Abstract
Altered structure, and hence function, of cellular macromolecules caused by oxidation can contribute to loss of physiological function with age. Here, we tested whether the lifespan of bats, which generally live far longer than predicted by their size, could be explained by reduced protein damage relative to short-lived mice. We show significantly lower protein oxidation (carbonylation) in Mexican free-tailed bats (Tadarida brasiliensis) relative to mice, and a trend for lower oxidation in samples from cave myotis bats (Myotis velifer) relative to mice. Both species of bat show in vivo and in vitro resistance to protein oxidation under conditions of acute oxidative stress. These bat species also show low levels of protein ubiquitination in total protein lysates along with reduced proteasome activity, suggesting diminished protein damage and removal in bats. Lastly, we show that bat-derived protein fractions are resistant to urea-induced protein unfolding relative to the level of unfolding detected in fractions from mice. Together, these data suggest that long lifespan in some bat species might be regulated by very efficient maintenance of protein homeostasis.—Salmon, A. B., Leonard, S., Masamsetti, V., Pierce, A., Podlutsky, A. J., Podlutskaya, N., Richardson, A., Austad, S. N., Chaudhuri, A. R. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis.
Keywords: longevity, comparative biology, oxidative stress, ubiquitin-proteasome, Chiroptera
Mammal species differ vastly in how long they live. For example, shrews live 1–2 yr and house mice (Mus musculus) rarely exceed a lifespan of 4 years even under protected laboratory conditions with abundant food and water and protection from predators and pathogens, whereas some whales may live more than 2 centuries despite enduring the challenges of life in the wild (1, 2). At least superficially, however, the aging process appears remarkably similar across mammalian species, even though the rate differs. To determine precisely what mechanistic processes are causally involved in the evolution of long life, it is critical to ascertain the cellular and molecular mechanisms by which long-lived species differ from short-lived species. A greater understanding of these aging regulatory mechanisms across mammalian species may help us better understand the generalities of how nature controls the aging process.
From a biogerontological perspective, bats (order Chiroptera) are exceptionally interesting, because after controlling for the effects of body size, bats are by far the longest-lived mammal group (3,4,5). This is particularly striking in that unlike most mammal longevity records, which come from captive populations such as zoos or research facilities, most of our knowledge about bat longevity has been adventitiously acquired during field studies for different purposes. Thus bats have survived as much as 10 times longer than expected for their body size and managed to retain for that long life the sensory acuity, agility, flight endurance, spatial memory, and general physiological integrity necessary to avoid predators, climatic catastrophes, famine, drought, and illness. The longest-lived bat species to date survived at least 41 yr in the wild (6), although as previously noted, because the documentation of bat longevity has been a casual enterprise, they may live considerably longer. The exceptional difference in lifespan between bats living in nature and short-lived mammals such as mice kept in laboratories and zoos holds true even after accounting for effects of hibernation and its attendant periods of reduced metabolism (4). The ability to fly, and thus reduce extrinsic mortality, has previously been suggested as a major reason for the evolution of their long lifespan in the wild. There must then be fundamental differences in the way that aging is regulated in these disparate species.
Oxidative stress is generally thought to be an important mechanistic contributor to the aging process (7). This theory proposes that aging and age-related disease at least partially result from the accumulation of oxidative damage to biological macromolecules. The root cause of this damage is hypothesized to be an imbalance between cellular prooxidant production, antioxidant defenses and repair of oxidative damage to these macromolecules (8). Oxidative damage to proteins may be a particularly important determinant of aging as proteins are integral to a wide range of cellular repair machinery. Moreover, most amino acids are sensitive to oxidation, and oxidation of critical protein-bound residues can negatively affect protein structure and function. Chronic oxidative stress can lead to protein unfolding, misfolding, and subsequent formation of higher-order protein structures. Protein homeostasis is maintained, in part, by ubiquitin-mediated targeting of misfolded proteins for proteasome-mediated degradation (9) and subsequent synthesis of new proteins. If not removed, damaged proteins can form insoluble aggregates, which have been shown to be associated with numerous age-related pathologies, particularly neurological disorders such as Alzheimer’s disease and Parkinson’s disease. Failure of proteome homeostasis has been hypothesized to affect the rate of aging itself (10).
Under the oxidative stress theory of aging, mechanisms that reduce the accumulation of oxidative damage might be predicted to prolong healthy lifespan. Among common laboratory animal models (e.g., Caenorhabditis elegans, Drosophila, and mice), there are conflicting data on whether oxidative stress and damage may affect aging rate within species (reviewed in ref. 8). Some evidence suggests that long-lived mammals display enhanced antioxidant defenses and/or decreased prooxidant production and/or increased resistance to oxidative stress when compared to short-lived mammals (11,12,13,14,15); however, these results have often been difficult to interpret, in part because they have generally neglected to account for the well-known correlation between body mass and longevity (1). When animals of similar size, yet greatly different lifespans, have been examined, there has been ambiguity in the role of oxidative stress in the regulation of aging. For instance, tissues from young naked mole rats (Heterocephalus glaber), mouse-sized rodents that live up to 30 yr, exhibit high levels of oxidative damage and no evidence of enhanced antioxidant defense compared with mice, despite living at least 8 times longer (16,17,18). However, unlike short-lived animals, naked mole rats do not appear to accumulate oxidative damage, increase prooxidant production, or reduce antioxidant defenses with age (18, 19). Furthermore, cultured cells from these animals, including primary fibroblasts and endothelial cells, tend to be resistant to some forms of oxidative stress (20, 21).
