Abstract
Protein S (PS) is a cofactor for activated protein C (APC), which inactivates coagulation factors (F) Va and VIIIa. Deficiency of protein C or PS is associated with risk of thrombosis. We found that PS also has APC-independent anticoagulant activity (PS-direct) and directly inhibits thrombin generated by FXa/FVa (prothrombinase complex). Here we report that PS contains Zn2+ that is required for PS-direct and that is lost during certain purification procedures. Immunoaffinity-purified PS contained 1.4 ± 0.6 Zn2+/mol, whereas MonoQ-purified and commercial PS contained 0.15 ± 0.15 Zn2+/mol. This may explain the controversy regarding the validity of PS-direct. Zn2+ content correlated positively with PS-direct in prothrombinase assays and clotting assays, but APC-cofactor activity of PS was independent of Zn2+ content. PS-direct and Zn2+ were restored to inactive PS under mildly denaturing conditions. Conversely, o-phenanthroline reversibly impaired the PS-direct of active PS. Zn2+-containing PS bound FXa more efficiently (Kdapp=9.3 nM) than Zn2+-deficient PS (Kdapp=110 nM). PS bound TFPI efficiently, independently of Zn2+ content (Kdapp=21 nM). Antibodies that block PS-direct preferentially recognized Zn2+-containing PS, suggesting conformation differences at or near the interface of 2 laminin G-like domains near the PS C terminus. Thus, Zn2+ is required for PS-direct and efficient FXa binding and may play a role in stabilizing PS conformation.—Heeb, M. J., Prashun, D., Griffin, J. H., Bouma, B. N. Plasma protein S contains zinc essential for efficient activated protein C-independent anticoagulant activity and binding to factor Xa, but not for efficient binding to tissue factor pathway inhibitor.
Keywords: blood coagulation regulation, zinc metalloprotein, vitamin K–dependent protein
Vitamin K-dependent protein S (PS) is an anticoagulant plasma glycoprotein whose heterozygous deficiency is associated with increased risk of venous thrombosis and stroke (1, 2), and whose homozygous deficiency can lead to life-threatening purpura fulminans at birth (3). PS is best known as a cofactor for activated protein C (APC) during proteolytic inactivation of procoagulant factor (F) Va and FVIIIa (4). PS also has anticoagulant functions independent of APC (PS-direct), via binding and inhibition of FXa and FVa (5,6,7). PS has also been reported to serve as a cofactor for tissue factor pathway inhibitor (TFPI) during inhibition of extrinsic FXase (8). Purified PS contains monomeric and multimeric forms, and it has been suggested that only artifactual multimeric forms of purified PS have PS-direct, measured as ability to inhibit the prothrombinase activity of FXa/FVa, by virtue of higher affinity for phospholipids (9). Yet we showed that when immunoaffinity-purified PS is separated into monomers and multimers, all forms have similar and efficient PS-direct and affinity for phospholipids on the basis of mass, comparable to the PS-direct of the PS in plasma (10). We also showed that plasma naturally contains monomeric and multimeric PS with similar PS-direct (11). Immunoaffinity-purified PS had antithrombotic activity in vivo in a baboon thrombosis model, even in the presence of blocking antibodies to APC, whereas a conventionally purified PS had weak activity (12).
Immunoaffinity-purified PS from our laboratory had good PS-direct, measured as inhibition of the prothrombinase activity of FVa/FXa in the presence of saturating phospholipids, whereas conventionally purified PS from several other laboratories had poor PS-direct, which leads to skepticism regarding the existence or importance of PS-direct. Yet at least 3 labs have shown that unpurified PS in plasma has PS-direct, supporting the validity of PS-direct (13,14,15). We therefore reviewed differences in purification methods. Conventional purification often includes anion exchange chromatography with MonoQ in the presence of EDTA, followed by MonoQ with a Ca2+ gradient (16). We hypothesized that PS may contain a divalent metal ion other than Ca2+ that might be removed by MonoQ in the presence of EDTA, or by elution of barium-adsorbed vitamin K-dependent factors from plasma with high concentrations of EDTA (17). Our procedure involves elution of vitamin K-dependent factors from barium citrate pellets with ammonium sulfate rather than EDTA (18), before immunoaffinity purification that produces PS with good PS-direct (6).
