Abstract
Tissue-engineered models that mimic in vivo tissue organization offer the potential of capturing complex signaling pathways in vitro. In the liver, hepatocytes and endothelial cells are closely associated but separated by the extracellular matrix of the space of Disse. This unique configuration was mimicked by embedding primary hepatocytes in collagen gel and overlaying the matrix with endothelial cells. We demonstrate that during the first few days of culture, the secretion of albumin and fibrinogen was 2-fold higher in cocultures compared to hepatocytes alone. Hepatocyte function in both cultures stabilized to a similar level during the second week, suggesting that endothelial cells can induce the early recovery of hepatocytes after isolation and seeding. Endothelial cell-conditioned medium reproduced the effect of coculture in a dose-dependent fashion, suggesting a role for endothelial cell-derived soluble factors. Endothelial cell-conditioned medium increased mRNA levels of various acute-phase proteins such as albumin, fibrinogen, transferrin, and α-macroglobulin in hepatocytes. Surprisingly, the effect of endothelial cell-conditioned medium was not mediated by growth factors or cytokines, or by secreted extracellular matrix, but by the release of the amino acid proline, which mediates endogenous collagen synthesis by hepatocytes. These findings suggest an important role for proline secretion by endothelial cells as a paracrine factor regulating hepatocyte function.—Jindal, R., Nahmias, Y., Tilles, A. W., Berthiaume, F., Yarmush, M. L. Amino acid-mediated heterotypic interaction governs performance of a hepatic tissue model.
Keywords: acute phase proteins · coculture · extracellular matrix · liver
In vitro models of the liver that capture critical aspects of the in vivo signaling microenvironment can provide an important platform for drug toxicity screening, as well as the study of liver regeneration, metabolism, development, or disease. These tissue-engineered models have the potential of bridging the gap between cell culture and animal experiments and serving as surrogate models of human studies. Prior studies have shown that long-term hepatocyte viability and function are maintained during coculture with fibroblasts or endothelial cells (1,2,3). It is thought that this interaction is mediated by cell-cell contacts, secreted extracellular matrix (ECM), and soluble growth factors (1, 4, 5). However, despite numerous studies, the exact role of the various factors involved in the long-term maintenance of liver-specific function remains unknown.
Even though traditional coculture models have provided useful insight into the role of cell-cell communication, they fail to capture the 3-dimensional (3-D)organization found in vivo. Hepatocytes in the liver are organized in structural units termed liver sinusoids, in which hepatocytes and endothelial cells are separated by a thin ECM layer called the space of Disse. This 3-D entrapment in matrix can be mimicked by embedding hepatocytes between two layers of a collagen gel. This collagen sandwich configuration maintains long-term liver-specific function and gene expression but requires 7 to 10 d to stabilize as the cells adjust to the new culture conditions (6, 7).
Although collagen sandwich culture offers a 3-D environment, it fails to capture heterotypic cell-cell interactions, which are known to be essential for proper liver function. One solution to this problem is layering nonparenchymal cells on top of hepatocytes, retaining a 3-D geometry as well as heterotypic cell support. Several investigators have reported cell-layering techniques using thermoresponsive polymers (8), polyelectrolyte multilayers (PEMs) (9), or magnetic liposomes (10). While these techniques offer interesting solutions to the problem, they all rely on artificial substrates for detachment, adhesion, or manipulation of the cells, which may result in the generation of artifactual responses.
In this report, we describe a layered coculture system consisting of hepatocytes, collagen, and an overlaying endothelial monolayer. Because collagen is one of the major components in the space of Disse (11), this approach relies on providing native cues to the hepatocyte and endothelial cell cocultures. We demonstrate that this culture configuration induces the early recovery of hepatocytes following cell isolation, with albumin and fibrinogen protein secretion and gene expression increasing by 2-fold by d 4 of culture. Surprisingly, early recovery was not caused by growth factors or cytokines, or by secreted extracellular matrix, but rather by the release of the amino acid proline by the endothelial cells, which promotes the hepatocellular production of new collagen.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle medium (DMEM), penicillin-streptomycin, epidermal growth factor (EGF), and fetal bovine serum (FBS) were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Glucagon was obtained from Lilly (Indianapolis, IN), insulin was purchased from Squibb (Princeton, NJ, USA), and hydrocortisone was obtained from Upjohn (Kalamazoo, MI). MCDB-131 medium and EndoGro supplement were obtained from VEC Technologies (Rensselaer, NY, USA). Laminin, fibronectin, proline, collagenase, and cis-hydroxyproline were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hepatocyte growth factor (HGF), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), neutralizing antibodies for HGF, TNF-α, IL-6 were all acquired from R&D Systems (Minneapolis, MN, USA). Matrix metalloproteinase-3 (MMP-3) was purchased from EMD Chemicals (Gibbstown, NJ, USA).
Culture of endothelial cells
Primary rat heart microvessel endothelial cells (RHMEC) were purchased from VEC Technologies and maintained in MCDB-131 medium supplemented with 10% FBS, 10 ng/ml EGF, 1 μg/ml hydrocortisone, 200 μg/ml EndoGro, 90 μg/ml heparin, and 1% antimycotic solution. RHMEC were cultured in a humidified incubator maintained at 37°C and 5% CO2. The endothelial cells were routinely trypsinized and used between d 5 and 7 after passage. The cells were used prior to passage 7.
Hepatocyte isolation and culture
Hepatocyte culture medium consisted of DMEM-supplemented with 20 ng/ml EGF, 14 ng/ml glucagon, 0.5 U/ml insulin, 7.5 μg/ml hydrocortisone, 200 U/ml penicillin, 200 μg/ml streptomycin, and 10% heat-inactivated FBS. Type I collagen was prepared by extracting acid-soluble collagen from rat tail tendons, as described previously (6). Hepatocytes were isolated from female Lewis rats (Charles River Laboratories, Wilmington, MA) weighing 180 to 200 g. All animals were treated in accordance with National Research Council guidelines, and the studies were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital. Hepatocytes were isolated by a 2-step collagenase perfusion technique originally described by Seglen (12) and modified by Dunn et al. (6). Isolation yields varied from 200 to 300 million hepatocytes per rat with viabilities ranging from 85% to 95%.