Limited data to date on oxidative stress in bats are similarly complex. For instance, tissues from the little brown bat [Myotis lucifugus, maximum lifespan (MLSP) 34 yr] were reported to produce lower levels of the prooxidant H2O2 than tissues from the much shorter-lived white-footed mouse (Peromyscus leucopus, MLSP 8 yr) and short-tailed shrew (Blarina brevicauda, MLSP 2 yr), but only when these levels are normalized to oxygen consumption (22). This same study found no difference in activity of the antioxidant enzyme, superoxide dismutase (SOD), between the bat and either shorter-lived species. Primary fibroblasts isolated from the little brown bat were also reported to be resistant to several forms of cellular stress, including H2O2, cadmium, UV light, and heat, relative to cells from mice (23). Cultured cells from this species also showed enhanced repair of γ-irradiation-induced DNA lesions (6). Another study of 5 South American bat species reported higher SOD activity, but lower catalase activity, in bats compared with mice (24).
In this study, we evaluate the hypothesis that enhanced proteome homeostasis in the form of reduced oxidative damage to proteins might contribute to the long lives of bats. Specifically, we examined in 2 bat species compared with house mice, the relative oxidation of liver proteins under basal and oxidatively challenged conditions. We quantified the presence of insoluble cellular aggregates and assessed how differences in protein degradation through the ubiquitin-proteasome system might influence our results. Finally, we explored whether bat proteins might better resist unfolding stress when compared with proteins from short-lived mice. We found that protein homeostasis in bats is superior by a variety of measures to that of mice.
MATERIALS AND METHODS
Animal species
Two bat species were used in this study. The Brazilian or Mexican free-tailed bat (Tadarida brasiliensis) is a 10–11 g cave-dwelling species that has been reported to live up to 12 yr, although as with other bat species, the limited number of field recaptures suggests that this longevity record could be a considerable underestimate. Our second species, the cave myotis (Myotis velifer), is a similar-size species also reported to live at least 12 yr in the wild. Other species in the genus Myotis have been documented to live as long as 41 yr in the wild (6). All bats were wild-caught locally and estimated to be young adults. They were sacrificed for experiments on the day of capture. Bat species were compared with wild-derived mice (M. musculus). These mice are fifth- and sixth-generation descendants of animals wild-caught in rural Idaho. They have previously been shown to live up to 4 yr in the laboratory (25). Mice were maintained on normal chow and given free access to food and water; all samples were from young adult mice (6–8 mo).
Measurement of protein carbonyls
Protein carbonyl content was determined using a method described by Chaudhuri et al. (26). In brief, liver tissues from all animals were homogenized in 50 mM phosphate buffer, pH 6.0; 0.5 mM MgCl2; and 10 mM EDTA containing cocktail of protease inhibitors (0.5 mM PMSF, 1 mg/ml leupeptin, and 1 mg/ml aprotinin). The crude extract was centrifuged at 4°C for 1 h at 100,000 g. The extract was incubated with 1% streptomycin sulfate for 10 min at 37°C. The cell extract was then centrifuged at 16,000 g for 10 min, and protein concentration of the supernatant was measured by the Bradford method (Bio-Rad, Hercules, CA, USA). Proteins (1 mg/ml) were incubated with 1 mM fluorescein-5-thiosemicarbazide (FTC) in deaerated phosphate buffer, pH 6.0, with 0.3 M guanidine for 2 h at 37°C in the dark. Proteins were then precipitated by addition of an equal volume of 20% trichloroacetic acid (TCA) on ice for 10 min. After 10 min centrifugation at 16,000 g at room temperature, protein pellets were broken up and washed 5 times with ethanol/ethyl acetate (1:1) (v/v). Protein pellets were resuspended in 20 mM phosphate buffer, pH 8.0, containing 8 M urea, then boiled in Laemmli buffer, and equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the image of the resolved fluorescently labeled proteins was captured using a Typhoon 9400 imager (excitation wavelength of 532 nm, emission filter at 526 nm, 40 nm bandpass; GE Healthcare Life Sciences, Piscataway, NJ, USA). The fluorescence intensity for each lane of the gel (from top to the bottom) was calculated using ImageQuant 5.0 software (Molecular Dynamics, Amersham Biosciences Corp., Piscataway, NJ, USA). The gel was then stained with Coomassie R250 (Sigma-Aldrich Corp., St. Louis, MO, USA) and destained with 10% acetic acid, 20% methanol solution, and the image of the Coomassie stained proteins was captured with an AlphaImage 3400 (Alpha Innotech Corp., San Leandro, CA, USA) for quantification.
Acute in vitro oxidative stress
Total protein lysates obtained after centrifugation at 100,000 g of liver homogenates from all animals were used to measure damage caused by in vitro oxidative stress generated from metal-catalyzed oxidation reaction (iron/ascorbate), as described by Chaudhuri et al. (26). In brief, equal amounts of protein were treated with or without iron (0.002 mM) and ascorbate (0.5 mM), followed by incubation at 37°C for 2 h with FTC (1 mM) to label protein carbonyls. Measurement of protein carbonyls was performed as described above.
Measurement of ubiquitinated proteins
Cytosolic protein homogenates from mouse and bat livers were assayed for protein ubiquitination by Western blot. Protein concentration was measured by BCA (bichronic acid) protein assay (Pierce Biotechnology, Rockford, IL, USA), and equal amounts of protein (15 μg) were separated by SDS-PAGE, transferred to nitrocellulose membrane, and subjected to Western blotting using a mouse polyclonal antibody against ubiquitin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The intensities of the bands were quantified by densitometry using ImageQuant 5.0. The results were expressed as ubiquitinated protein normalized to the total amount of protein measured by Coomassie blue.