PS has ∼10 divalent metal ion binding sites that are thought to be occupied by Ca2+ (19). Most Ca2+ is located in the γ-carboxyglutamic acid domain at the N terminus, where it contributes to exposure of hydrophobic residues that mediate membrane binding. Three high-affinity Ca2+ sites are thought to be located in epidermal growth factor (EGF) domains 2–4 of PS (20, 21). Based on the crystal structure of the laminin G-1 (LG-1) domain of sex hormone binding globulin (SHBG) (22), a Ca2+ binding site was postulated to reside in the LG-1 domain of the PS SHBG-like domain (23). In the crystal structure of the homologue Gas6, the same residues of LG-1 and an additional residue in the LG-2 domain form a Ca2+ binding site that is postulated to strengthen the arrangement of the LG domain pair (24). There is scant evidence, however, that all of the metal ion binding sites in PS or in other vitamin K-dependent proteins are naturally occupied by Ca2+. We set about to identify any non-Ca2+ divalent metal ions in PS and to correlate them with PS-direct.
MATERIALS AND METHODS
Proteins and reagents
PS and protein C were prepared from citrated plasma by barium adsorbtion, then elution with 33% saturated ammonium sulfate (18) before further purification.
For immunoaffinity purification of PS, the barium eluate was dialyzed against Tris-buffered saline (TBS; 0.05 M Tris and 0.1 M NaCl, pH 7.4), then TBS-1 mM sodium citrate. Complexes of PS with C4b-binding protein were removed by precipitation with 3.75% (final concentration) polyethylene glycol. Crude PS was then chromatographed on a Sepharose column coupled with PS monoclonal antibody S7 and eluted with either glycine, pH 2.7, or 6 M urea. Fractions were adjusted to neutral pH and subjected to SDS-PAGE and ELISA. Selected fractions were pooled, concentrated by membrane filtration, and dialyzed twice against Hepes-buffered saline, pH 7.4 (HBS).
For conventional purification, two methods were used, each beginning with barium eluate. The first was a variation of a commonly used method (16). It employed dialysis and MonoQ chromatography (quaternary amine resin from Amersham, Uppsala, Sweden) in the presence of 1 mM EDTA, then MonoQ chromatography with a Ca2+ gradient. The second method was novel and employed hexyl agarose (ProMetic, Isle of Mann, UK) chromatography of the barium eluate, with a wash of 25% saturated ammonium sulfate in HBS 1:5 and a linear gradient beginning with the wash solution and ending with HBS 1:5. A second chromatography step employed heparin Sepharose (Amersham) and a gradient beginning with 0.05 M MES and 0.01 M NaCl, pH 6.5, and ending in the same solution containing 0.5 M NaCl. This second method yielded PS that was 50% pure and avoided mild denaturants or anion exchange chromatography. The other methods used yielded PS that was > 95% homogenous as judged by SDS-PAGE.
Several lots of conventionally purified PS, as well as FXa, prothrombin, and fibrinogen, were from Enzyme Research Laboratories (South Bend, IN, USA); tissue factor (TF; Innovin) was from Dade (Marburg, Germany). A PS sample was kindly donated by Dr. Brian Grinnell (Eli Lily, Indianapolis, IN, USA) and was purified by his method (16). FV was prepared/activated as described (5). Thrombin substrate PefaTH was obtained from Centerchem (Norwalk, CT, USA), and full-length TFPI activity standard was obtained from American Diagnostica (Stamford, CT, USA). Phospholipid vesicles, 80% phosphatidyl choline/20% phosphatidyl serine, were prepared by sonication (6). Sheep anti-protein C was a gift from Dr. Peter Schwarz (Baxter, Vienna, Austria) and was affinity purified. All metal ions were used in the chloride form. Pooled normal human plasma (NHP) and PS-immunodepleted plasma (PSdP) were from Affinity Biologicals (Hamilton, ON, Canada).
Rabbit neutralizing antibodies against TFPI was a kind gift from Prof. Samuel I. Rapaport, Dr. L. Vijay Mohan Rao, and Dr. Bonnie Warn-Cramer (formerly of the University of California at San Diego School of Medicine, San Diego, CA, USA). TFPI-depleted plasma (TFPIdP) was prepared by passing NHP over a column of sheep anti-TFPI (Affinity Biologicals) coupled to activated CNBr-Sepharose (Amersham). The first undiluted fraction eluted was frozen in small aliquots for plasma clotting assays. To ensure that no TFPI remained during clotting assays, the TFPIdP was incubated with rabbit neutralizing anti-TFPI antibodies for 4 min just before use. The concentration of antibodies was chosen on the basis of full neutralization of TFPI activity in plasma and of full neutralization of purified TFPI in extrinsic FXase assays (8).