Isolated hepatocytes were suspended in ice-cold hepatocyte culture medium at a concentration of 2.4 × 106 cells/ml. Collagen solution was prepared by mixing 9 parts of 1.25 mg/ml collagen solution with 1 part of 10× DMEM on ice. Hepatocytes were embedded in collagen gel by mixing the cell suspension with a collagen solution in a 1:1 volume ratio. Typically, 1000 or 400 μl of this mixture was introduced in one well of a 6-well or a 12-well tissue culture plate, respectively. The cell suspension was allowed to gel at 37°C for 75 min before culture medium was added on top of the gel. In another set of experiments, hepatocytes were cultured on top of the collagen gel. In this case, 200 μl of collagen solution was introduced in each well of a 12-well plate and allowed to gel at 37°C for 60 min. Next, hepatocytes were seeded on top of the gel at similar seeding densities. In all cases, hepatocytes were maintained in hepatocyte culture medium for the first 24 h. Coculture with endothelial cells and treatment with conditioned medium were started on the following day (d 1).
Coculture of hepatocytes and endothelial cell
Endothelial cells were trypsinized and suspended in hepatocyte culture medium at a concentration of 2.4 × 106 cells/ml. Endothelial cells were introduced on top of the collagen gels at a 2:1 ratio with respect to hepatocytes. The height of the separating gel was measured using a computer controlled motorized 3-axis microscope stage set on an inverted Zeiss 200 M microscope (Carl Zeiss Inc., Thornwood, NY). Distance was defined from one optical plane to the next at ×16. In some experiments, endothelial cells were labeled with the fluorescent dye CM-DiI (Invitrogen, Carlsbad, CA, USA) prior to cell seeding. Medium was replaced daily in all cultures.
Growth factors and neutralizing antibodies
In some experiments, hepatocyte culture medium was supplemented with neutralizing antibodies for HGF, IL-6, and TNF-α at concentrations of 25, 5, and 25 μg/ml, respectively (13). The medium containing neutralizing antibodies was introduced in the coculture on the first day of culture and replenished daily. The recombinant forms of HGF, IL-6, and TNF-α were also added to hepatocyte culture medium at concentrations ranging from 1 to 200 ng/ml, 10 to 1000 pg/ml, and 10 to 500 pg/ml, respectively. The medium containing the recombinant proteins was used to stimulate hepatocytes embedded in a collagen gel on the first day of culture and replenished daily.
Endothelial cell-conditioned medium
Endothelial cells were grown to confluence in the endothelial culture medium described above. The cells were carefully washed with PBS and transferred to a serum-free version of the hepatocyte culture medium for 48 h. Following the conditioning step, the medium was centrifuged at 500 g for 30 min to remove cell debris, and the supernatant was supplemented with 10% heat-inactivated FBS. A stock solution of conditioned medium was prepared by mixing serum supplemented supernatant and fresh hepatocyte culture medium at a ratio of 3:1 by volume and henceforth called 75% conditioned medium. Different dilutions of conditioned medium were prepared by mixing the conditioned medium with fresh hepatocyte culture medium. Conditioned medium, like fresh culture medium, was replenished daily.
Matrix and metalloproteinase
In some experiments, laminin, fibronectin, and MMP-3 were added to the culture medium at concentrations of 10, 10, and 0.33 or 1 μg/ml, respectively. MMP-3 was specifically added to the endothelial cell-conditioned medium and incubated for 24 h prior to the addition of serum, to minimize the potential of serum inhibition of its activity.
Amino acid analysis
Amino acid composition of conditioned and control medium was determined using a Waters HPLC apparatus and the Accu-Tag protocol, as described in detail elsewhere (14).
Proline stimulation and inhibition
Proline was added to hepatocyte culture medium (control medium) at a concentration of 20 μg/ml. Stock solution of cis-hydroxyproline was prepared in control medium at a concentration of 1 mg/ml. Working concentrations of cis-hydroxyproline were prepared by mixing stock with conditioned or control medium. In all of the experiments, medium was introduced on d 1 and continuously replenished daily.
Real-time polymerase chain reaction (PCR)
On d 4 of culture, hepatocytes embedded in collagen and those cultured on top of collagen were treated with 1 mg/ml collagenase at 37°C for 30 min. The isolated cells were then pelleted and frozen at −80°C until further analysis. RNA was extracted from cells using nucleospin RNA II kit (Macherey-Nagel Inc., Bethlehem, PA), according to the manufacturer’s instructions. Quantitative reverse transcription PCR (qRT-PCR) was performed using the Superscript III Two-Step qRT-PCR kit purchased from Invitrogen (Carlsbad, CA). Cellular RNA (100 ng) was reverse transcribed, according to the manufacturer’s directions. Real-time quantitative PCR was performed using the Stratagene (La Jolla, CA, USA) MX5000P QPCR system. Each reaction was carried out with 15 ng cDNA and 0.2 μM primers. During amplification, the cycling temperatures were 95°C for 15 s, 55°C for 1 min, and 72°C for 30 s. The following primers were used for amplifying DNA: albumin forward primer, ATTACTCCGTGTCCCTGCTG; albumin reverse primer, CTGAGGTGCTTTCTGGGTGT; α fibrinogen forward primer, GTGGACCAGGGTCGAAGATA; α fibrinogen reverse primer, AGGCTTAAGGTTCCCAGAGC; transferrin forward primer, AGATGGAGGTGGAGATGTGG; transferrin reverse primer, GAGCCACAACAGCATGAGAA; α macroglobulin forward primer, CCCACAGAGACTAGGCGAAG; α macroglobulin reverse primer, ATTGGACCACAGGAGACAGG; β-actin forward primer, GTCGTACCACTGGCATTGTG; and β-actin reverse primer, CTCTCAGCTGTGGTGG TGAA. Threshold cycles (Ct) were determined using the Stratagene MX-Pro QPCR software using settings with an amplification-based threshold and adaptive baseline. Relative changes in gene expression were calculated using the 2−ΔΔCt method (15). Expression of albumin, α fibrinogen, transferrin, and α-macroglobulin was measured relative to β-actin and normalized to unstimulated hepatocytes.
