassed the size threshold described above. We only considered target store events in which the participant arrived at that store without it leaving the field of view, and only considered neutral store events in which no passenger was visible (we only considered a passenger to be present if it occupied more than 0.35% of the screen at any time during the event). We identified a total of 2421, 2068, and 1725 target, non-target, and neutral events, respectively, but we discarded a substantial proportion of these events because of eye-movement artifacts leaving 1225, 1494, and 1014 target, non-target, and neutral events, respectively.4 The median time interval between successive events of the same kind (considering only artifact-free events) was 62 s, 55 s, and 63 s for target, non-target, and neutral events, respectively; the median time between any two artifact-free events was 23 s.
To minimize potential issues arising from variability of store detection relative to the threshold described above and to investigate high as well as low oscillatory frequencies in EEG activity, we calculated the oscillatory power for different frequencies at each trial and electrode and only averaged measures derived from this oscillatory power rather than averaging raw voltages as is done in ERP analyses. We calculated oscillatory power using Morlet wavelets with a width of 5 for 23 logarithmically spaced frequencies between 2 and 90.5 Hz ({2x}, where x ∈ [1, 1.25, 1.5,…,6.5]; cf. Jensen & Tesche, 2002; Tallon-Baudry, Bertrand, Delpuech, & Permier, 1997). In contrast to other electrophysiological measures such as phase-locking values and ERPs, this procedure is relatively robust to variations in the true detection times with respect to the threshold defined above.
Results
As in previous studies investigating spatial navigation in simple virtual towns where passenger search phases allowed for ample exploration (Newman et al., 2007), participants learned the layout of the towns quickly and took efficient routes to the target stores (for each town, the majority of deliveries consisted of routes that exceeded the city-block distance between the passenger pick-up location and the target store by less than one unit). Because navigation performance was so high across towns and because our main focus is the perception of target, non-target, and neutral stimuli, rather than spatial navigation or learning, we aggregate across towns and deliveries in all subsequent analyses. Detailed behavioral analyses of spatial navigation and learning in a very similar task to the one used here are reported by Newman et al. (2007).
To investigate how oscillations in EEG activity covary with high-level perception, we compared oscillatory activity after the onset of target, non-target, and neutral stores with activity for a baseline period before store onset. As detailed below, we found strong and widespread effects in response to all stores in the lower frequency bands that differentiated between the high-level store categories in the frontal ROIs.
Specifically, we aggregated oscillatory power (for each participant at each session) over ROIs (cf., Figure 1) and over time windows of 0.3 s starting 0.6 s before the onset of the store (as defined by the threshold described above) until 1.5 s after store onset. The first time window (0.6–0.3 s before store onset) served as a baseline, and power in all time windows was z-transformed by subtracting the mean baseline power and dividing by the standard deviation of baseline power. We chose this baseline because the associated 300 ms buffer between its end and the store onset allowed us to reasonably assume that it mainly indexed electrophysiological activity prior to the detection of the store. Thus the transformed values index the standardized change in power associated with the detection of a store.
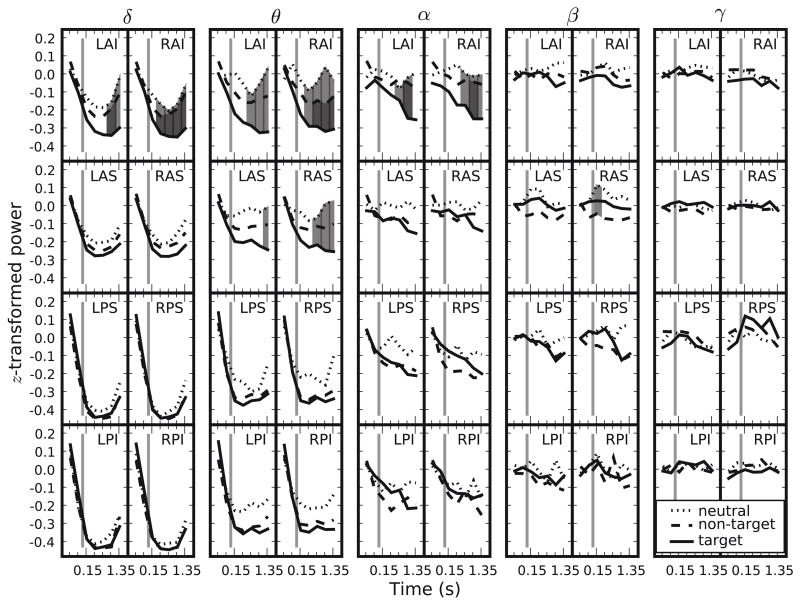
We then aggregated the data into frequency bands by averaging over frequencies in the delta (2–4 Hz), theta (4–8 Hz), alpha (8–16 Hz), beta (16–32 Hz), and gamma (≥ 32 Hz) ranges. Figure 3 shows the z-transformed power for target, non-target, and neutral events in the different frequency bands and ROIs as a function of time.