Determination of resistance to protein unfolding using photoincorporation of BisANS assay
Resistance to protein unfolding was measured by incubating cytosolic protein extracts from bat and mouse liver with different concentrations of urea treatment followed by UV-induced photoincorporation of 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid (BisANS) to proteins, as described previously by Pierce et al. (27). Briefly, extracts were diluted to 1 mg/mL in labeling buffer containing 50 mM Tris-HCl; 10 mM MgSO4, pH 7.4; and protease inhibitors. The samples were treated with and without different concentrations of urea for 2 h at room temperature, followed by addition of 100 mM BisANS and immediate vortexing. Samples (200 μl) were added to a clear 96-well plate and incubated on ice (to minimize local heating) for 1 h under direct exposure of a 115-V 0.16-A handheld longwave UV lamp (365 nm, model UVL-21; Ultraviolet Products, Inc., Cambridge, UK). After photoincorporation of BisANS, proteins were dissolved in Laemmli buffer and separated by SDS-PAGE. After electrophoresis, gels were removed and illuminated with 365 nm UV light, and BisANS fluorescence was captured with an AlphaImage 3400 for quantification. The data were expressed as fluorescent units/mg protein.
20S proteasome activity assay
Liver samples from mice and bats were homogenized in homogenization buffer (50 mM Tris-CL, pH 8.0; 1 mM EDTA; 0.5 mM DTT) using a dounce tissue homogenizer, then measured for protein concentrations by BCA assay. For each sample, 100 μg total protein was assayed in triplicate in 96-well plates using a 20S proteasome fluorometric (AMC) assay kit as per instructions from the vendor (Calbiochem, San Diego, CA, USA). In brief, the release of free AMC from the fluorogenic peptide Suc-Leu-Leu-Val-Tyr-AMC was measured over time at 37°C using a microplate fluorescence spectrophotometer. 20S activity was calculated by the slope of free AMC release over time after an ∼10 min period of normalization. Quantity of 20S proteasome per sample was measured by Western blot. Total cellular protein extract (20 μg) was prepared in 6× SDS sample buffer, subjected to SDS-PAGE, and transferred to PVDF membrane (Millipore, Billerica, MA, USA), then incubated with an anti-rabbit antibody against 20S proteasome subunit (28). The intensities of the bands were quantified by densitometry using ImageQuant 5.0. 20S proteasome specific activity was calculated by normalizing 20S activity to the quantity of 20S proteasome as measured by Western blot; data were expressed as AMC release per second per protein.
Statistical analyses
Basal carbonyl levels were normalized to total protein (Coomassie blue) and were analyzed by 2-tailed Student’s t test when experiments were run separately (supernatant; one bat compared with mouse) and by 1-way ANOVA when samples from 3 species were run concurrently (pellet) with post hoc Newman-Keul’s test to determine which samples differed from the others. In vivo oxidative stress-induced carbonyl levels were analyzed by 2-tailed Student’s t test. In vitro oxidative stress assays were analyzed both by 1-way ANOVA (within a treatment) and by ANOVA with species and treatment as the two factors with post hoc Newman-Keul’s test to determine which samples differed from the others. Ubiquitin levels were normalized to total protein (Coomassie blue) and analyzed by 2-tailed Student’s t test. Normalized 20S proteasome activity was analyzed by 1-way ANOVA. Differences in BisANS intensity between species at each urea concentration were analyzed by 2-tailed Student’s t test, and the data sets as a whole were analyzed by repeated-measures ANOVA. All data were analyzed using NCSS software (NCSS, Kaysville, UT, USA) or SigmaStat software (Aspire Software, Ashburn, VA, USA).
RESULTS
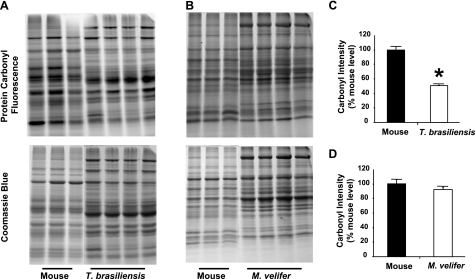
To measure protein oxidation, we utilized a fluorescence-based protein carbonyl assay we have previously shown to be adaptable to multiple species (17). We compared samples from each bat species to those from a wild-derived, genetically heterogeneous mouse strain mice known to be smaller, mature more slowly, have lower levels of several hormones, and live significantly longer than typical inbred mouse strains (25). This comparison was made, in part, because typical inbred laboratory mouse strains have been raised under artificial conditions for many hundreds of generations under conditions that have generated strain-specific physiological characteristics and pathologies that may be confounding factors in multispecies comparisons. Using this assay, we found that unstressed, soluble liver proteins from young T. brasiliensis had significantly lower carbonyl levels than samples from young mice (Fig. 1A, C; t test, P<0.01). M. velifer exhibited a trend toward lower carbonyl levels than mice, although this difference was smaller (∼10%) and did not reach statistical significance (Fig. B, D; t test, P=0.22).
Figure 1.
Protein carbonyl levels are relatively lower in samples from bat species relative to those from mice. A) Representative SDS-PAGE gel showing carbonyl probe fluorescence and total protein as measured by Coomassie blue stain in samples from liver homogenates from house mice and T. brasiliensis. B) As in A, comparing liver homogenates from house mice and M. velifer. C) Relative carbonyl levels for mice (n=3 animals) and T. brasiliensis (n=4 animals), presented as carbonyl intensity (carbonyl fluorescence/total protein as measured by Coomassie blue) relative to average intensity for mice. Bars represent means ± se. *P < 0.01; t test. D) As in C, comparing carbonyl levels for mice (n=3 animals) and M. velifer (n=4 animals).