Measurement of albumin, fibrinogen, and urea
Albumin secreted in the medium was analyzed by enzyme-linked immunosorbent assay (ELISA), as described previously (6) using a polyclonal antibody to rat albumin (Cappel Laboratories, Aurora, OH, USA). Fibrinogen concentration in the medium was evaluated using commercially available ELISA kit (Assaypro, St. Charles, MO, USA). Urea content was determined with a commercially available kit (StanBio Laboratory, Boerne, TX, USA). Standard curves were generated by dissolving different concentrations of rat albumin, fibrinogen, or urea in culture medium. Data reported are means of triplicate cultures for each condition and normalized to that of 1 million hepatocytes.
Measurement of cytochrome P-450 activity
Activity of cytochrome P-450 IA1 (CYPIA1) was measured according to the procedure described previously (16). Briefly, cells were washed with HBSS for 15 min. Next, HBSS containing 2 μM of substrate ethoxy-resorufin and 80 μM of dicuramol was added to hepatocyte cultures. Samples were withdrawn at regular intervals to measure formation of the fluorescent product, resorufin, using an Fmax fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 530-nm excitation and 590-nm emission wavelengths. Activity of CYPIA1 was determined from the rate of formation of resorufin in culture supernatant.
Measurement of collagen synthesis
In 6-well plates, cells were maintained in their respective mediums for 4 d prior to spiking of 1 ml of medium with 10 μCi of [3H]glycine. After 24 h of exposure, medium was collected and analyzed for freshly synthesized collagen by estimating incorporation of [3H]glycine into collagenase-sensitive protein, according to a previously described procedure (17) with slight modifications. Briefly, Amicon centrifugal filters (Millipore, Billerica, MA, USA) with MWCO 5000 were used for reconstituting the medium components in collagenase buffer (volume exchange by a factor of 1000). The sample was divided into two aliquots (200 μl) with one aliquot treated overnight with 90 μg of collagenase at 37°C, while control buffer was added into the other aliquot. After digestion, 500 μg of bovine serum albumin was added to each aliquot, and proteins were precipitated by adding 10% trichloroacetic acid/0.5% tannic acid solution. The precipitate was centrifuged down, and radioactivity was counted in the supernatant. The amount of collagen synthesized was directly proportional to the difference in counts per minute (cpm) between undigested and digested aliquots for each sample.
Imaging
Images were acquired using a Zeiss 200 M microscope (Carl Zeiss, Inc., Thornwood, NY). The phase contrast and fluorescence images were captured using a CCD camera (Carl Zeiss) and Zeiss imaging software (Axiovision LE).
Statistical analysis
Results are reported as mean ± sd. Statistical analysis was performed using the Student’s t test, with P < 0.05 considered significant.
RESULTS
Organotypical model of hepatocytes and endothelial cells
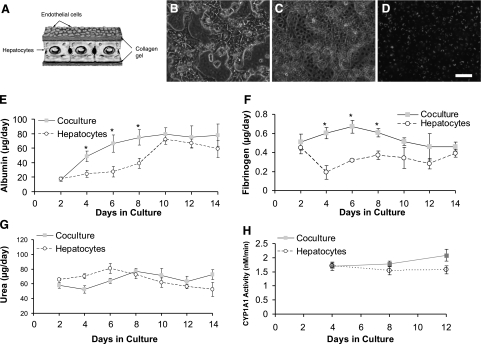
Endothelial cells have long been known to maintain long-term function of hepatocytes in direct coculture (2, 18). However, in vivo hepatocytes and endothelial cells are separated by the space of Disse. To study the effects of endothelial cells on hepatocyte function in an in vivo-like configuration, we embedded hepatocytes in a thin collagen gel and overlaid the matrix with endothelial cells (Fig. 1A). Figure 1B, C shows phase contrast images of hepatocytes and endothelial cells in different planes of the layered coculture structure. Hepatocytes exhibited typical polygonal morphology. To better visualize the endothelial cells on top of the hepatocytes, the cells were fluorescently labeled prior to seeding (Fig. 1D). The endothelial cells attached and spread on top of the collagen gel to form a confluent monolayer displaying typical cobblestone morphology (19). The thickness of the separating matrix was measured to be 150 ± 10 μm on d 4 but decreased over time in culture to 60 ± 10 μm.
Figure 1.
Organotypical model of hepatocytes and endothelial cells. A) Schematic showing hepatocytes embedded in collagen gel and endothelial cells overlaid on top of gel. B) Phase-contrast image of hepatocytes on bottom layer of construct on d 9 of culture. C) Phase-contrast image of endothelial cells on top layer of construct (d 9); note cobblestone morphology. D) Fluorescent micrograph of CM-DiI dyed endothelial cells on top of gel (d 9). Scale bar = 50 μm. E–G) Albumin (E), fibrinogen (F), and urea (G) secretion profiles of hepatocyte and endothelial cell cocultures compared to hepatocytes in single cultures. H) Cytochrome P-450IA1 (CYP1A1) activity of hepatocytes in coculture with endothelial cells compared to monoculture of hepatocytes. *P < 0.05 vs. hepatocytes in single culture.
