Abstract
Autoantigen presentation to T cells is crucial for the development of autoimmune disease. However, the mechanisms of autoantigen presentation are poorly understood. In this study, we show that splenic phagocytes play an important role in autoantigen presentation in murine lupus. Nucleosomes are major autoantigens in systemic lupus erythematosus. We found that nucleosome-specific T cells were stimulated dominantly in the spleen, compared with lymph nodes, lung, and thymus. Among splenic APCs, F4/80+macrophages and CD11b+CD11c+ dendritic cells were strong stimulators for nucleosome-specific T cells. When splenic phagocytes were depleted in (NZB × NZW) F1 (NZB/W F1) mice, nucleosome presentation in the spleen was dramatically suppressed. Moreover, depletion of splenic phagocytes significantly suppressed anti-nucleosome Ab and anti-dsDNA Ab production. Proteinuria progression was delayed and survival was prolonged in phagocyte-depleted mice. The numbers of autoantibody-secreting cells were decreased in the spleen from phagocyte-depleted mice. Multiple injections of splenic F4/80+ macrophages, not those of splenic CD11c+ dendritic cells, induced autoantibody production and proteinuria progression in NZB/W F1 mice. These results indicate that autoantigen presentation by splenic phagocytes including macrophages significantly contributes to autoantibody production and disease progression in lupus-prone mice.
Systemic lupus erythematosus (SLE)3 is an autoimmune disease characterized by autoantibody production and various types of organ damages. Hyperactivation of T (1) and B cells (2) has been observed in human and murine lupus. Several groups have reported that intrinsic abnormalities in APCs are associated with SLE. Dendritic cells (DCs) contribute to the pathogenesis of lupus by producing cytokines or chemokines (3). Monocytosis in BXSB mice (4) and increased macrophages in NZB/W F1 and MRL/lpr mice have been documented (5). The efficiency of macrophage clearance of apoptotic bodies has been associated with lupus-like disease in mice. For example, MFG-E8−/− (6) mice and Merkd mice (7) produced high titers of autoantibodies. Though the autoantigen load leads to autoimmunity, autoantigen presentation by APCs is poorly understood in lupus-prone mice.
APCs play multiple roles in the immune system: clearance of Ags, cytokine production, and Ag presentation to T cells. DCs are thought to be the most potent cells in Ag presentation, including autoantigens (8). Macrophages produce immunosuppressive and anti-inflammatory cytokines like IL-10 and TGF-β after ingesting apoptotic cells (9 –11) Ag presentation by macrophages may induce a tolerogenic response in T cells. In contrast, macrophages are capable of producing proinflammatory cytokines such as TNF-α or type 1 IFN and express costimulatory molecules in response to stimulation of Toll-like receptors by self nucleic acids (12). Thus, activation of macrophages could promote immune responses to self by virtue of inflammatory cytokine production and through its APC function.
Nucleosomes are major immunogens for T cells and are targets for pathogenic autoantibody production in lupus-prone mice (13, 14). Nucleosomes are ubiquitous autoantigens generated by apoptosis of cells (15, 16). Moreover, antinucleosome Ab titers have better specificity and diagnostic confidence than anti-dsDNA Ab titers in human SLE (17). Antinucleosome Abs can be detected earlier than anti-dsDNA Abs in lupus-prone mice (18).
In our previous study, we reconstituted nucleosome-specific T cells and found that nucleosome hyperpresentation in the spleen from prenephritic NZB/W F1 mice (19).
The purpose of the present study is to determine the pathogenic effect of autoantigen presentation by splenic phagocytes. We demonstrated that nucleosome presentation was dominant in the spleen, and that splenic F4/80+ macrophages presented nucleosomes efficiently. In NZB/W F1 mice, depletion of splenic phagocytes, including macrophages, suppressed nucleosome presentation in the spleen, autoantibody production, and proteinuria progression. The numbers of autoantibody-secreting cells were decreased in the spleen. Repeated injections of splenic macrophages into prenephritic mice induced autoantibody production and proteinuria progression. These findings demonstrate that autoantigen presentation by splenic phagocytes is immunogenic and contributes to the development of murine lupus.
Materials and Methods
Mice
NZB/W F1, BALB/c, NZB, and NZW mice were obtained from Japan SLC. SWR mice were obtained from The Jackson Laboratory. SNF1 mice were bred at our laboratory. All animal experiments were conducted in accordance with the institutional and national guidelines.
Plasmid construction
pMXW-AN3α and pMXW-AN3β were used to generate nucleosome-specific TCR as previously described (19). pMX-DOTAE and pMX-DOTBE were used to generate OVA-specific DO11.10 TCR as previously described (20).
Production of retroviral supernatants and retroviral transductions
Total splenocytes were cultured for 48 h in the presence of Con A (10 μg/ml) and mIL-2 (50 ng/ml) (R&D Systems). Retroviral supernatants were obtained by transfection of pMXW-AN3α, pMXW-AN3β, pMX-DOTAE, or pMX-DOTBE into PLAT-E packaging cell lines using Fu-GENE 6 transduction reagent (Roche Diagnostic Systems), as previously described (21). Retroviral gene transduction was performed as described (19, 20). In brief, Falcon 24-well plates (BD Biosciences) were coated with the recombinant human fibronectin fragment CH296 (Retronectin; Takara Shuzo). The viral supernatant was preloaded into each well of the CH296-coated plate, and the plate was spun at 2400 rpm for 3 h at 32°C. This procedure was repeated three times. The viral supernatant was washed away, and Con A-stimulated splenocytes were placed into each well (1 × 106 per well). Cells were cultured for 36 h to allow infection to occur.