Metal analysis
Before analysis, each PS was dialyzed 17 h against TBS containing 1 mM citrate to remove any loosely bound or contaminating metal ions. It was then dialyzed twice against TBS without citrate for a minimum of 5 h each time. PS antigen concentration was determined, and metals were quantified using inductively coupled plasma-optical emission spectrometry. Measurements were obtained on a Varian VISTA AX CCD simultaneous spectrometer (Varian, Inc., Palo Alto, CA, USA) equipped with a Teflon™ nebulizer and sample uptake tubing. Calibration curves were established using metal standards (Inorganic Ventures, Lakewood, NJ, USA). All standards and samples were spiked with a yttrium internal standard at a final concentration of 10 ppm to normalize any irregularities in nebulization. Metal concentrations were determined by monitoring multiple wavelengths (2–4 for Ca, 6 for Mg, and 4–6 for transition metals) and averaging the resulting values. Concentrations were corrected for any trace amounts of metals in the final dialysis buffer and compared to [PS] to yield metal loadings.
Incorporation of Zn2+ into PS with poor PS-direct
MonoQ-purified PS was placed in a series of dialysis bags and treated overnight in TBS containing 1 g/L of the chelator, Chelex-100 (Bio-Rad, Hercules, CA, USA). The bags were then moved for 4 h to solutions containing 10 μM Zn2+ in the following: 0.1 M glycine and 0.05 M NaCl, pH 2.7; TBS adjusted to pH 8; 6 M urea in TBS; or TBS containing 1 mM Mg2+ and 2 mM Ca2+. They were then moved to TBS for 4 h, then to TBS with 1 mM citrate for 17 h, followed by 2 dialyses in TBS. PS was removed from the bags, assayed for antigen level, and tested for PS-direct.
On a larger scale, the conditions of 6 M urea or pH 2.7 were chosen for incorporation of Zn2+ at 20 μM. When low pH was used, pH was brought to neutral by addition of 1 M Tris base, and serial dialyses were as described above. In parallel, untreated PS was dialyzed without Zn2+ and without denaturant. Antigen level, PS-direct, and Zn2+ were measured.
Plasma clotting assays
Endogenous thrombin potential (ETP) assays were performed by mixing 50 μl of NHP, PSdP, or TFPIdP with 11 μg/ml neutralizing antibodies to APC with or without added PS at 37°C for 5 min. TF, phospholipids, and Ca2+ (10 μl) were added to 0.1 pM, 10 μM, and 15 mM, followed by 40 μl of warm fluorogenic thrombin substrate ZGGR-aminomethylcoumarin (Bachem, Torrance, CA, USA). Thrombin generation was measured as the first derivative of the fluorescence signal over time.
Surface plasmon resonance (SPR)
A CM5 chip (Biacore, Piscataway, NJ, USA) was coupled with high-affinity PS monoclonal antibody S7 or with an irrelevant monoclonal antibody, then blocked according to the manufacturer’s instructions. Three hundred response units of PS was captured on the chip in HBS containing 2 mM CaCl2, 1 mM MgCl2, 0.1 M NaCl, and 0.025% BSA, with or without 5 μM phospholipid vesicles. Dissociation rate was negligible. Either TFPI or FXa in a series of concentrations in the same buffer was bound to the PS, then dissociated with the buffer. Binding from a blank channel treated the same way without antibody or from a blank channel containing irrelevant antibody was subtracted. Little difference was seen in results using the two types of blank. Global analysis of binding sensograms of the series of concentrations of FXa or TFPI was performed using Biacore 3000 software. Peak binding in response units in each case was separately plotted.
Recognition of various PS preparations by antibodies against PSP14 (PS residues 621–635)
Rabbit anti-PSP14 antibodies were prepared and immunoaffinity purified as previously described (25). Several PS preparations were coated at variable concentrations to the wells of microtiter plates, followed by blocking of the wells with I-block (Tropix, Bedford, MA, USA). Anti-PSP14 (2 μg/ml) was incubated in the wells for 20 min, followed by washing. Bound antibody was detected with biotin-anti-rabbit IgG, followed by streptavidin-peroxidase (Pierce, Rockford, IL, USA), then o-phenylenediamine. After sufficient color development, the reaction was stopped with 1 M HCl, and product was measured at 490 nm in a plate reader.