Figure 3.
Z-transformed power for neutral, non-target, and target store views. The labels on the abscissa show the mean time point of each bin (the first two time points in each panel, separated by a gray vertical line, show data from before the store presentation exceeded threshold). Because of the z-transformation, the means in each panel are constrained to start near 0 (because of different numbers of neutral, non-target, and target observations the central tendency of these means can deviate slightly from 0). The 5 columns show z-transformed power for the δ (2–4 Hz), θ (4–8 Hz), α (8–16 Hz), β (16–32 Hz), and γ (≥ 32 Hz) frequency bands. From top to bottom the panels show data from the left and right anterior inferior ({L,R}AI), anterior superior ({L,R}AS), posterior superior ({L,R}PS), and posterior inferior ({L,R}PI) regions of interest, respectively. Shaded gray areas show significant differences—except for the β frequency band, which shows a significant difference between neutral and non-target in the RAS region, light gray areas show significant differences between neutral and target store views, whereas dark gray areas denote significant differences between non-target and target store views.
As is evident from the figure, oscillatory power in the lower frequencies decreased around the onset of a store. Aggregating across store categories, ROIs, and time windows after store onset confirmed significantly negative z-transformed power for the delta, theta, and alpha frequency bands (t(19) = −9.14, −8.78, and −3.12, respectively; SE = 0.03, 0.02, and 0.03 respectively; p <.01 for all comparisons). Furthermore, oscillatory power in the anterior ROIs exhibited a tendency to diverge for target, non-target, and neutral store views. To determine whether power values for these different event types differed significantly, we performed three permutation tests, one for each possible pairwise comparison. In analogy to t-tests, the permutation tests were based on the relative differences of z-transformed power between two event types across participants. To compare target and non-target events, for example, we subtracted the mean non-target power for each participant at each ROI and time window from the corresponding mean target power and averaged this difference across participants. We then divided this mean difference in power for each ROI and time window by its standard error to get the relative differences of power between these two conditions.
We compared this relative difference in power between two conditions to the distribution of relative differences that is obtained by permuting the condition labels at the level of participants. For 20 participants there are 220 possible ways to permute the labels for two conditions, but because inverting all labels just changes the sign of the relative difference only half of these need to be considered. To illustrate this process, consider comparing data for target and non target events: Each combination of frequency band, ROI, and time window yields a relative difference between these two conditions. A permutation distribution can be obtained by flipping the two labels for subsets of the participants such that for some participants the data from target events are treated as data from non-target events and vice versa (as described above, there are 220 different ways to do this). If z-transformed power does not distinguish between target and non-target events we expect the observed relative difference of power between these two conditions to be close to the center of the distribution of relative differences obtained from these permutations. We compared the observed absolute relative difference in power to the distribution of absolute relative differences based on these permutations and deemed a difference to be statistically significant if it exceeded the 95th percentile of this distribution. By using only one overall significance criterion per test, the family-wise Type I error rate for each of the 3 comparisons is fixed at 5% across all frequency bands, ROIs, and time windows (cf. Maris & Oostenveld, 2007; Nichols & Holmes, 2001).