Cell purification
A CD4+ T cell population was prepared by negative selection with MACS using anti-CD19 mAb, anti-CD11c mAb, and anti-CD8 mAb. CD11c+ DCs were prepared as previously described (19). In brief, spleen cells were digested with collagenase type IV (Sigma Aldrich) and DNase I, and the CD11c+ DCs were selected twice by positive selection with MACS CD11c microbeads and magnetic separation columns. The purity of the isolated CD11c+ cells was consistently >90%. F4/80+ macrophages were isolated by incubating single cell suspensions with anti-F4/80-biotin mAb (Caltag Laboratories) and anti-CD11c-conjugated MicroBeads. After negative selection for CD11c positive cells, negative fraction was additionally incubated with streptavidin-conjugated microbeads, and F4/80+ macrophages were isolated with two rounds of positive selection using magnetic separation columns (Miltenyi Biotec). The purity of the isolated F4/80+ cells was consistently >90%, and the contamination was <0.5% for CD11c+ cells and 3.5% for CD19+ cells. Cell viability was always >97%, as detected by trypan blue exclusion. CD11b+ CD11c+ DCs and CD8+ CD11c+ DCs were prepared by positive selection with MACS using the indicated Abs and a FITC MultiSort Kit (Miltenyi Biotec).
Transfer experiments
The indicated number of cells suspended in PBS was i.v. injected into mice. Cell viability was always >97%, as detected by trypan blue exclusion.
FACS analysis
For analyzing MHC class II expression levels of splenic DCs or macrophages, splenocytes were stained with anti-CD11c-FITC mAb, anti-F4/80-PE mAb, and anti-I-A/I-E-biotin mAb followed by streptavidin-allophycocyanin. Plasma cells (PCs) were stained with anti-CD138-PE mAb (BD Pharmingen) as previously described (22). For analyzing CFSE-labeled AN3-transduced T cells in the spleen, splenocytes isolated from recipient mice were stained with anti-CD69-PE mAb, anti-Vβ4-biotin mAb followed by streptavidin-allophycocyanin, and anti-CD4-allophycocyanin-Cy7 mAb. Cytometric analysis was performed using a FACSVantage cytometer (BD Biosciences) with CellQuest software (BD Biosciences).
Proliferation assay
At 24 h post infection, purified CD4+ T cells were cultured at 1 × 104 cells/well, with 1 × 104 cells/well of irradiated APCs or 5 × 105 cells/well of irradiated splenocytes in 96-well flat-bottom plates in volumes of 100 μl of complete medium.
In the experiment of OVA presentation, indicated number of purified DO11.10 TCR-transduced CD4+ T cells were cultured with 1 × 104 cells/well of irradiated APCs in 96-well flat-bottom plates in volumes of 100 μl of complete medium with 3 μM chicken OVA323–339 peptide. [3H] thymidine incorporation was determined as previously described (19).
In vivo depletion of splenic phagocytes
Splenic phagocytes were depleted in vivo using dichloromethylene diphosphonate (Cl2MDP) encapsulated in liposomes. Cl2MDP-liposomes and PBS-liposomes were prepared as described (23). Cl2MDP was a gift of Roche Diagnostics. Cl2MDP-liposomes or PBS-liposomes (0.1 ml/10g body weight) were i.v. injected into female NZB/W F1 mice at 20 and 22 wk of age.
In the experiment of OVA presentation, purified OVA-reactive CD4+ T cells were cultured at 1 × 105 cells/well, with 5 × 105 cells/well of irradiated splenocytes from Cl2MDP- or PBS-liposome-treated mice in the presence of 100 μg/ml whole OVA protein in 96-well flat-bottom plates in volumes of 100 μl of complete medium.
Immunohistochemistry
Cryopreserved sections of spleen were incubated with a rat Alexa488-labeled mAb to CD4, Alexa546-labeled mAb to F4/80 or CD11c, and Cy5-labeled mAb to B220 (Vector Laboratories). To detect the deposition of immune complexes at glomeruli, we incubated sections with FITC-labeled goat Abs to mouse IgG or to C3 (ICN Pharmaceuticals).
Evaluation of nephritis
Kidneys were fixed in 10% formalin for 24 h at 4°C. Paraffinized sections of kidneys were stained with H&E, and periodic acid-Schiff reagents. Histopathologic findings in glomerular lesions were graded on a scale of 0 –3, where 0 = normal, 1 = mild (cell proliferation and/or cell infiltration), 2 = moderate (cell proliferation and/or cell infiltration with membrane proliferation), and 3 = severe (cell proliferation and/or cell infiltration, membrane proliferation, and crescent formation and/or hyalinosis). The glomerular lesion index was calculated from the sum of the scores for 40 random glomeruli per kidney as previously described (24 –26).