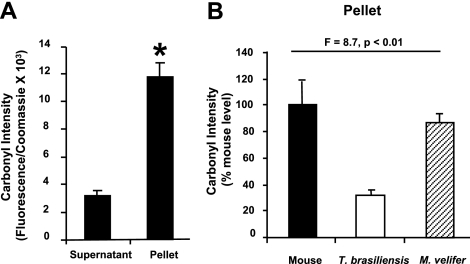
Protein oxidation and misfolding can lead to insoluble protein aggregates not represented in the supernatant; thus, measurement of only the supernatant may underestimate the carbonyl content of the total proteome. In mice, for example, we noted that proteins in the insoluble, pellet-bound fraction had carbonyl levels nearly 4 times higher than the proteins in the soluble supernatant (Fig. 2A). Thus, we compared carbonyl levels of the pellet fraction from all three species (Fig. 2B). We found a significant difference between species in their insoluble protein fraction carbonyls (1-way ANOVA, F=8.7, P<0.01), with post hoc Newman-Keul’s test showing that samples from T. brasiliensis were significantly different from those of either mice or M. velifer. Carbonyl levels of pellets from T. brasiliensis were ∼40% of those found in samples from mice, and we found a smaller difference between M. velifer and mice in the carbonyl content of this insoluble protein fraction; the carbonyl content in M. velifer samples represented ∼85% of that found in mouse samples.
Figure 2.
Protein carbonyl levels are relatively lower in samples from bats in both supernatant and pellet fractions of protein homogenates. A) Relative carbonyl levels in supernatant fraction and insoluble pellet fraction of liver homogenates from mice (n=3 animals) presented as carbonyl intensity (carbonyl fluorescence/total protein as measured by Coomassie blue). Bars represent means ± se. *P < 0.01; t test. B) Relative carbonyl levels in pellet fraction of liver homogenates from mice (n=5 animals), T. brasiliensis (n=5 animals), and M. velifer (n=5 animals), presented as carbonyl intensity (carbonyl fluorescence/total protein as measured by Coomassie blue) relative to average intensity for mice. Bars represent means ± se. F and P values represent 1-way ANOVA for data comparing all three species.
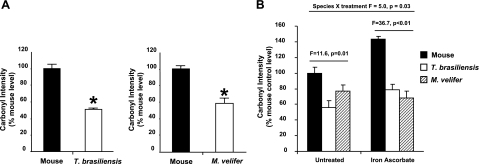
The oxidative stress theory of aging predicts that long-lived species should show relatively greater resistance to oxidative stress and the oxidative damage associated with this stress. Previous results have shown that cells from another bat species, M. lucifugus, tend to be resistant to multiple forms of acute oxidative stress (6, 23). In this study, we addressed whether these two bat species were resistant to oxidative damage in vivo relative to mice. γ-Irradiation is a well-known generator of oxygen radicals in cells and tissues (29). Bats and laboratory mice were exposed to whole-body 9 Gy γ-irradiation, then sacrificed 17 h later. After irradiation, the relative intensity of the carbonyl probe in the liver-derived soluble protein fraction from both bat species was significantly lower (∼50% for T. brasiliensis, P < 0.001; ∼40% for M. velifer, P = 0.002) than in mice (Fig. 3A, B). Together, these data suggest that both bat species are protected from the accumulation of oxidative damage to proteins caused by acute exposure to γ-irradiation.
Figure 3.
Protein carbonyl levels following oxidative stress are relatively lower in samples from bat species than those from mice. A) Relative carbonyl levels in liver homogenates from irradiated mice (n=3 animals) and T. brasiliensis (n=4 animals) or for irradiated mice (n=3 animals) and M. velifer (n=4 animals), presented as carbonyl intensity (carbonyl fluorescence/total protein as measured by Coomassie blue) relative to average intensity for mice. Bars represent means ± se. *P < 0.01; t test. B) Relative carbonyl levels in liver homogenates from mice (n=3 animals), T. brasiliensis (n=3 animals), and M. velifer (n=3 animals) after treatment with iron ascorbate. Bars represent means ± se. F and P values represent 1-way ANOVA for data within either control or treated samples.
Interpretation of oxidative stress resistance in whole animals can be complicated by the number of buffering systems used by mammals to maintain homeostasis. To determine whether bats have developed inherent cellular mechanisms of protein oxidation resistance, we tested whether total tissue homogenates from T. brasiliensis and M. velifer differed from those from mice in carbonyl formation following exposure to a metal-catalyzed oxidation reaction (iron/ascorbate). We found relatively lower carbonyl levels in samples from both bat species relative to mice in both treated and untreated homogenates (Fig. 3B; 1-way ANOVA, F=11.6, P=0.01; F=36.7, P<0.01, respectively). When comparing the data set as a whole, we found a significant species × treatment interaction (ANOVA, F=4.97, P=0.03) by Newman-Keul’s multiple comparison, with mice being strongly affected by the treatment, and no effect in samples from T. brasiliensis and M. velifer. These data then show that tissue homogenates from mice are significantly more prone to oxidation in vitro than are those from T. brasiliensis and M. velifer; thus, bat-derived proteins are resistant to oxidative stress, as predicted by the oxidative stress theory of aging.