Figure 3 shows significant differences in oscillatory power between neutral and target stores which were prevalent in the left and right anterior inferior ROIs for the delta, theta, and alpha frequency bands; this difference can also be observed in the left and right anterior superior ROI for the theta frequency band and was always such that oscillatory power was lower for target stores than for neutral stores. In the anterior inferior ROIs (with the exception of the left anterior inferior ROI) there are also significant differences between oscillatory power for target and non-target events at the same three lowest frequency bands such that oscillatory power was lower for target stores than for non-target stores. Furthermore, the right anterior superior ROI showed significantly lower oscillatory power for non-target than for neutral events directly after store onset (all other effects only reached significance at least 0.3 s after store onset). Because differences in low frequency oscillations in EEG activity (most notably theta) have been linked to differences in (virtual) movement (e.g., Caplan et al., 2003; Ekstrom et al., 2005), we repeated our analyses using only a subset of events where participants moved continuously from the beginning of the baseline period until 1.5 s after store onset. Despite being considerably more noisy due to the reduced amount of data, these results showed the same qualitative pattern as the full data set.
Discussion
We investigated oscillations in EEG activity relative to the onset of stimuli that, on some trials, were the targets of spatial search during navigation in a virtual environment. Our analysis focused on comparing electrophysiological activity in response to goal stimuli (target stores) to that for the same stimuli on trials where either a stimulus from the same category was the target (i.e., non-target stores when a store was the target) or when participants searched for categorically different stimuli (i.e., neutral stores when participants searched for passengers). We observed a widespread decrease in low-frequency oscillatory power around the time of store onset for all store types. This decrease was especially pronounced for target stimuli such that frontal oscillatory power for these stimuli was significantly lower than that for neutral stimuli, predominantly in the theta range, but also extending to neighboring frequency ranges (cf. Figure 3). Differences between target and non-target stimuli showed a similar pattern of results (albeit with smaller effect size), but with the exception of one time point at one ROI, the differences between neutral and non-target stimuli failed to reach significance. Strikingly, all significant differences between conditions were confined to the frontal ROIs.
These results extend the findings of Caplan et al. (2003) who observed greater theta power for passenger search than for store search without conditioning on store view in a similar taxi driver game. Even though the natural and interactive nature of our task makes it impossible to completely rule out behavioral confounds that may have contributed to these results (such as potential differences in small eye movements across conditions and/or in response to all stores), we note that we would expect different levels of micro-saccades to produce artefactual results in the gamma band (Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008) where we found no differences between conditions and no difference in oscillatory power from baseline. We also note that our results are characterized by widespread and robust low-frequency desynchronizations that vary in strength across stimulus classes at frontal ROIs — an unlikely pattern to be due to muscle artifacts, especially given the fact that we matched the different event types as closely as possible in terms of their perceptual and navigational characteristics. The fact that the same stimuli served as target, non-target, and neutral stores suggests that any differences between conditions are likely due to top-down processes that match stores to the current goal.
Another possible concern are potentially different cognitive strategies between passenger search and store search phases of the experiment. We note that such differences would likely have affected non-target store views (during store search phases) and neutral store views (during passenger search phases) differently, yet the differences between these conditions were (with one exception) small and not statistically significant. Our analyses in terms of relative change with respect to baseline periods before store onset likely substantially reduced or eliminated potential effects from different cognitive strategies and therefore these results are not well suited to evaluate global effects of different strategies for spatial search.
It is particularly interesting that oscillatory brain activity reliably distinguishes between visual stimuli based only on their trial-specific role. To perceive a given stimulus as “target” on some trials and not on others requires the integration of ongoing sensory input with the current goals and knowledge. It is tempting to conclude from our results that widespread cortical networks that (de-)synchronize at low frequencies directly index processes that are sensitive to the detection of salient stimuli with frontal networks specializing in the formation of high-level percepts based on the current goals. The special role of the frontal ROIs seems compatible with several imaging studies that particularly implicate frontal brain regions in visual categorization (see, e.g., Vogels, Sary, Dupont, & Orban, 2002, for a review). This interpretation suggests that decreases in low-frequency oscillatory power may also be able to index target detection in other tasks where targets may not be well separated in time and/or space from other salient stimuli, such as visual search or RSVP studies. This would make low-frequency oscillatory power an important dependent variable to consider when studying target detection, even when the task is not well suited for conventional stimulus-locked EEG analyses.