ELISA
IgG anti-DNA Abs were measured using ELISA plate coated with λ-phage-derived purified dsDNA (MESACUP DNA-II TEST; Medical & Biological Laboratories). The DNA-bindings activities were expressed in units, referring to a standard curve obtained by serial dilutions of a standard serum pool from 7- to 9-mo-old NZB/W F1 mice, containing 1000 U/ml (19). IgG anti-nucleosome Abs were quantified using ELISA plates coated with human nucleosome (Orgentec).
Quantification of Ab-secreting cells by ELISPOT
Single cell suspensions of the spleen and bone marrow (BM) were filtered through a Falcon cell strainer (70 mm), washed, and resuspended in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Anti-dsDNA Ab-secreting cells and anti-nucleosome Ab-secreting cells were analyzed using a modified ELISPOT technique as previously described (22, 27). ELISA plates coated with λ-phage-derived purified dsDNA (Medical & Biological Laboratories) or human nucleosome (Orgentec Diagnostika) were blocked with PBS containing 3% BSA for 1 h at room temperature. The phage-derived purified dsDNA gave an OD260/280 of 1.8. No contamination of proteins was seen by agarose gel electrophoresis and silver staining in the phage-derived purified dsDNA. The purity of human nucleosome was 88%. Subsequently, splenocytes or BM cells were added to each well (5 × 106 cells/well) and incubated for 16 h at 37°C in a humid atmosphere with 5% CO2. Then, the cells were analyzed for DNA-specific or nucleosome-specific Ab-secreting cells. The absolute numbers of Ab-secreting cells in total BM were calculated as previously described (27).
Repeated injections of splenic phagocytes
Five × 105 splenic F4/80+ macrophages or CD11c+ DCs were transferred in NZB/W F1 mice at the age of 8, 12, 16, and 20 wk. Proteinuria progression and serum Ab levels were assessed.
Statistical analysis
Statistical analyses were performed using the Student’s t test, the Mann-Whitney U test for nonparametric data, or one-way ANOVA followed by Bonferroni correction. Proteinuria and survival data were analyzed using Kaplan-Meier curves and the log-rank test. A p value of <0.05 was considered to be significant.
Results
Splenic phagocytes presented nucleosome spontaneously in lupus-prone mice
We have previously reported a reconstitution of nucleosome specificity in NZB/W F1 CD4+ T cells by TCR gene transfer (19, 28). Retroviral vectors with nucleosome-specific AN3 TCR (pMXW-AN3β and pMXW-AN3β) were used for the gene transfer. T cells expressing AN3 TCR recognize nucleosomes in the context of I-Ad (13, 14, 19, 29). Because the TCR β-chain of AN3 belongs to Vβ4 subfamily, retroviral infection of the AN3 TCR genes into NZB/W F1 splenocytes resulted in a 40 – 45% increase of the Vβ4+ population in CD4+ T cells compared with pMXW-infected splenocytes (Fig. 1A). The efficacy of the Vβ4 introduction into the CD4+Vβ4− population was calculated to be 50 – 60%. Thus, the proportion of clonotypic AN3-TCR-expressing cells was estimated to be 25–36% in CD4+ T cells. These cells were referred to as AN3 CD4+ T cells, and pMXW-infected CD4+ T cells were referred to as mock CD4+ T cells. When CFSE-labeled AN3 or mock CD4+ T cells were transferred to NZB/W F1 mice, extensive proliferation of AN3 CD4+ T cells was observed in the spleen whereas only slight proliferation was observed in the inguinal lymph nodes (LNs), lung or thymus (Fig. 1B). Because AN3 TCR was originally derived from (SWR × NZB) F1 (SNF1) mice, proliferation of AN3 or mock CD4+ T cells was also examined in SNF1 mice. As with NZB/W F1 mice, AN3 CD4+ T cells proliferated predominantly in the spleen of SNF1 mice (data not shown). Together, these results indicate that nucleosome-specific CD4+ T cells are mostly activated in the spleens of lupus-prone mice.
FIGURE 1.
Splenic F4/80+ macrophages are potent activators of nucleosome responsive T cells. Ten-wk-old NZB/W F1 mice were used for the experiments. A, Anti-CD4 and anti-Vβ4 staining of pMXW (mock)- or AN3-transduced splenocytes. B, CFSE-labeled AN3- or mock-transduced CD4+ T cells were i.v. transferred into 10-wk-old NZB/W F1 mice. Forty-eight h later, single-cell suspensions of spleen, inguinal LNs (iLNs), lung or thymus from recipient mice were examined for CFSE+Vβ4+CD4+-gated cells. C, Proliferation of mock- or AN3-transduced CD4+ T cells to splenic APC subsets. Results represent means ± SD of triplicate wells. D, The MHC class II expression of splenic F4/80+ macrophages and CD11c+ DCs from BALB/c, NZB/W F1, NZB, or NZW mice. *, Significant difference (p < 0.05) compared with BALB/c mice or NZW mice. E, Proliferation of mock- or DO11.10-transduced CD4+ T cells to splenic F4/80+ macrophages or CD11c+ DCs with 3 μM OVA323–339 peptide. F, Proliferation of mock- or AN3-transduced CD4+ T cells to F4/80+ macrophages from indicated organs. Results represent means ± SD of triplicate wells. Data shown are representative of three independent experiments with similar results.