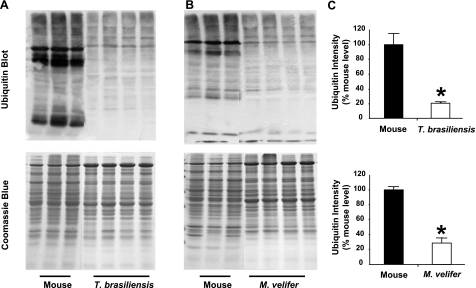
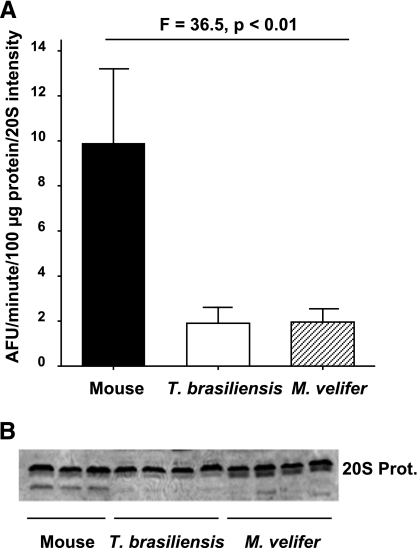
One way cells defend against the accumulation of protein oxidative damage during aging is by degradation of misfolded or aggregated proteins through the ubiquitin-proteasome system and subsequent synthesis of new proteins (9). To determine whether bat proteins might experience higher turnover rates than mouse proteins, we measured both ubiquitination and proteasome activity in total protein homogenates from all three species. Western blots using a primary antibody for ubiquitin indicate that basal levels of ubiquitination are drastically lower in samples from both bat species than they are in samples from mice (Fig. 4A, B); samples from T. brasiliensis showed only ∼20% of the total ubiquitin levels of samples from mice (P<0.001), and those from M. velifer showed only ∼30% of those from mice (P<0.001) (Fig. 4C). Such a large difference in protein ubiquitination could indicate that mouse proteins turn over more slowly, thus are on average older, and hence are more damaged and more likely marked by ubiquitination for proteasomal degradation. Alternatively, mouse protein turnover could be similar or even slower than in bats, and these results could indicate enhanced protein stability in bats. As a measure of protein turnover, we assayed 20S proteasome chymotrypsin-like activity of liver homogenates from all three species. Specific activity of each sample was calculated by normalizing to 20S protein content quantified from a Western blot of liver proteins incubated with a primary antibody to 20S proteasome that was generated from purified 20S proteasome subunits generated from rat liver. We found the specific 20S proteasome activity was drastically reduced in both bat species relative to mice (Fig. 5A). There was no significant difference in proteasome content between bats and mice as measured by this Western blot (Fig. 5B). Thus, the lower protein carbonyl levels and lower protein ubiquitination we see in these two bat species suggest that their soluble liver proteins resist damage and misfolding relative to mouse proteins.
Figure 4.
Protein ubiquitination levels are relatively lower in samples from bat species relative to those from mice. A) Representative Western blot showing signal obtained after incubation with primary antibody for ubiquitin and total protein as measured by Coomassie blue stain in samples from liver homogenates from house mice and T. brasiliensis. B) As in A, comparing liver homogenates from house mice and M. velifer. C) Relative ubiquitin levels for mice (n=3 animals) and T. brasiliensis (n=4 animals) or for mice (n=3 animals) and M. velifer (n=4 animals), presented as ubiquitin intensity (ubiquitin signal/total protein as measured by Coomassie blue) relative to the average intensity for mice. Bars represent means ± se. *P < 0.01; t test.
Figure 5.
Tissue homogenates from bat species show relatively low 20S proteasome chymotrypsin-like activity. A) 20S proteasome-specific activity in liver homogenates from mice (n=3 animals), T. brasiliensis (n=4 animals), and M. velifer (n=4 animals), presented as specific activity (fluorescence units/minute/100 μg protein/intensity of 20S signal from Western blot). Bars represent means ± se. F and P values represent 1-way ANOVA for data within either control or treated samples. B) Representative Western blot showing intensity of signal following incubation with primary antibody for 20S proteasome. Each lane represents 20 μg total protein from liver homogenates.
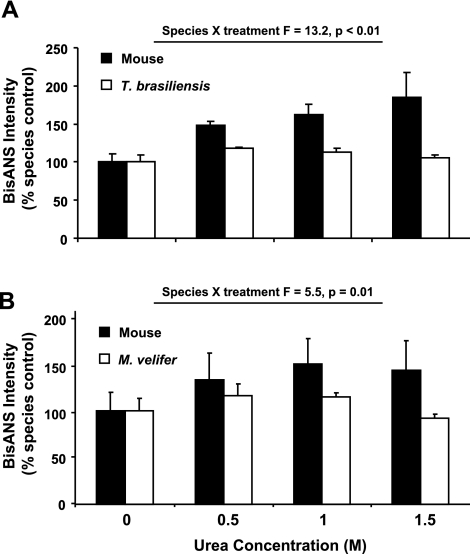
The data above suggest that bats may employ some specific mechanisms whereby they are able to maintain their proteome in its properly folded, and thereby optimally functional, state. Our lab has shown that exposed hydrophobic sites in proteins, such as those caused by unfolded and denatured proteins, can be labeled using the apolar fluorescent probe BisANS (27). Using this technology, we measured the resistance of total protein homogenates to unfolding and denaturing caused by incubation with different concentrations of the reducing agent urea. Because misfolding stress causes more hydrophobic sites to be exposed on the surface of proteins, more BisANS will bind to these sites, thereby increasing fluorescence intensity. Therefore, we incubated proteins isolated from the livers of T. brasiliensis, M. velifer, and mice in different concentrations of urea to impose an unfolding stress and, after treatment, labeled the proteins with BisANS; protein samples from the same animals were tested under each of 4 conditions (control and 3 concentrations of urea). The BisANS signal of untreated samples was not significantly different between species, but tended to be ∼10–15% higher in bat species (data not shown). We found that increasing urea concentration led to a significant increase in BisANS intensity in mice, yet the same urea doses failed to increase BisANS incorporation in samples from either bat species (Fig. 6). These differences were significant for both by repeated-measures ANOVA comparing species and treatment (mouse vs. T. brasiliensis species × treatment, F=13.2, P<0.01; mouse vs. M. velifer species × treatment, F=5.5, P=0.01), suggesting that the proteome in long-lived bats is protected from structural perturbation in response to an unfolding stressor. In sum, our data suggest that the mechanisms that slow aging may also affect the processes that maintain protein homeostasis.
Figure 6.