Liver-specific function of hepatocyte-endothelial cell models
The secretion of acute-phase proteins, such as albumin and fibrinogen, and urea production are essential functions of hepatocytes, which are rapidly lost during short-term culture (20). To characterize the function of hepatocytes in our organotypical model, we evaluated albumin (Fig. 1E), fibrinogen (Fig. 1F), and urea secretion (Fig. 1G) and cytochrome P-450 activity (Fig. 1H) in the cocultures over a 2-wk period. Albumin and fibrinogen secretion were 2-fold higher for hepatocytes cocultured with endothelial cells compared to monoculture of hepatocytes (embedded in collagen) during the first week of culture (Fig. 1E, F). However, by the end of the second week in culture, albumin and fibrinogen secretion were similar for both systems. Urea secretion and cytochrome P-450 activity were unaffected by endothelial coculture (Fig. 1G, H).
Endothelial cell-secreted factors induce a dose-dependent early recovery of hepatocytes
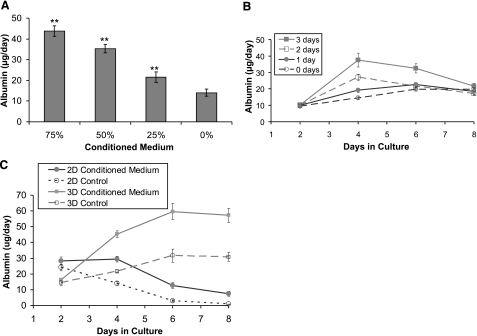
The early recovery of albumin and fibrinogen secretion in hepatocytes separated from endothelial cells suggests a role for endothelial cell-secreted factors in this phenomenon, rather than a direct cell-cell contact mediated event. To further identify this factor, we stimulated hepatocytes cultured alone with endothelial cell conditioned medium diluted in freshly prepared hepatocyte culture medium. Figure 2A demonstrates that the effect of endothelial cell-conditioned medium was dose dependent. At 75 and 25% conditioned medium, hepatocyte albumin secretion was 200 ± 20 and 50 ± 17% (P<0.01) higher than control, respectively. Similar dose-dependent effect was observed for fibrinogen secretion (Supplemental Fig. S1A).
Figure 2.
Dose and time effect of endothelial cell-conditioned medium on albumin secretion. A) Endothelial cell-conditioned medium induces a dose-dependent increase in hepatocyte albumin secretion on d 4 of culture. **P < 0.01 vs. control medium. B) Albumin secretion profile of hepatocytes embedded in collagen gel exposed to 75% endothelial cell-conditioned medium for either 1, 2, or 3 d and then switched to control medium for remainder of culture duration. C) Albumin secretion profile of hepatocytes embedded in collagen gel (3-D) compared to hepatocytes seeded on top of a collagen gel (2-D) during continuous stimulation with 75% endothelial-cell conditioned medium.
Figure 2B shows the effect of exposing hepatocytes to 75% conditioned medium for either 1, 2, or 3 d and then switching to control medium for the remainder of culture duration. Hepatocytes showed a transient increase in albumin function, which eventually reduced to the level of control (0 d) by the eighth day of culture as the medium was switched from conditioned to control medium. The increase in albumin secretion was highest and most sustained for cells treated for 3 d, while cells exposed to conditioned medium for 1 d demonstrated minimal effect, both in terms of magnitude and duration. Taken together, these results demonstrate a dose and time-dependent response, suggesting that the secreted factors were not present in excess.
The previous experiments were carried out on hepatocytes embedded in a 3-D collagen matrix, a culture condition that eventually stabilizes long-term function. To evaluate whether the early recovery of hepatocytes by endothelial cell-conditioned medium is specific to this model, we compared albumin (Fig. 2C) and fibrinogen (Supplemental Fig. S1B) secretion in our 3-D configuration to hepatocytes cultured on top of a collagen gel in a 2-D configuration. Both culture conditions were exposed to 75% conditioned medium. Figure 2C demonstrates that in the 2-D model, which is inherently an unstable culture model (6, 7), endothelial cell-induced early recovery was only partial, while sustained maintenance of albumin secretion also required the presence of collagen on top of hepatocytes.
Endothelial cell-induced early recovery occurs at the RNA level
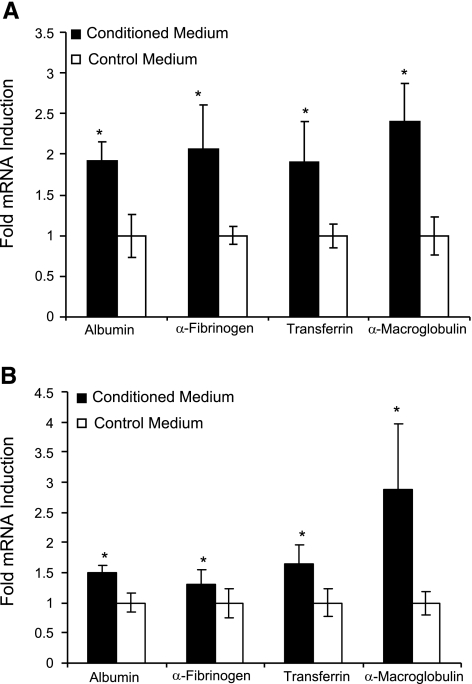
Regulation of acute-phase protein secretion can occur both at the transcriptional and translational levels. To investigate whether endothelial cell-conditioned medium increases the transcription of acute-phase proteins in collagen-embedded hepatocytes, we evaluated 4 genes on d 4 of culture following stimulation with 75% conditioned medium. Figure 3A demonstrates that all four genes were up-regulated on d 4 of culture. Albumin mRNA increased by 90% compared with nonstimulated control (P<0.05). In a similar fashion, the transcription of fibrinogen, transferrin, and α-macroglobulin were increased by 105, 90, and 140%, respectively. Similar results were observed for hepatocytes cultured in 2-D configuration and exposed to conditioned medium (Fig. 3B). These results suggest that the endothelial cell-induced enhancement of hepatocyte function is not limited to albumin and fibrinogen secretion.
Figure 3.