We next determined the specific APC subsets that presented nucleosomes in the spleen. In prenephritic NZB/W F1 and SNF1 mice, F4/80+ macrophages as well as CD11b+CD11c+ DCs stimulated AN3 CD4+ T cells (Fig. 1C and data not shown). As we reported previously, (19), CD19+ B cells did not stimulate AN3 CD4+ T cells (data not shown). When we examined phenotype of F4/80+ macrophages and CD11c+ DCs in NZB/W F1 and control BALB/c mice, the MHC class II expression levels of NZB/W F4/80+ macrophages were ~3 times as high as those of BALB/c F4/80+ macrophages. NZB/W and NZB F4/80+ macrophages exhibited significantly increased expression levels of MHC class II compared with BALB/c and NZW F4/80+ macrophages (Fig. 1D). The expression levels of CD40 and CD86 showed no significant elevation in NZB/W F4/80+ macrophages (data not shown). In contrast, MHC class II expression levels of NZB/W CD11c+ DCs were comparable to those of BALB/c CD11c+ DCs. The MHC class II expression levels of NZB/W F4/80+ macrophages were similar in 4 wk old and 8 wk old NZB/W F1 mice and were comparable to those of NZB/W CD11c+ DCs (Fig. 1D). With regard to exogenous Ag presentation, the OVA peptide presentation of F4/80+ macrophages to DO11.10 TCR-transduced CD4+ T cells (DO11.10 CD4+ T cells) was essentially similar to that of CD11c+ DCs in NZB/W F1 mice (Fig. 1E).
Because F4/80+ macrophages showed increased expression of costimulatory molecule and the number of F4/80+ macrophages was ~3 to 5 times as large as that of CD11c+ DCs in the spleen (Fig. 2A and data not shown), we examined nucleosome presentation of F4/80+ macrophages derived from other organs where apoptosis occurred spontaneously. F4/80+ macrophages directly isolated from the BM hardly stimulated AN3 T cells. F4/80+ macrophages from the thymus stimulated AN3 T cells significantly more weakly than those from the spleen (Fig. 1F). We also checked exogenous Ag presentation using DO11.10 CD4+ T cells. F4/80+ macrophages from the spleen, BM, and thymus stimulated DO11.10 CD4+ T cells similarly in the presence of OVA323–339 peptide (data not shown). These data indicate that nucleosome presentation by splenic F4/80+ macrophages is a relatively specific phenomenon.
FIGURE 2.
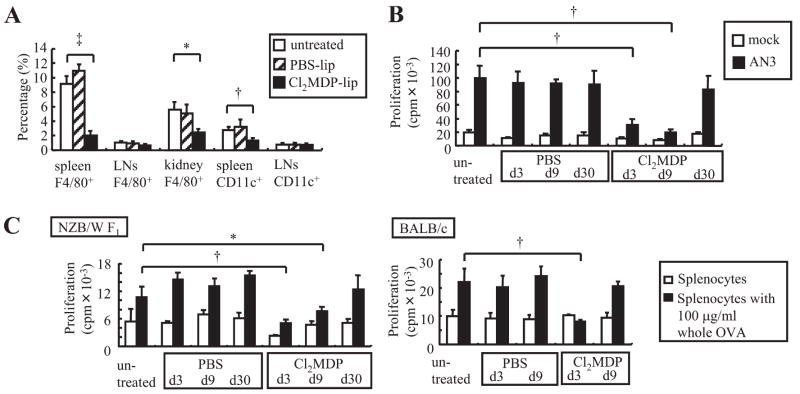
Depletion of phagocytes suppresses autoantigen presentation in the spleen. A, Ten-wk-old NZB/W F1 mice were treated with i.v. injection of Cl2MDP- or PBS-liposomes. Three days later, single-cell suspensions from the spleen, inguinal LNs, and kidneys were prepared and analyzed by FACS. The percentages of CD11c+ DCs and F4/80+ macrophages in the indicated organ are shown. Results represent means ± SD of nine mice/group. B, Proliferation of nucleosome-specific T cells to splenocytes harvested 3, 9, and 30 days after liposome treatment. C, Proliferation of OVA-primed CD4+ T cells to splenocytes from liposome-treated mice and untreated mice in the presence or absence of whole OVA. In B and C, results represent means ± SD of triplicate wells. Data shown are representative of three independent experiments with similar results. *, Significant difference (p < 0.05) compared with untreated mice. †, Significant difference (p < 0.01) compared with untreated mice. ‡, Significant difference (p < 0.001) compared with untreated mice.
In vivo depletion of splenic phagocytes reduced autoantigen presentation
To determine the relevance of these in vitro observations in vivo, NZB/W F1 mice were treated with Cl2MDP (dichloromethylene diphosphonate)-liposomes. As described in previous publications, Cl2MDP-liposomes are artificial lipid vesicles that encapsulate Cl2MDP solution (23). Splenic macrophages are depleted dominantly by i.v. administration of Cl2MDP-liposomes (23). Three days after Cl2MDP-liposome injection to NZB/W F1 mice, splenic F4/80+ macrophages showed an 80% decrease in number (Fig. 2 A). In contrast, splenic CD11c+ DCs decreased in number by <50%. Intravenous injection of Cl2MDP-liposome produced an only marginal effect on F4/80+ macrophages and CD11c+ DCs in the LNs.