An equally plausible explanation for our results, however, is that the observed differences index associated processes that depend on the formation of high-level percepts (rather than indexing the processes responsible for forming such percepts directly). Such secondary processes could include those associated with orienting and route planing: Even though the environment was well learned, it is reasonable to assume that the relative routes between stores were especially well encoded such that viewing a store decreased uncertainty about the location within the environment and about which way to proceed. To the extent that low-frequency oscillatory activity reflects ongoing orienting and route planing processes, viewing a store should decrease the demand of those processes. This could explain the widespread decrease in low-frequency oscillatory power for all store types as well as the stronger decrease for target stores in frontal ROIs (because target stores signal the goal of the ongoing navigation). Likewise, the decrease in demand of these processes should be least when all stores are neutral with respect to target status, just as we observed. This interpretation of our results is consistent with studies that show the recruitment of frontal brain areas for route learning (e.g., Shelton & Gabrieli, 2002) and single unit studies that highlight the importance of frontal regions in goal-directed navigation (e.g., Hok, Save, Lenck-Santini, & Poucet, 2005; Poucet et al., 2004).
Alternatively, our results may reflect attentional and/or working memory effects: While searching for a particular store, encountering this goal is likely associated with a release of working memory (i.e., the current goal does not have to be actively maintained anymore) and attention likely remains focused on the target store. Encountering a non-target store, on the other hand, only helps to narrow down the target search and attention likely shifts away from that stimulus as it is passed. Similarly, neutral stores are of little relevance during passenger search and we would therefore expect any attentional or working memory effects to be smallest for these stimuli. This interpretation of our results is consistent with several studies relating theta oscillations to working memory and task demands (e.g., Jensen & Tesche, 2002; Sauseng, Hoppe, Klimesch, Gerloff, & Hummel, 2007). A particular sensitivity of frontal regions to attentional effects seems compatible with a recent imaging study assessing the processing of unexpected events during spatial navigation in humans (Iaria, Fox, Chen, Petrides, & Barton, 2008).
If our results index secondary processes that depend on the formation of high-level percepts, the extent to which our findings generalize to different tasks such as visual search and RSVP studies would depend on the specific nature of these processes. Attentional processes may produce similar results even for superficially very different tasks, whereas other processes, such as those involved in orienting and route planing, would be more specific to tasks involving spatial navigation.
Future work is required to better characterize whether the measures we used assess the formation of high-level percepts directly or indirectly. Regardless of the ultimate source of the effects, their clear correspondence with high-level percepts can establish strong constraints for theories addressing their formation and time course and, in turn, for those on decision making, categorization, and other aspects of human cognition.
Acknowledgments
The authors acknowledge Igor Korolev’s help with the data aquisition and helpful discussions with Joshua Jacobs, Jeremy Manning, Katherine McEldoon, Sean Polyn, and Per Sederberg that benefitted this paper. This work was supported by a post-doctoral fellowship to Christoph Weidemann from the German Academic Exchange Service (DAAD), as well as by grants NIMH 55678 and NIMH 61975 to Michael Kahana.
Footnotes
This particular design was inspired by the the studies of Newman et al. (2007) before we decided to conduct the analyses presented here.
Even though we did not separately measure familiarity with the stores, we take the fact navigation performance was high (see results section) to indicate that participants were highly familiar with the stores. We also note that the set of different stores was relatively small (only 5 stores per town). To the extent that participants were not familiar enough with a particular store, this should have equally affected target, non-target, and neutral store views, because every store served each of these roles.
Navigation required only slight movements of the left thumb to press down different sides of a round pad on the controller. Speed could vary with the amount of pressure that was applied on the pad, but participants tended to navigate at full speed.
In order not to interfere with the interactive and dynamic task, participants received no special instructions to avoid eye blinks or eye movements. Because of the ±75 μV threshold on scalp electrodes not all of these events contributed data for every electrode.