Relative changes in gene expression measured by qRT-PCR for albumin, α-fibrinogen, transferrin, and α-macroglobulin on d 4 of culture in response to stimulation with conditioned medium (75%). A) Hepatocytes were embedded in collagen gel (3-D culture). Gene expression was normalized to that of hepatocytes embedded in collagen gel and exposed to nonconditioned hepatocyte culture medium. B) Hepatocytes were cultured on top of collagen gel (2-D culture). Gene expression was normalized to that of hepatocytes seeded on top of collagen gel and treated with nonconditioned hepatocyte culture medium. *P < 0.05 vs. control medium.
Endothelial cell-induced recovery is independent of HGF, IL-6, and TNF-α
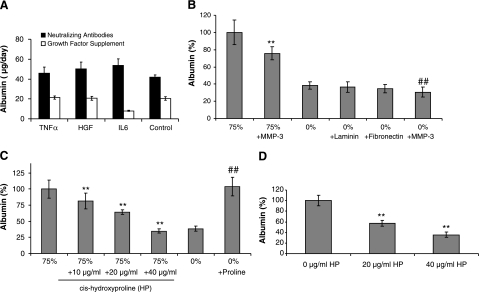
Endothelial cells are known to secrete various growth factors and cytokines, which could modulate hepatic protein secretion. For example, VEGF has previously been shown to stimulate the secretion of HGF and protect the liver against toxic damage (21). On the other hand, inflammatory cytokines such as IL-6 and TNF-α, which are secreted by activated endothelial cells, could up-regulate the secretion of acute-phase proteins. To investigate the role of HGF, IL-6, and TNF-α, two sets of experiments were carried out. First, we added the recombinant proteins to culture media and demonstrated that there was no significant increase in albumin secretion over control (Fig. 4A and Supplemental Fig. S4A). Second, we added neutralizing antibodies against HGF, IL-6, or TNF-α to coculture of hepatocytes and endothelial cells. Figure 4A shows that introduction of neutralizing antibodies against HGF, IL-6, or TNF-α did not abrogate the effect of endothelial cells on hepatocyte function. This data suggest that despite the current paradigm in the field (22), this early enhancement of function is not mediated by HGF, IL-6, or TNF-α.
Figure 4.
Evaluating the role of growth factor, matrix, and amino acid in the induction of early recovery in hepatocytes cultured in 3-D configuration. A) Hepatocyte albumin secretion on d 4 of culture following addition of neutralizing antibodies against HGF, IL-6, or TNF-α to coculture of hepatocytes and endothelial cells (black bars) or exposure of monocultures of hepatocytes to culture medium supplemented with recombinant HGF (200 ng/ml), IL-6 (1 ng/ml), or TNF-α (0.5 ng/ml) (white bars). B) Albumin secretion by hepatocytes on d 4 of culture following stimulation with 75% endothelial cell-conditioned medium compared to conditioned medium treated with MMP-3 (1 μg/ml); albumin secretion by hepatocytes on d 4 of culture in hepatocyte culture medium (0%) supplemented with laminin (10 μg/ml), fibronectin (10 μg/ml), or MMP-3 (1 μg/ml). Results are normalized to hepatocytes cultured in conditioned medium (75%).**P < 0.01 vs. conditioned medium; ##P < 0.01 vs. control medium. C) Dose-dependent inhibition of albumin secretion by hepatocytes (d 4) exposed to 75% endothelial cell-conditioned medium supplemented with cis-hydroxyproline (HP). Results are normalized to hepatocytes cultured in conditioned medium (75%). Addition of exogenous proline dramatically increases the albumin secretion of nonstimulated hepatocytes. **P < 0.01 vs. conditioned medium; ##P < 0.01 vs. control medium. D) Dose-dependent inhibition of albumin secretion (d 4) in cocultures of hepatocytes and endothelial cells stimulated with control medium (0%) supplemented with cis-hydroxyproline (HP). Results are normalized to cocultures exposed to control medium (0%). **P < 0.01 vs. control medium.
Endothelial cell-induced recovery is not mediated by endothelial cell-secreted matrix
Previously several investigators have demonstrated that endothelial cells secrete ECM such as laminin and fibronectin during coculture with hepatocytes (2, 18). It was postulated that this secreted matrix is, in part, responsible for the maintenance of hepatocyte function (23, 24). To determine whether ECM production plays a role in the effect of endothelial cells on hepatocellular function, we added MMP-3 to the culture medium. MMP-3 is a broad matrix metalloproteinase that cleaves a number of ECM molecules, including laminin, fibronectin, heparan and chondroitin sulfate, as well as collagen III, IV, and V (25). Figure 4B and Supplemental Fig. S4B show that the addition of MMP-3 to 75% conditioned medium at concentrations of 1 μg/ml and 330 ng/ml reduced hepatocyte albumin secretion by ∼25% (P<0.01) and 15% (P<0.01), respectively. However, hepatocytes treated with MMP-3 in the absence of conditioned medium also demonstrated a decrease in albumin secretion. Thus, the reduction caused by MMP-3 may involve effects on both the endothelial cell-derived factors in the conditioned medium as well as directly on the hepatocytes themselves. Supplementing the standard culture medium with either laminin or fibronectin did not increase hepatocellular albumin secretion. These results suggest that the endothelial cell-secreted factor is not likely to be an ECM molecule but could exert its effect by modulating ECM secretion by hepatocytes. In fact, hepatocyte-secreted collagen has been previously implicated in enhanced albumin secretion (17).
Proline as a mediator of enhanced albumin secretion
Past reports have shown that various amino acids, including proline (17, 26), and histidine (27), can modulate albumin secretion in hepatocytes. As proline is essential for collagen production, it suggests that endothelial cells may modulate hepatocyte function by secreting the amino acid proline. To investigate whether endothelial cells secrete proline, we quantified the differences in amino acid composition of endothelial cell-conditioned medium compared to freshly made hepatocyte culture medium. HPLC analysis revealed a dramatic increase in proline levels, increasing from 2.5 to 21 μg/ml. To evaluate whether this increase in proline concentration was responsible for higher hepatocellular albumin secretion, two sets of experiments were conducted. First, proline was added exogenously to the control fresh medium and second, cis-hydroxyproline, a competitive inhibitor of proline, was added to endothelial cell-conditioned medium. cis-hydroxyproline blocks the enhancing effect of proline on albumin secretion by preventing the formation of functional collagen triple helices (17). Figure 4C demonstrates that the addition of proline significantly enhanced albumin secretion in fresh culture medium (P<0.01). In addition, cis-hydroxyproline decreased albumin production in the conditioned medium in a dose-dependent manner (Fig. 4C). These results strongly suggest that the active factor in the endothelial cell-conditioned medium responsible for higher hepatocellular albumin secretion is proline. Finally, cis-hydroxyproline was added to our hepatocyte-endothelial cell organotypical model characterized above. As expected, cis-hydroxyproline decreased secretion of albumin in our model in a dose-dependent manner (Fig. 4D).