We then examined nucleosome presentation by splenocytes after phagocyte depletion. Compared with PBS-liposome treatment, Cl2MDP-liposome treatment resulted in a highly significant reduction of AN3 T cell proliferation at days 3 and 9, but the T cell response recovered at day 30 (Fig. 2B). Because depletion and recovery of phagocytes reflected the nucleosome presentation capacity, it was concluded that phagocytes were responsible for nucleosome presentation in the spleen.
To confirm that Ag uptake of splenocytes was reduced by Cl2MDP-liposome treatment, we cultured OVA-reactive T cells with splenocytes from PBS- or Cl2MDP-liposome-treated mice in the presence of whole OVA protein. In NZB/W F1 mice, splenocytes harvested at 3 or 9 days after Cl2MDP-liposome treatment stimulated OVA-reactive T cells more weakly than those from PBS-liposome-treated mice. In NZB/W F1 mice, splenocytes regained their OVA presentation capacity at 30 days after Cl2MDP-liposome treatment as well as their nucleosome presentation capacity (Fig. 2C, left panel). These results verified that Cl2MDP-liposomes resulted in a transient reduction in APC function in the spleen. However, the course of recovery was different between NZB/W F1 and BALB/c mice. In BALB/c mice, splenocytes recovered their OVA presentation capacity 9 days after Cl2MDP-liposome treatment (Fig. 2C, right panel). This result suggests that NZB/W F1 mice have a reduced turnover of phagocytes.
Depletion of splenic phagocytes suppresses autoantibody production and proteinuria progression
We next investigated whether depletion of splenic phagocytes altered autoantibody titers. We i.v. injected Cl2MDP-liposomes to NZB/W F1 mice only twice at 20 and 22 wk of age. Though the number of splenic CD11c+ DCs was almost comparable between PBS-liposome- and Cl2MDP-liposome-treated mice at 28 wk of age, splenic F4/80+ macrophages of Cl2MDP-liposome-treated mice were markedly reduced (Fig. 3A). Interestingly, Cl2MDP-liposome treatment suppressed IgG anti-nucleosome Ab titers at 24 and 32 wk of age (Fig. 3B). IgG anti-dsDNA Ab titers were also suppressed at 32 wk of age. Serum total IgG levels were not affected in Cl2MDP-liposome-treated mice (data not shown). Moreover, Cl2MDP-liposome treatment at 20 and 22 wk of age significantly suppressed proteinuria progression and prolonged survival in NZB/W F1 mice (Fig. 3C). In immunofluorescense analysis, though PBS-liposome-treated mice showed severe glomerulonephritis with thickening of the capillary walls with marked deposition of IgG and complement, Cl2MDP-liposome-treated mice showed mild glomerular lesions and deposition of IgG and complement was only restricted to the mesangial area (Fig. 3D). The histological index of glomerular lesion was significantly reduced by Cl2MDP-liposome treatment (Fig. 3E). These results strongly suggest that nucleosome presentation by splenic phagocytes promotes pathogenic anti-nucleosome autoantibodies responsible for nephritis.
FIGURE 3.
Splenic phagocytes contribute to autoantibody production and disease progression. NZB/W F1 mice were treated with Cl2MDP- or PBS-liposome at 20 and 22 wk of age. A, At 28 wk of age, spleen sections from PBS-liposome-treated and Cl2MDP-liposome-treated mice were stained with Alexa546-labeled anti-F4/80 or anti-CD11c (red), Alexa488-labeled anti-CD4 (green), and Cy5-labeled anti-B220 (blue). B, Serum autoantibody levels of NZB/W F1 mice treated with Cl2MDP- or PBS-liposome (n = 12/group). C, Kaplan-Meier plots of proteinuria progression and overall survival of mice treated with liposomes (n = 19/group). D, At 36 wk of age, kidney sections from PBS- and Cl2MDP-liposome-treated mice were stained with H&E, anti-IgG, or anti-C3. E, Evaluation of glomerulonephritis in PBS- and Cl2MDP-liposome-treated mice at 36 wk of age. The degree of tissue damage was graded as described in Materials and Methods. Values are the mean and SD index of glomerular lesions (IGL; 40 random glomeruli per kidney) (n = 6/group). Images of A and D are representative of three mice per group. *, Significant difference (p < 0.05) compared with PBS-liposome-treated mice. †, Significant difference (p < 0.01) compared with PBS-liposome-treated mice. ‡, Significant difference (p < 0.001) compared with PBS-liposome-treated mice.
To determine whether Ag-priming capacity of s.c. LNs was preserved after Cl2MDP-liposome treatment, we immunized NZB/W F1 mice with OVA in the footpad 3 days after liposome treatment. When analyzed at day 21, Cl2MDP-liposome-treated, PBS-liposome-treated and untreated mice had equivalent IgG anti-OVA Ab titers (data not shown). This preservation of the immune response to peripheral immunization is consistent with the marginal effects of Cl2MDP-liposomes on the APCs in the draining LN (Fig. 2A). Therefore, depletion of splenic phagocytes had a relative specific effect on pathogenic autoantibodies in NZB/W F1 mice.