Other assays
Prothrombinase assays for PS-direct were performed as described previously (6), using 20 pM FVa; 0.5 nM FXa; 25 μM phospholipids; 0.3 μM prothrombin; 0.5% BSA Hepes-buffered saline, pH 7.4; and 5 mM CaCl2. Briefly, FXa, FVa, and phospholipids were preincubated 10 min with PS before addition of prothrombin. Aliquots were taken over time and quenched in buffer containing EDTA in the wells of microtiter plates. Chromogenic substrate was added, and the rate of thrombin generation was determined using a kinetic plate reader.
APC cofactor activity of purified PS was measured as follows. Mixtures of PSdP and NHP corresponding to 0, 0.825, 1.65, and 3.33 μg/ml of free PS (15 μl) were mixed with 10 μl of 9 mg/ml fibrinogen, 50 μl of 0.2 μg/ml APC, 50 μM phospholipids, and 50 μl of 0.8 nM FVa in HBS with 0.5% BSA, and preincubated at 37°C for 2 min. Then 25 μl of warm 25 mM CaCl2 was added, and clotting time was measured. Purified PS was used to reconstitute PSdP, and clotting times were compared to those of standard mixtures of PSdP and NHP.
PS antigen was measured by ELISA (14). For immunoblots, PS was transferred from 8% native-PAGE gels (Invitrogen, Carlsbad, CA, USA) to nitrocellulose membranes (Bio-Rad) and was detected with peroxidase-coupled rabbit anti-PS (Dako, Carpenteria, CA, USA) and chemiluminescent substrate.
Statistical comparisons were made using a 2-tailed t test with GraphPad software (GraphPad, San Diego, CA, USA). A value of P < 0.05 was considered significant. Curve fitting was also performed with GraphPad.
RESULTS
Correlation of Zn2+ content with PS-direct in purified component assays
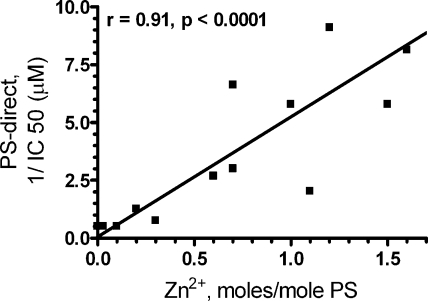
Immunoaffinity-purified PS had good PS-direct, measured as inhibition of prothrombinase activity, whereas MonoQ-purified and commercial PS had weak PS-direct. PS-direct was measured in saturating phospholipids to reflect direct inhibition of FXa/FVa rather than competition for phospholipids. We subjected a number of immunoaffinity-purified PS preparations and conventionally purified PS preparations to divalent metal analysis. PS contained Zn2+, Mg2+, and Ca2+ ions (Table 1), but not Cu2+, Mn2+, Fe2+, Co2+, Ni2+, or Ba2+. Immunoaffinity-purified PS contained significantly more Zn2+ than MonoQ-purified and commercial PS (P=0.002), whereas Mg2+ and Ca2+ did not significantly vary by preparation method (P=0.56 and 0.35, respectively). PS purified by the hexyl agarose method contained significant levels of Zn2+. Zn2+ content of all PS preparations correlated with PS-direct, measured as ability to inhibit thrombin generation by FXa and FVa (r=0.91, P<0.0001; Fig. 1). The data suggest that each PS molecule naturally contains 1 atom of Zn2+ and 1 atom of Mg2+ in addition to ∼8 mol of Ca2+, and that Zn2+ is lost during some purification methods.
TABLE 1.
Metal content and PS-direct of PS preparations purified by different methods
| Purification method (from barium-adsorbed plasma) | n | Zn/mol | Mg/mol | Ca/mol | IC50 (μM)a | PS-direct (1/IC50) |
|---|---|---|---|---|---|---|
| Immunoaffinity methods | 7 | 1.4 ± 0.6 | 0.9 ± 0.6 | 8.5 ± 2.2 | 0.13 ± 0.06 | 7.7 |
| MonoQ/EDTA + monoQ/Ca2+b | 2 | 0.25 ± 0.05 | 0.7 ± 0.1 | 7.5 ± 0.3 | ≥2c | ≤0.5 |
| Commercial, unspecified | 3 | 0.1 ± 0.1 | 0.6 ± 0.3 | 7.0 ± 2.2 | >2 | <0.5 |
| Hexyl agarose, heparin sepharose | 4 | 0.8 ± 0.5 | 0.9 ± 0.2 | 7.8 ± 1.9 | 0.14 ± 0.04 | 7.1 |
[PS] required to inhibit by 50% the rate of thrombin generated in the standardized prothrombinase assay.