References
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. Journal of Neuroscience. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM. Types and tokens in visual processing: A double dissociation between the attentional blink and repetition blindness. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:738–755. doi: 10.1037//0096-1523.23.3.738. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlated of recollection and familiarity. Memory & Cognition. 2004;42:1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Curran T, DeBuse C, Lenynes PA. Conflict and criterion setting in recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:2–17. doi: 10.1037/0278-7393.33.1.2. [DOI] [PubMed] [Google Scholar]
- Curran T, DeBuse C, Woroch B, Hishrman E. Combined pharmacological and electrophysiological dissociation of familiarity and recollection. Journal of Neuroscience. 2006;27:1979–1985. doi: 10.1523/JNEUROSCI.5370-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Friedman W. ERP old/new effects at different retention intervals in recency discrimination tasks. Cognitive Brain Research. 2004;8:107–120. doi: 10.1016/j.cogbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Curran T, Hancock J. The FN400 indexes familiarity-based recognition of faces. NeuroImage. 2007;36(2):464–471. doi: 10.1016/j.neuroimage.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behavior Research Methods, Instruments and Computers. 1998;30:34–43. [Google Scholar]
- Ekstrom AD, Caplan J, Ho E, Shattuck K, Fried I, Kahana M. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proceedings of the National Academy of Sciences. 2005;102(12):4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DE, Shiffrin RM, Lyle KB, Ruys KI. Perception and preference in short-term word priming. Psychological Review. 2001;108(1):149–182. doi: 10.1037/0033-295x.108.1.149. [DOI] [PubMed] [Google Scholar]
- Hwang G, Jacobs J, Geller A, Danker J, Sekuler R, Kahana MJ. EEG correlates of verbal and nonverbal working memory. Behavioral and Brain Functions. 2005;1:20. doi: 10.1186/1744-9081-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Fox CJ, Chen JK, Petrides M, Barton JJS. Detection of unexpected events during spatial navigation in humans: Bottom-up attentional system and neural mechanisms. European Journal of Neuroscience. 2008;27:1017–1025. doi: 10.1111/j.1460-9568.2008.06060.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. NeuroImage. 2006;15(2):978–87. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. The cognitive correlates of human brain oscillations. Journal of Neuroscience. 2006;26(6):1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher NG. Repetition blindness: Type recognition without token individuation. Cognition. 1987;27:117–143. doi: 10.1016/0010-0277(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval oparations. Journal of Experimental Psychology. 1971;90:227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW. Meaning, memory structure, and mental processes. Science. 1976;192:27–33. doi: 10.1126/science.1257753. [DOI] [PubMed] [Google Scholar]
- Newman EL, Caplan JB, Kirschen MP, Korolev IO, Sekuler R, Kahana MJ. Learning your way around town: How virtual taxicab drivers learn to use both layout and landmark information. Cognition. 2007;104(2):231–253. doi: 10.1016/j.cognition.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucet B, Lenck-Santini PP, Hok V, Save E, Banquet JP, Gaussier P, et al. Spatial navigation and Hippocampal place cell firing: The problem of goal encoding. Reviews in the Neurosciences. 2004;15:89–107. doi: 10.1515/revneuro.2004.15.2.89. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. European Journal of Neuroscience. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. NeuroImage. 2006;32(3):1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- Shelton AL, Gabrieli JDE. Neural correlates of encoding space from route and survey perspectives. Journal of Neuroscience. 2002;22(7):2711–2717. doi: 10.1523/JNEUROSCI.22-07-02711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17(2):722–34. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- Vogels R, Sary G, Dupont P, Orban GA. Human brain regions involved in visual categorization. NeuroImage. 2002;16:401–414. doi: 10.1006/nimg.2002.1109. [DOI] [PubMed] [Google Scholar]
- Weidemann CT, Huber DE, Shiffrin RM. Confusion and compensation in visual perception: Effects of spatiotemporal proximity and selective attention. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:40–61. doi: 10.1037/0096-1523.31.1.40. [DOI] [PubMed] [Google Scholar]
- Weidemann CT, Huber DE, Shiffrin RM. Prime diagnosticity in short-term repetition priming: Is primed evidence discounted, even when it reliably indicates the correct answer? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:257–281. doi: 10.1037/0278-7393.34.2.257. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. London, UK: Psychology Press; 1998. pp. 13–75. [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]