Abundant PCs have been detected in the spleen and kidneys of NZB/W F1 mice (27) and both long- and short-lived PCs contain anti-DNA Ab-secreting cells (22). We therefore examined whether phagocyte depletion affected autoantibody-secreting PCs. After two injections of Cl2MDP-liposomes, CD138-positive PCs were decreased in the spleen (Fig. 4A). In contrast, neither PC nor F4/80 + macrophage was significantly affected by Cl2MDP-liposome treatment in the BM. Consistent with the decrease of PCs, the ELISPOT assay revealed that both anti-dsDNA Ab-secreting cells and nucleosome-specific PCs were decreased in the spleen after Cl2MDP-liposome treatment (Fig. 4B). In the BM, Cl2MDP-liposome treatment affected nucleosome-specific PCs more strongly than dsDNA-specific PCs. These results suggest that phagocytes either directly or indirectly contribute to the survival of autoantigen-specific PCs in the spleen.
FIGURE 4.

Splenic plasma cells were decreased by Cl2MDP-liposome treatment. NZB/W F1 mice were treated with Cl2MDP- or PBS-liposomes at 20 and 22 wk of age (n = 8/group). At 24 wk of age, single-cell suspensions from the spleen or BM were prepared and analyzed. A, The numbers of CD138+ plasma cells and F4/80+ macrophages in the spleen and BM are shown. B, The numbers of autoantibody-secreting cells in the spleen and BM are shown. Ag-specific IgG secreting cells were detected by ELISPOT and quantified from individual organs. Results represent means ± SD. Data shown are representative of three independent experiments with similar results. *, Significant difference (p < 0.05) compared with PBS-liposome-treated mice. †, Significant difference (p < 0.01) compared with PBS-liposome-treated mice. ‡, Significant difference (p < 0.001) compared with PBS-liposome-treated mice.
Because Cl2MDP-liposome treatment depleted both F4/80+ macrophages and CD11c+ DCs in the spleen, we next evaluated the pathogenic effect of the adoptive transfer of splenic F4/80+ macrophages or CD11c+ DCs. We injected splenic F4/80+ macrophages or CD11c+ DCs repeatedly to prenephritic NZB/W F1 mice. Injections of splenic macrophages significantly induced proteinuria progression, compared with PBS or splenic DCs (Fig. 5A). Serum anti-nucleosome Ab levels were increased in F4/80+ macrophage-injected mice, but serum anti-dsDNA Ab levels were not increased (Fig. 5B).
FIGURE 5.
Splenic F4/80+ macrophages contribute to disease progression and autoantibody production. A, Five × 105 splenic macrophages or DCs were transferred in NZB/W F1 mice at 8, 12, 16, and 20 wk of age. Kaplan-Meier plots of proteinuria progression are shown (n = 20/group). *, Significant difference (p < 0.05) compared with PBS-injected mice. B, Serum autoantibody levels in mice treated as described in A (n = 6/group). The horizontal lines represent the mean value of each group. †, Significant difference (p < 0.01) compared with PBS-injected mice.
In addition, we investigated the effect of Cl2MDP-liposome treatment on other lupus mice strains. In MRL/lpr mice, Cl2MDP-liposome treatment also suppressed anti-nucleosome Ab titers (data not shown) 2 wk after Cl2MDP-liposome treatment.
Nucleosome-specific T cell retention in the splenic red pulp
Many of the lymphocytes entering the spleen are released in the marginal zone. Some of these cells pass to the outer region of the marginal zone and then to the red pulp or directly into venous sinuses. A fraction of the lymphocytes take a different route to appear within the B and T cell areas of the white pulp (30). We next investigated whether nucleosome-specific T cells migrate to the red pulp, where most of splenic F4/80+ macrophages reside. AN3-transduced CD4+ T cells were CFSE labeled and i.v. transferred to 10-wk-old NZB/W F1 mice. Because about half of AN3-transduced CD4+ T cells were Vβ4 negative (Fig. 1A), we compared the kinetics of CFSE+Vβ4+ (AN3+) and CFSE+Vβ4− (AN3−) CD4+ T cells. The percentage of Vβ4+ cells in CFSE+ CD4+ T cells in red pulp was significantly higher than that in white pulp 3 and 5 h after the transfer (Fig. 6A). The difference disappeared 18 h after the transfer. Though basal CD69 expression was comparable between CFSE+Vβ4+ CD4+ cells and CFSE+ Vβ4− CD4+ T cells, CD69 expression of CFSE+Vβ4+ CD4+ cells was significantly increased compared with that of CFSE+Vβ4− CD4+ T cells 8 h after the transfer (Fig. 6B). These results indicated that nucleosome-specific T cells were initially retained in the red pulp and activated during this retention phase.
FIGURE 6.