Proteins from bat species are relatively resistant to unfolding caused by incubation with urea. A) Relative BisANS intensity for total protein homogenates from mice and T. brasiliensis incubated in different concentrations of urea. Bars represent means ± se. For each species, BisANS intensity for each sample was normalized to the BisANS intensity for the untreated sample from that species. B) As in A, comparing samples from mice and M. velifer.
DISCUSSION
One of the fundamental questions in biogerontology is why different species age at different rates. Yet, there have been limited attempts to define the molecular mechanisms behind these differences. For over 60 yr, one of the most studied mechanistic theories on how aging is regulated has been the oxidative stress theory of aging. In this study, we have shown that long-lived bat species differ from short-lived mice in maintaining relatively low rates of protein oxidation and are resistant to the effects of acute oxidative stress, both of which may contribute to maintenance of proteins in their correctly folded, and thereby functional, state.
One prediction of the oxidative stress theory of aging is that slow aging should be associated with reduction in the accumulation of oxidative damage, thus long-lived species should show relatively low oxidation even at young ages. The data in this study partially support this prediction; protein oxidation in long-lived T. brasiliensis was significantly less than that of short-lived mice, but protein oxidation in M. velifer was not statistically different from mice, though it tended to be lower. On the other hand, when oxidatively stressed, both bat species proteins displayed enhanced stability compared with mice, suggesting that mechanistic differences underlying different aging rates may only be revealed when the regulatory systems are stressed. Our data are in some agreement with a previous report showing reduced lipid peroxidation, measured by thiobarbituric acid reactive substances (TBARS) level, in liver samples from 5 South American bat species compared to liver samples from mice (24).
The relation between resistance to oxidative stress and longevity has been well-established in single-gene mouse mutants of extended lifespan (30, 31), calorie restriction (32), and across several mammalian species with different maximum lifespans (15, 20, 23). We have shown here, too, that bats seem to be relatively resistant to the increase in protein oxidation caused by irradiation-induced in vivo oxidative stress or chemically induced in vitro oxidative stress. Thus, our data are consistent with the prediction that one mechanism responsible for the long lifespan of these bat species may be a reduction in protein oxidation. However, at present we do not have any evidence whether particular amino acids such as cysteine and methionine, which are highly sensitive to oxidation, are in some way protected from oxidation in bat species. There are many different protein oxidation moieties that may contribute to protein misfolding (33), and it will be of interest to investigate further whether other protein oxidation moieties, such as cysteine (disulfides) or methionine sulfoxides, are also reduced in bats.
It is still unclear how bat species are able to maintain lower levels of oxidative damage relative to mice. One hypothesis is that long-lived species possess more efficient mitochondria and produce fewer reactive oxygen species (ROS) for a given amount of oxygen consumption than short-lived species (34, 35). Previously, little brown bats (M. lucifugus), which live up to 34 yr in the wild, have been shown to have diminished H2O2 production compared to shorter-lived rodents when ROS production was normalized to oxygen consumption (22).
Alternatively, long-lived species might exhibit greater antioxidant capacities than shorter-lived species, although the evidence for this effect is decidedly mixed. For example, Brunet-Rossinni (22) found no difference between bats and short-lived rodents in their superoxide dismutase activity, and a second report showed no elevation in catalase activity in bat species relative to mice or rats (24). However, superoxide dismutase and catalase represent only two of the many antioxidants and oxidation-repair enzymes that handle cellular oxidative stress (36); hence, it remains to be seen whether there are significant differences in either the activity or tissue specificity of most of these antioxidants though evidence to date supporting the idea that antioxidant activities correlate with lifespan is weak and inconsistent (37).
It seems likely that no one particular antioxidant, or other stress response protein, is the proximate cause of long lifespan; rather, a coordinate up-regulation of many stress defense systems, including oxidative stress defenses, may have a greater role in regulating aging rate. Under this hypothesis, resistance to acute oxidative stress, or other forms of stress, may be a more important predictor of long life than the activity of any single antioxidant. As we have shown here, both bat species are resistant to acute oxidative challenge, both in vivo and in vitro, suggesting fundamental differences in how cells and macromolecules in bats deal with oxidative challenge. This is consistent with previous reports that cells from M. lucifigus are resistant to cell death caused by some forms of oxidative stress (23) and show enhanced DNA repair capacity following γ-irradiation (6). In addition, cells from parti-colored bats (Vespertilio murinus) also appear oxidation resistant; these cells were relatively resistant to growth crisis, the general progression to cell senescence caused by oxygen-dependent accumulation of mutations and chromosomal rearrangements characteristic of mouse cells grown under 20% O2 concentrations (38, 39). However, others found no relation between in vitro cell crisis and species longevity using cells from several mammalian species (40).
The low level of protein oxidation in young T. brasiliensis (and to some degree, in M. velifer) is even more surprising considering the high metabolic demands of bats. During hibernation, the heart rate of bats may be as low as 10 beats/min, but during flight it may be as high as 1000 beats/min (41). The expenditure of energy during flight is associated with bursts of high levels of acute oxidative stress that could presumably contribute to carbonyl formation, as well as other forms of oxidation. However, bat mitochondria do seem to produce less H2O2 in vitro than do mitochondria from shorter-lived species (22). In addition, several bat species have a heterogeneous compliment of mitochondrial DNA, which has been suggested to represent selection for mitochondrial profiles that minimize free radical production (41). These findings are similar to those from another long-lived group of flying animals; birds generally live much longer than similarly sized, nonvolant mammal species, yet have relatively high metabolism relative to those same species (42). The sparse data using bird species to test mechanisms of oxidative stress also suggest that, like bats, birds have evolved efficient means to reduce production of free radicals from mitochondria (34, 43) and show no clear relation between circulating antioxidant levels and survival rate (44), and that bird-derived cells are resistant to oxidative stress relative to those from mice (45, 46). Evolutionary theories of aging have predicted that birds and bats are long lived in part due to their ability to fly and thus reduce their extrinsic mortality; that is, there is lower risk of predation on flying animals. The similarities between bats and birds in their oxidative profiles suggest perhaps that the demands of flight also coordinately up-regulate pathways that play a role in slowing the aging process. It will be of interest to explore whether patterns of protein homeostasis in birds are similar to those we show for bats in this study.