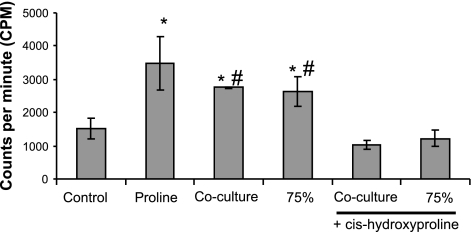
Previous reports have indicated that proline enhances albumin secretion by promoting collagen synthesis by hepatocytes. To investigate the involvement of collagen in our system, we measured collagen synthesis by hepatocytes cultured under different conditions. Figure 5 shows that both conditioned medium and coculture with endothelial cells leads to enhancement of collagen synthesis by hepatocytes. Furthermore, addition of cis-hydroxyproline to coculture or conditioned medium attenuates collagen synthesis by hepatocytes.
Figure 5.
Estimation of freshly synthesized collagen by hepatocytes embedded in collagen gel between d 4 and 5 of culture. Control, proline, and 75% refer to monoculture of hepatocytes exposed continuously to control medium, proline (20 μg/ml)-supplemented control medium, and endothelial cell-conditioned medium, respectively. Cocultures of hepatocytes and endothelial cells were continuously treated with control medium. Under certain conditions, control or conditioned medium was supplemented with cis-hydroxyproline (40 μg/ml). *P < 0.05 vs. control; #P < 0.05 vs. cis-hydroxyproline supplemented cultures.
DISCUSSION
This paper describes the effect of culturing endothelial cells on top of collagen embedded hepatocytes. This culture configuration resulted in a 2-fold increase in albumin and fibrinogen secretion during the first week of coculture in comparison to hepatocytes embedded in collagen, the current state of the art. However, long-term secretion profiles became similar in coculture and monoculture during the second week of culture. These results suggest that endothelial cells lead to an earlier recovery of hepatocytes following isolation. Replacing endothelial cells with the conditioned medium resulted in a similar albumin secretion profile, suggesting a role for endothelial cell-secreted factors. Treatment with conditioned medium resulted in a significant enhancement of hepatocyte gene expression of albumin, fibrinogen, α-macroglobulin, and transferrin compared to control, suggesting this stimulation was not limited to albumin alone.
Even though several groups, including ours, reported an increase in hepatocyte function as a result of heterotypic cell-cell interaction, the exact mechanism that leads to this enhancement remains unknown. It has been suggested that 3T3-J2 fibroblasts stimulate hepatocyte function by N-cadherin-mediated cell contact, the secretion of the extracellular matrix decorin, and by the secretion of the growth factor EGF (28). Similar mechanisms were suggested for endothelial cells (13). In our system, direct cell-cell contact was absent as hepatocytes and endothelial cells were separated by 60-μm-thick collagen gel. However, this collagen sandwich configuration has been implicated in the maintenance of hepatocyte differentiated function, even in the absence of heterotypic cell-cell interaction (7). We conducted experiments to separate out the contribution of collagen on top of hepatocytes from factors secreted by the endothelial cells in the maintenance of hepatocyte differentiated function in the layered coculture system.
In the past reports, conditioned medium had limited success in the maintenance of hepatocyte function (1). This suggests that soluble factors are not sufficient for the stabilization of hepatocyte function. In our experiments, hepatocytes cultured on top of collagen and exposed to endothelial cell-conditioned medium failed to maintain albumin secretion beyond 4 d. However, hepatocytes embedded in collagen and treated with conditioned medium resulted in similar albumin secretion as those in coculture. These experiments indicate that collagen on top of hepatocytes and soluble factors secreted by endothelial cells acted synergistically in maintaining differentiated function of hepatocytes in our model.
Endothelial cells secrete growth factors such as HGF, which has been suggested to be a mediator of hepatocyte regeneration following injury (21). However, in our system, the addition of HGF exogenously to the control medium or its neutralization in the coculture did not change albumin secretion, indicating that it was not the factor responsible for the early recovery. In a similar fashion, IL-6 and TNF-α failed to exhibit a response.
Our work led to the identification of proline as the soluble factor present in endothelial cell-conditioned medium that induced the early recovery of hepatocyte function. The amino acid proline was 8-fold higher in endothelial cell-conditioned medium compared to control, while its exogenous addition dramatically increased albumin secretion in hepatocyte cultures. Furthermore, the effect of proline was blocked by introducing cis-hydroxyproline in the conditioned medium. It has been reported that cis-hydroxyproline is incorporated into newly synthesized proteins, including collagen, and disrupts proper folding of the triple-helix collagen molecule (29). Our experiments support the notion that proline exerts its effect by promoting synthesis of collagen by hepatocytes. Involvement of hepatocyte-secreted matrix in this early recovery was also supported by experiments in which MMP-3, a matrix metalloproteinase that degrades several types of collagen, was added to the conditioned medium, leading to a decrease in albumin secretion. Finally, cis-hydroxyproline reduced albumin secretion in cocultures of hepatocytes and endothelial cells, further establishing proline as the factor secreted by endothelial cells that resulted in enhanced albumin function.