Nucleosome-specific T cells are stimulated in the splenic red pulp. CFSE-labeled AN3-transduced T cells were i.v. transferred into 10-wk-old NZB/W F1 mice. A, Percentage of CFSE+Vβ4+CD4+ T cells (AN3 CD4+ T cells) among CFSE+ CD4+ T cells was shown. Three, 5 and 18 h later, spleen sections from recipient mice were stained with Alexa546-labeled anti-F4/80 (red) and biotin-labeled anti-Vβ4 followed by streptavidin-Cy5 (blue). *, Significant difference (p < 0.05) compared with white pulp. †, Significant difference (p < 0.01) compared with white pulp. B, Expression of CD69 on CFSE+Vβ4+CD4+ T cells (AN3+CD4+ T cells) and CFSE+Vβ4−CD4+ T cells (AN3−CD4+ T cells) were examined before and eight hours after the transfer. Percentages of CD69highCD4+ T cells among CFSE+Vβ4+CD4+ T cells and CFSE+Vβ4−CD4+ T cells were shown. Data shown are representative of three independent experiments with similar results. †, Significant difference (p < 0.01) compared with Vβ4−CD4+ T cells.
Discussion
In this study, we observed potent nucleosome autoantigen stimulation of T cells by splenic phagocytes, including F4/80+ macrophages. Reconstitution of nucleosome-specific T cells enabled better understanding of autoantigen presentation in lupus-prone mice. Because F4/80+ macrophages from the thymus and BM presented nucleosomes very weakly, apoptosis in lymphoid organs may not simply explain nucleosome presentation. Splenic macrophages play a significant role in trapping particulate Ags in circulation (31, 32). This is the reason for the severe depletion of splenic macrophages by Cl2MDP-liposome treatment. Nucleosomes are abundant particulate autoantigens generated by cellular apoptosis. Because serum nucleosome levels have been reported to be elevated in SLE patients (33), it suggests that nucleosomes are generated in various organs and enter the circulation. Therefore, we speculate that splenic phagocytes including F4/80+ macrophages take up apoptotic debris and present nucleosomes specifically to T cells.
The increased expression of MHC class II in NZB/W F4/80+ macrophages may be associated with pathogenic autoantigen presentation of this APC population. Because NZB/W and parental NZB F4/80+ macrophages exhibited similar MHC class II expression levels (Fig. 1D), the increased expression of MHC class II in NZB/W F4/80+ macrophages is probably influenced by genetic background. The increased expression of MHC class II in NZB F4/80+ macrophages may be associated with autoimmune hemolytic anemia in NZB mice. In contrast, unknown apoptotic mechanisms specific to the spleen may be associated with splenic nucleosome presentation.
Our data also reveals an apparent paradoxical role of phagocytes in lupus. As shown in MFG-E8−/− mice (6) and Merkd mice (7), impaired clearance of apoptotic cells by macrophages predisposes to lupus-like disease. In contrast, macrophages themselves are responsible for autoantigen presentation, which drives autoimmunity. In addition to our study, macrophages promote development and activation of β cell-specific T cells in NOD mice (34). The balance between clearance of apoptotic cells and autoantigen presentation seems important, because 10 –15 injections of Cl2MDP-liposomes exacerbated lupus disease (35). Moreover, reduction of autoantibody titer may not fully ameliorate advanced stage of the disease. Clodronate-liposome insensitive population (e.g., cytotoxic CD8 T cells and neutrophils) may contribute to the late phase of disease progression. In our experiment, a few injections of Cl2MDP-liposomes delayed the development of lupus. Transient depletion of splenic phagocytes was effective in suppressing autoantigen presentation, autoantibody production, and disease progression.
Depletion of splenic phagocytes was associated with the reduction of autoantigen-specific PCs. In Fig. 3B, production of anti-nucleosome Ab was severely impaired after depletion of splenic phagocytes. This profound suppression of anti-nucleosome Ab is consistent with the decrease of nuc-specific PCs both in the spleen and in the BM described in Fig. 4B. In contrast, the suppression of anti-dsDNA titer was only around 50% after depletion of splenic phagocytes. We suppose that this partial suppression of anti-dsDNA Ab is associated with the survival of dsDNA-specific PCs in the BM. The spleens of NZB/W F1 mice contain ~10 times more PCs than those of normal mice, though PCs are not significantly increased in the BM (22). Less than 60% of the splenic Ab-secreting cells are short-lived PCs. These splenic short-lived PCs are sensitive to cyclophosphamide (short-lived PCs are eliminated 1 wk after cyclophosphamide treatment). Investigators have shown that survival of long-lived PCs is dependent on a supportive environment, i.e., specific survival niches which can be found in the BM, inflamed tissue, and the normal spleen (36). Whether splenic phagocyte depletion by Cl2MDP-liposomes affects survival niches for PCs directly or indirectly should be addressed further.
F4/80+ macrophages are located in the splenic red pulp where plasmablasts and PCs migrate after Ag-specific differentiation (32, 37). We showed activation-associated retention of nucleosome-specific T cells in red pulp. Because nucleosome-specific T cells appeared to migrate to white pulp 18 h after the transfer, initial activation of T cells in red pulp may be an important stimulation for autoantigen priming.
In summary, splenic phagocytes present autoantigen significantly and support autoantibody production in lupus-prone mice. Suppressing autoantigen presentation by splenic phagocytes without affecting the clearance of apoptotic bodies would be a novel therapeutic approach for systemic autoimmune disease.
Acknowledgments
We are grateful to Yayoi Tsukahara and Kayako Watada for excellent technical assistance.