In general, data from bat species in our study show a greater similarity to each other than do the comparisons of data from each bat to mice. While our main assertion is that these data explain the longevity differences between species, they may also be explained by the fact that closely related species, such as T. brasiliensis and M. velifer, are more likely to share life history features due to their recent common ancestry than they are to share those features with more distantly related species such as mice. Our analysis using each species as a separate data point may not accurately assess the degree to which evolution has resulted in correlated change between longevity and other variables; thus, the long evolutionary distance between bats and mice could have confounded results from some of our experiments. Phylogeny might also have an effect on the actual experiments, because most molecular biology and biochemistry techniques have been optimized on human or murine samples. Experiments utilizing functional activity (such as proteasome activity) or the formation of particular biological products (such as protein carbonyls or BisANS binding) are unlikely to be affected by phylogeny; however, it is possible that ubiquitin in bats is less well-recognized than that in mouse by the polyclonal antibody used in our assays. While plausible, this seems relatively unlikely, because the sequence of ubiquitin differs only 2–3 amino acids in sequence across invertebrate and vertebrate classes (47). However, it must also be mentioned that ubiquitin-labeling of proteins is used by the cell for other purposes than marking for degradation; it is possible that the difference we see between species could represent different subsets of proteins marked by post-translation modification for these purposes and not for protein removal.
It is interesting that we found reduced 20S proteasome chymotrypsin-like activity in liver samples from both bat species compared to samples from mice, even though 20S proteasome levels appeared equal across species (Fig. 5). The mechanisms behind this are unclear, though the low protein ubiquitination we saw in bats could suggest less need for ubiquitin-linked protein removal in these species. Consistent with this finding is a previous report showing that calpain activity was 5- to 7-fold lower in brains from T. brasiliensis and the pallid bat (Antrozous pallidus) compared to the activity in mice (48). Calpains are calcium-dependent cysteine proteases that degrade proteins associated with the cell cytoskeleton and have been implicated in degenerative processes in muscles and neurons. Together, these findings suggest that protein degradation may be relatively low in general in bats, possibly because bats are able to maintain their protein structure and function better than shorter-lived animals, thereby reducing demand for removal of damaged and misfolded proteins. Our data showing that protein homogenates from bats are resistant to urea-induced protein unfolding are consistent with the idea that bats have enhanced mechanisms of protein homeostasis. The mechanisms by which bat proteins are better maintained in their folded states still needs to be determined; it may be that bats show high expression of protein chaperones such as heat shock proteins or differences in the cellular matrix that may better buffer against potentially damaging stresses. Alternatively, it may be that bats have evolved proteins with more stable conformation, or express a subset of particularly stable proteins that are expressed at lower levels, or not at all, in mice. It might be argued that enhanced maintenance of proteins, rather than removal of damaged proteins, may be one of the potential underlying mechanisms for maintenance of normal physiology of bats even at their extreme lifespans relative to mice.
Much can be learned about the mammalian aging process by studying how treatments such as calorie restriction or genetic mutations can alter the aging rate of a single species such as the mouse. Studies involving comparison between species with different aging rates may clarify the role specific molecular mechanisms play on the generalities of aging throughout animal species. For instance, naked mole rats live longer than both bat species tested herein, yet exhibit high levels of oxidative damage relative to mice, unlike what we have shown here for T. brasiliensis and M. velifer (16,17,18). However, unlike short-lived animals, naked mole rats do not appear to accumulate oxidative damage (18, 19) and tend to be resistant to some forms of oxidative stress (20, 21), much like what has been found for multiple bat species, both here and elsewhere (6, 23). Thus, some molecular patterns, such as oxidative stress resistance or enhanced protein homeostasis, may be necessary for long mammalian longevity, while others, such as the steady-state oxidation levels, may have less effect on the determination of lifespan. Testing whether patterns exist in the regulation of aging processes across a broad phylogenetic spectrum of species may yield valuable insight into the generalities of aging in nature and utilization of animals with extreme longevity, such as bat species, may help us better understand the physiological, biochemical, and molecular process required for healthy aging. Our data herein support the hypothesis that one of the mechanisms by which nature can generate species with remarkable longevity may be through modulation of the processes responsible for maintaining proper protein structure and function.
Acknowledgments
The authors thank Bertran Friguet (Université Paris, Paris, France) for providing 20S proteasome antibody. We also thank Tom Kunz and Louise Allen for help in obtaining our bat samples. This work was supported by K07 AG025063 04 (A.R.C., A.R., S.N.A.); NIH training grant T32 AG021890-05 (A.B.S.); and NIH grants AG23843, R37 AG26557 (A.R.), and AG022873 (S.N.A.).