The proline profile in the supernatant of hepatocytes in coculture and monoculture (Supplemental Fig. S2) further supports proline’s involvement in inducing faster recovery of hepatocytes in coculture with endothelial cells. The proline concentration in the supernatant of cocultured hepatocytes and endothelial cells was higher during the first week of culture, which eventually became similar to monoculture of hepatocytes. This parallels the albumin and fibrinogen secretion profiles in coculture and monoculture. Furthermore, albumin (Supplemental Fig. S3A) and fibrinogen (Supplemental Fig. S3B) secretion profiles on exogenous addition of proline to control medium capture the coculture effect, further implicating proline as the mediator of faster recovery of hepatocytes in coculture with endothelial cells.
The mRNA levels of albumin, fibrinogen, transferrin, and α-macroglobulin were enhanced in hepatocytes exposed to endothelial cell-conditioned medium. This suggests that proline enhanced protein secretion, at least in part, at the transcriptional level. Further work is required to fully establish the transcriptional and translational control of protein secretion mediated by proline. However, the plausible role of endogenously synthesized collagen, by hepatocytes utilizing proline, in regulating transcription of albumin is consistent with other reports in which ECM molecules, such as laminin, have been implicated in influencing albumin mRNA levels (24). The presence of ECM response element in the promoter region of albumin has also been suggested in the literature (30). However, the activation of this response element was related to hepatocytes seeded on collagen gel. More work is warranted to investigate whether similar activation occurs by endogenously synthesized collagen.
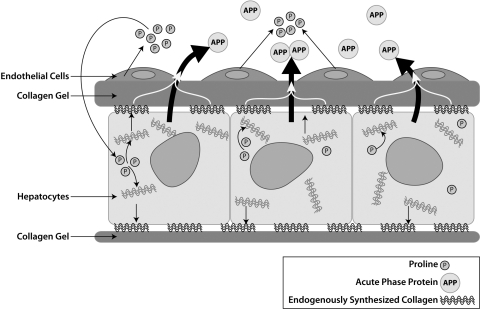
Our results are consistent with the following mechanism (Fig. 6) for endothelial cell-induced early recovery of hepatocytes. In the layered coculture, endothelial cells secrete proline, which is taken up by hepatocytes for increasing synthesis of collagen. Hepatocytes secrete freshly synthesized collagen, which dynamically modifies the ECM microenvironment of the cells. In the 3-D configuration, collagen gel present on top of hepatocytes acts as a scaffold for accumulating endogenously synthesized collagen. The actively synthesized collagen enhances the acute-phase protein secretion by hepatocytes. By contrast, for hepatocytes cultured on top of collagen gel (2-D configuration), there is no scaffolding gel on top that allows retention of endogenously synthesized collagen by hepatocytes exposed to conditioned medium. However, in addition to the loss of actively secreted collagen, other factors such as differences in organization of cytoskeleton and expression of cytoskeletal proteins could also account for the differential response of hepatocytes cultured in 3-D and 2-D configuration (31).
Figure 6.
Schematic illustrating proposed mechanism of endothelial cell-induced early recovery of hepatocytes. In the layered coculture, endothelial cells enhance proline levels in the supernatant during the first week of culture. Hepatocytes take up the proline to synthesize increased levels of collagen. The original collagen gel on top of hepatocytes acts as a scaffold for retaining freshly synthesized collagen. This endogenously synthesized collagen enhances acute-phase protein secretion by hepatocytes during the early phase of culture.
One plausible reason for earlier recovery of hepatocytes in the layered coculture is that the rate of collagen secretion is higher during the first week of culture, in comparison to monoculture of hepatocytes embedded in collagen gel. As mentioned above, the collagen gel on top of hepatocytes provides a scaffold for continuous deposition of collagen and remodeling of ECM around hepatocytes. The level of collagen deposition required for reaching maximum acute-phase protein secretion is reached faster in coculture as compared to monoculture. As culture time progresses, eventually the level of proline becomes similar in the supernatants of both coculture and monoculture, which, in turn, should result in similar levels of collagen synthesis and acute-phase protein secretion.
In our coculture model, hepatocytes were embedded in collagen gel, and endothelial cells were overlaid on top of the gel. Hepatocytes were embedded in gel by mixing them with collagen solution and allowing the mixture to gel. Traditionally, collagen sandwich culture of hepatocytes is prepared by seeding cells on top of the collagen gel and then the next day overlaying a second layer of collagen gel. Comparison of albumin secretion profiles across embedded (Fig. 1E) and sandwich models (7) suggests that the difference in timing of 1 d in introducing collagen on top of hepatocytes does not change the model performance appreciably.
In our layered model, the collagen between hepatocytes and endothelial cells resembles the space of Disse that separates these two cell types in vivo. Although, in vivo, the space of Disse separates hepatocytes from liver sinusoidal endothelial cells (LSECs), we employed primary rat heart microvessel endothelial cells due to their capacity to proliferate and ease of maintaining them in culture. LSECs have limited proliferation capacity. Furthermore, long-term monocultures of these cells are difficult to maintain, which further complicates obtaining endothelial cell-conditioned medium for experimentation. We reasoned that primary rat heart endothelial cells will be a compromise in terms of ease of maintaining them in culture, while at the same time not omitting interactions mediated by growth factors and cytokines, as they belong to the same species as the hepatocytes. Moreover, a wide variety of cell type, including cells belonging to tissues other than liver and even those obtained from different species, have shown remarkable ability of modulating hepatocyte function (1).
The space of Disse contains multiple extracellular matrix proteins, including collagen types I, III, IV, VI; fibronectin; laminin; perlecan; and tenascin (11). Past reports indicate that hepatocytes synthesize collagen, fibronectin, and laminin in collagen sandwich culture (31). Our results also support the secretion of collagen by hepatocytes, which results in modification of the original extracellular matrix composition (collagen I) surrounding hepatocytes, although the exact variation in matrix composition remains unknown.