Footnotes
This study was supported by Program and Project Grant funding from Japan Society for the Promotion of Science; Ministry of Health, Labour and Welfare; and Ministry of Education, Culture, Sports, Science and Technology. Keith B. Elkon is supported by Grant RO1 AR48796 from the National Institutes of Health (NIAMS).
Abbreviations used in this paper: SLE, systemic lupus erythematosus; DC, dendritic cell; BM, bone marrow; LN, lymph node; PC, plasma cell; Cl2MDP, dichloromethylene diphosphonate.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Vratsanos GS, Jung S, Park YM, Craft J. CD4+ T cells from lupus-prone mice are hyperresponsive to T cell receptor engagement with low and high affinity peptide antigens: a model to explain spontaneous T cell activation in lupus. J Exp Med. 2001;193:329–337. doi: 10.1084/jem.193.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–1402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wofsy D, Kerger CE, Seaman WE. Monocytosis in the BXSB model for systemic lupus erythematosus. J Exp Med. 1984;159:629–634. doi: 10.1084/jem.159.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller M, Emmendorffer A, Lohmann-Matthes ML. Expansion and high proliferative potential of the macrophage system throughout life time of lupus-prone NZB/W and MRL lpr/lpr mice: lack of down-regulation of extramedullar macrophage proliferation in the postnatal period. Eur J Immunol. 1991;21:2211–2217. doi: 10.1002/eji.1830210932. [DOI] [PubMed] [Google Scholar]
- 6.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 7.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald PP, V, Fadok A, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 11.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 12.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 13.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 17.Bruns A, Blass S, Hausdorf G, Burmester GR, Hiepe F. Nucleosomes are major T and B cell autoantigens in systemic lupus erythematosus. Arthritis Rheum. 2000;43:2307–2315. doi: 10.1002/1529-0131(200010)43:10<2307::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Amoura Z, Chabre H, Koutouzov S, Lotton C, Cabrespines A, Bach JF, Jacob L. Nucleosome-restricted antibodies are detected before anti-dsDNA and/or antihistone antibodies in serum of MRL-Mp lpr/lpr and +/+ mice, and are present in kidney eluates of lupus mice with proteinuria. Arthritis Rheum. 1994;37:1684–1688. doi: 10.1002/art.1780371118. [DOI] [PubMed] [Google Scholar]
- 19.Fujio K, Okamoto A, Tahara H, Abe M, Jiang Y, Kitamura T, Hirose S, Yamamoto K. Nucleosome-specific regulatory T cells engineered by triple gene transfer suppress a systemic autoimmune disease. J Immunol. 2004;173:2118–2125. doi: 10.4049/jimmunol.173.3.2118. [DOI] [PubMed] [Google Scholar]
- 20.Fujio K, Misaki Y, Setoguchi K, Morita S, Kawahata K, Kato I, Nosaka T, Yamamoto K, Kitamura T. Functional reconstitution of class II MHC-restricted T cell immunity mediated by retroviral transfer of the α β TCR complex. J Immunol. 2000;165:528–532. doi: 10.4049/jimmunol.165.1.528. [DOI] [PubMed] [Google Scholar]
- 21.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi S, Nose M, Sasaki J, Yamamoto T, Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991;147:515–519. [PubMed] [Google Scholar]
- 25.Inoue A, Hasegawa H, Kohno M, Ito MR, Terada M, Imai T, Yoshie O, Nose M, Fujita S. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2005;52:1522–1533. doi: 10.1002/art.21007. [DOI] [PubMed] [Google Scholar]
- 26.Muraoka M, Hasegawa H, Kohno M, Inoue A, Miyazaki T, Terada M, Nose M, Yasukawa M. IK cytokine ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2006;54:3591–3600. doi: 10.1002/art.22172. [DOI] [PubMed] [Google Scholar]
- 27.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol. 2001;31:2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Fujio K, Jiang Y, Zhao J, Tada N, Sudo K, Tsurui H, Nakamura K, Yamamoto K, Nishimura H, et al. Dissection of the role of MHC class II A and E genes in autoimmune susceptibility in murine lupus models with intragenic recombination. Proc Natl Acad Sci USA. 2004;101:13838–13843. doi: 10.1073/pnas.0405807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaels MA, Kang HK, Kaliyaperumal A, Satyaraj E, Shi Y, Datta SK. A defect in deletion of nucleosome-specific autoimmune T cells in lupus-prone thymus: role of thymic dendritic cells. J Immunol. 2005;175:5857–5865. doi: 10.4049/jimmunol.175.9.5857. [DOI] [PubMed] [Google Scholar]
- 30.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med. 2003;197:353–361. doi: 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol. 2003;171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 32.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 33.Amoura Z, Piette JC, Chabre H, Cacoub P, Papo T, Wechsler B, Bach JF, Koutouzov S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 1997;40:2217–2225. doi: 10.1002/art.1780401217. [DOI] [PubMed] [Google Scholar]
- 34.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, Lee KD, Gavalchin J, Kaplan MJ. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 36.Manz RA, Arce S, Cassese G, Hauser AE, Hiepe F, Radbruch A. Humoral immunity and long-lived plasma cells. Curr Opin Immunol. 2002;14:517–521. doi: 10.1016/s0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 37.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]