References
- Finch C E. Chicago: University of Chicago Press; Longevity, Senescence and the Genome. 1990 [Google Scholar]
- George J C, Bada J, Zeh J, Scott L, Brown S E, O'Hara T, Suydam R. Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Can J Zool. 1999;77:571–580. [Google Scholar]
- Austad S N, Fischer K E. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol A Biol Sci Med Sci. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Wilkinson G S, South J M. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Jürgens K D, Prothero J. Scaling of maximal lifespan in bats. Comp Biochem Physiol. 1987;88A:361–367. doi: 10.1016/0300-9629(87)90498-1. [DOI] [PubMed] [Google Scholar]
- Podlutsky A J, Khritankov A M, Ovodov N D, Austad S N. A new field record for bat longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1366–1368. doi: 10.1093/gerona/60.11.1366. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Glickman M H, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Herczenik E, Gebbink M F B G. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22:2115–2133. doi: 10.1096/fj.07-099671. [DOI] [PubMed] [Google Scholar]
- Tolmasoff J M, Ono T, Cutler R G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol B Biochem System Environ Physiol. 1998;168:149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- Sohal R S, Sohal B H, Brunk U T. Relationship between antioxidant defenses and longevity in different mammalian species. Mech Ageing Dev. 1990;53:217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sohal R S. Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species. Exp Gerontol. 1996;31:365–372. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Boulton M E, Kirkwood T B. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor T P, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech Ageing Dev. 2005;126:1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor T P, Qi W, DeWaal E M, Pierce A, Chaudhuri A R, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z I. Vascular aging in the longest-living rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- Salmon A B, Sadighi Akha A, Buffenstein R, Miller R A. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, UV light, and ER stress. J Gerontol A Biol Sci Med Sci. 2008;63:232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2· and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291:H2698–H2704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni A K. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech Ageing Dev. 2004;125:11–20. doi: 10.1016/j.mad.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Harper J M, Salmon A B, Leiser S F, Galecki A T, Miller R A. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho D W, Althoff S L, Dafrè A L, Boveris A. Antioxidant defenses, longevity and ecophysiology of South American bats. Comparative Biochem Physiol C Toxicol Pharmacol. 2007;146:214–220. doi: 10.1016/j.cbpc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Miller R A, Harper J M, Dysko R C, Durkee S J, Austad S N. Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med. 2002;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A R, de Waal E M, Pierce A, Van Remmen H, Ward W F, Richardson A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Pierce A, deWaal E, Van Remmen H, Richardson A, Chaudhuri A. A novel approach for screening the proteome for changes in protein conformation. Biochemistry. 2006;45:3077–3085. doi: 10.1021/bi052031i. [DOI] [PubMed] [Google Scholar]
- Bulteau A L, Szweda L I, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304. doi: 10.1006/abbi.2001.2663. [DOI] [PubMed] [Google Scholar]
- Friedberg E D, Walker G, Siede W, Wood R, Schultz R, Ellenberger T. Washington D.C.: ASM Press; DNA Repair and Mutagenesis. (2nd ed.) 2006 [Google Scholar]
- Murakami S, Salmon A, Miller R A. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Salmon A B, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller R A. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Richardson A, Liu F, Adamo M L, Remmen H V, Nelson J F. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Hsieh-Wilson L. A molecular switchboard-covalent modifications to proteins and their impact on transcription. Org Biomol Chem. 2004;2:1–7. doi: 10.1039/b312466e. [DOI] [PubMed] [Google Scholar]
- Barja G, Cadenas S, Rojas C, Perez-Campo R, Lopez-Torres M. Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Radic Res. 1994;21:317–327. doi: 10.3109/10715769409056584. [DOI] [PubMed] [Google Scholar]
- Lambert A J, Boysen H M, Buckingham J A, Yang T, Podlutsky A, Austad S N, Kunz T H, Buffenstein R, Brand M D. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Davies K J A. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Rohme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc Natl Acad Sci U S A. 1981;78:5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuttil R A, Rubio M, Dolle M E, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–294. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Lorenzini A, Tresini M, Austad S N, Cristofalo V J. Cellular replicative capacity correlates primarily with species body mass not longevity. Mech Ageing Dev. 2005;126:1130–1133. doi: 10.1016/j.mad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni A, Austad S. Ageing studies on bats: a review. Biogerontology. 2004;5:211–222. doi: 10.1023/B:BGEN.0000038022.65024.d8. [DOI] [PubMed] [Google Scholar]
- Holmes D J, Ottinger M A. Birds as long-lived animal models for the study of aging. Exp Gerontol. 2003;38:1365–1375. doi: 10.1016/j.exger.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech Ageing Dev. 1998;103:133–146. doi: 10.1016/s0047-6374(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Cohen A, McGraw K, Wiersma P, Williams J, Robinson W, Robinson T, Brawn J, Ricklefs R. Interspecific associations between circulating antioxidant levels and life history variation in birds. Am Nat. 2008;172:178–193. doi: 10.1086/589456. [DOI] [PubMed] [Google Scholar]
- Ogburn C E, Austad S N, Holmes D J, Kiklevich J V, Gollahon K, Rabinovitch P S, Martin G M. Cultured renal epithelial cells from birds and mice: Enhanced resistance of avian cells to oxidative stress and DNA damage. J Gerontol A Biol Sci Med Sci. 1998;53:B287–B292. doi: 10.1093/gerona/53a.4.b287. [DOI] [PubMed] [Google Scholar]
- Ogburn C E, Carlberg K, Ottinger M A, Holmes D J, Martin G M, Austad S N. Exceptional cellular resistance to oxidative damage in long-lived birds requires active gene expression. J Gerontol A Biol Sci Med Sci. 2001;56:B468–B474. doi: 10.1093/gerona/56.11.b468. [DOI] [PubMed] [Google Scholar]
- Wilkinson K D. Roles of ubiquitinylation in proteolysis and cellular regulation. Annu Rev Nutr. 1995;15:161–189. doi: 10.1146/annurev.nu.15.070195.001113. [DOI] [PubMed] [Google Scholar]
- Baudry M, Dubrin R, Beasley L, Leon M, Lynch G. Low levels of calpain activity in Chiroptera brain: implications for mechanisms of aging. Neurobiol Aging. 1986;7:255–258. doi: 10.1016/0197-4580(86)90004-7. [DOI] [PubMed] [Google Scholar]