Most studies indicate that hepatocytes maintain their function by direct cell-cell contact mediated interaction with other cell types. In our layered coculture model, hepatocytes and endothelial cells interact by soluble factor and not by direct cell-cell contact. Nevertheless, maximum albumin secretion (3.3 μg−1·h−1·106 cells−1) observed in our system is comparable to that observed in coculture where hepatocytes were in direct contact with endothelial cells (1). One way of introducing direct cell-cell contact under 3-D configuration is by adding a second layer of collagen on top of hepatocytes cocultured in direct contact with another cell type. Unfortunately, this is not compatible with cocultures that involve endothelial cells. On introduction of second layer of collagen on top, endothelial cells change their morphology from cobblestone to tubular. This change in morphology is associated with induction of apoptosis in endothelial cells (32). However, direct contact coculture in 3-D configuration should work with other cell types such as fibroblasts.
In conclusion, coculture influences hepatocyte function in a number of ways. In this report, we have established that a change in amino acid composition is another modality through which coculture can influence hepatocyte function.
Supplementary Material
Acknowledgments
We thank Chris Pohun Chen, Avrum Leeder, Luke Selby, and Carley Shulman for the isolation of cells from rat livers. We appreciate the help of Dr. David Yarmush in conducting collagen measurement studies. This work was supported by NIH BioMEMS Resource Center grant P41 EB-002503 and NIH grant RO1AI063795. Microscopic imaging studies were made possible by a core morphology facility, and gene expression measurements were carried out at the Genomic and Proteomic Facility at Boston’s Shriners Burns Hospital.
References
- Bhatia S N, Balis U J, Yarmush M L, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Morin O, Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986;129:103–110. doi: 10.1002/jcp.1041290115. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Novikoff P M, Greenwel P, Rubin J, Rojas-Valencia L, de Carvalho A C, Stockert R, Spray D, Hertzberg E L, Wolkoff A W. Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol. 1995;146:1508–1520. [PMC free article] [PubMed] [Google Scholar]
- Bhatia S N, Balis U J, Yarmush M L, Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J Biomater Sci Polym Ed. 1998;9:1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- Hui E E, Bhatia S N. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J C, Tompkins R G, Yarmush M L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Dunn J C, Yarmush M L, Koebe H G, Tompkins R G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- Harimoto M, Yamato M, Hirose M, Takahashi C, Isoi Y, Kikuchi A, Okano T. Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res. 2002;62:464–470. doi: 10.1002/jbm.10228. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Shen C J, Berthiaume F, Tilles A W, Toner M, Yarmush M L. Polyelectrolyte nano-scaffolds for the design of layered cellular architectures. Tissue Eng. 2006;12:1553–1563. doi: 10.1089/ten.2006.12.1553. [DOI] [PubMed] [Google Scholar]
- Ito A, Takizawa Y, Honda H, Hata K, Kagami H, Ueda M, Kobayashi T. Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10:833–840. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta P S. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- Seglen P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush M L. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–265. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- Arai K, Lee K, Berthiaume F, Tompkins R G, Yarmush M L. Intrahepatic amino acid and glucose metabolism in a D-galactosamine-induced rat liver failure model. Hepatology. 2001;34:360–371. doi: 10.1053/jhep.2001.26515. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Behnia K, Bhatia S, Jastromb N, Balis U, Sullivan S, Yarmush M L, Toner M. Xenobiotic metabolism by cultured primary porcine hepatocytes. Tissue Eng. 2000;6:467–479. doi: 10.1089/107632700750022125. [DOI] [PubMed] [Google Scholar]
- Lee J, Morgan J R, Tompkins R G, Yarmush M L. Proline-mediated enhancement of hepatocyte function in a collagen gel sandwich culture configuration. FASEB J. 1993;7:586–591. doi: 10.1096/fasebj.7.6.8472895. [DOI] [PubMed] [Google Scholar]
- Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology. 1988;8:1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- Deroanne C F, Lapiere C M, Nusgens B V. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res. 2001;49:647–658. doi: 10.1016/s0008-6363(00)00233-9. [DOI] [PubMed] [Google Scholar]
- Nahmias Y, Berthiaume F, Yarmush M L. Integration of technologies for hepatic tissue engineering. Lee K, Kaplan D, editors. Berlin: Springer; 2006:309–329. doi: 10.1007/10_029. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Moritz D R, Li B, Phillips G L, Liang X H, Gerber H P, Hillan K J, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- Davidson A J, Zon L I. Love, honor, and protect (your liver) Science. 2003;299:835–837. doi: 10.1126/science.1082006. [DOI] [PubMed] [Google Scholar]
- Bissell D M, Arenson D M, Maher J J, Roll F J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J M. Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol Cell Biol. 1990;10:1239–1243. doi: 10.1128/mcb.10.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- Lee J W, Morgan J R, Tompkins R G, Yarmush M L. The importance of proline on long-term hepatocyte function in a collagen gel sandwich configuration-regulation of protein secretion. Biotechnol Bioeng. 1992;40:298–305. doi: 10.1002/bit.260400214. [DOI] [PubMed] [Google Scholar]
- Honda H, Nojima T, Kobayashi T. Effect of additives such as antioxidative agent on albumin secretion from primary culture of rat hepatocyte. Biotechnol Lett. 1995;17:365–370. [Google Scholar]
- Khetani S R, Szulgit G, Del Rio J A, Barlow C, Bhatia S N. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40:545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- Uitto J, Hoffman H, Prockop D J. Retention of nonhelical procollagen containing cis-hydroxyproline in rough endoplasmic reticulum. Science. 1975;190:1202–1204. doi: 10.1126/science.1198105. [DOI] [PubMed] [Google Scholar]
- Liu J K, DiPersio C M, Zaret K S. Extracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Mol Cell Biol. 1991;11:773–784. doi: 10.1128/mcb.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume F, Moghe P V, Toner M, Yarmush M L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- Peters K, Troyer D, Kummer S, Kirkpatrick C J, Rauterberg J. Apoptosis causes lumen formation during angiogenesis in vitro. Microvasc Res. 2002;64:334–338. doi: 10.1006/mvre.2002.2438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.