MonoQ chromatography: first with a salt gradient in the presence of EDTA; second, with a gradient of CaCl2. Second preparation employed a similar Fast Flow Q resin, with EDTA, then CaCl2 (16).
Highest [PS] tested was 2 μM, which is 6-fold higher than the plasma [PS].
Figure 1.
PS-direct correlates with Zn2+ content. PS-direct of each PS is plotted as a function of its Zn2+ content. 1/IC50, 1/[PS] required to inhibit the rate of thrombin generation by 50% in the standardized prothrombinase assay. Typical initial rate of thrombin generation in the control without PS was 7 nM/min.
Correlation of Zn2+ content with PS-direct in plasma clotting assays
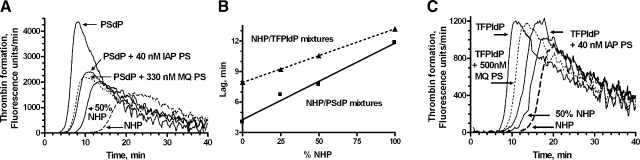
Using endogenous thrombin potential assays in reconstituted PSdP, Zn2+-containing immunoaffinity-purified PS had similar PS-direct as PS in NHP, while Zn2+-deficient commercial PS had 8-fold weaker activity, similar to differences in PS-direct in Table 1 (Fig. 2A), and confirming our previous results using a different plasma assay (10). Coagulation assays were performed in the presence of neutralizing anti-APC antibodies; therefore, the observed anticoagulant activity of PS was not due to its cofactor activity for APC. NHP that contained PS exhibited a lag of 11.8 min before measurable thrombin was generated, compared to a 3-fold shorter lag for PSdP (Fig. 2A). Peak thrombin generation was 2.8-fold greater for PSdP than for NHP, and area under the curve was 1.5-fold greater, but lag time was the parameter that demonstrated a linear response to dose of NHP mixed with PSdP (Fig. 2B). Addition of 40 nM of immunoaffinity-purified Zn2+-containing PS to PSdP resulted in a lag time similar to that obtained by addition of 330 nM conventionally purified commercial Zn2+-deficient PS, and similar to that of 25% NHP/75% PSdP. Thus, immunoaffinity-purified Zn2+-containing PS was ∼8-fold more potent than commercial Zn2+-deficient PS in down-regulating thrombin generation in ETP assays using PSdP. Neutralizing rabbit anti-PS antibodies (Dako) converted the thrombin generation profile of NHP to one similar to that of PSdP (data not shown).
Figure 2.
PS containing Zn2+ expresses PS-direct in plasma. A) Endogenous thrombin potential assays were performed as described in Materials and Methods. NHP (dashed curve) or PSdP or mixtures of the 2 plasmas were compared to PSdP supplemented with 2 doses each of immunoaffinity-purified PS (IAP PS) that contained Zn2+ and conventionally MonoQ-purified PS (MQ PS, dotted curve) that was Zn2+ deficient. For clarity, only 1 dose of each PS and 1 mixture of NHP and PSdP are shown. B) Linear response of lag time in the ETP assay for various mixtures of NHP/PSdP or NHP/TFPIdP. Standard curves were used to estimate the PS-direct of Zn2+-containing or Zn2+-deficient PS added to PSdP or TFPIdP. C) Similar experiment as in A, but using TFPIdP in place of PSdP. All results were confirmed on separate dates.
In similar experiments using TFPIdP in place of PSdP, Zn2+-containing PS also exerted an anticoagulant effect, whereas conventionally purified commercial Zn2+-deficient PS had little effect (Fig. 2C). Although PS may have TFPI-dependent effects, the data illustrated in Fig. 2 demonstrate that PS can have TFPI-independent activity in clotting plasma. It was previously shown that the PS-direct of our PS in purified component prothrombinase assays is unaffected by neutralizing antibodies to TFPI, and is therefore TFPI-independent in these assays (5, 11).
Enhanced PS-direct after Zn2+ incorporation
When MonoQ-purified PS with weak PS-direct was incubated in 10–1000 μM Zn2+, there was no gain of PS-direct. Since this PS could have been in a conformation unfavorable for binding Zn2+, it was treated with Chelex to remove divalent metal ions, followed by incubation with Zn2+ under several conditions of mild denaturation and renaturation that might be conducive to Zn2+ incorporation. Treatment with Zn2+ at low pH or in urea, followed by dialysis in neutral buffer, promoted enhanced PS-direct, whereas several other conditions did not (Fig. 3A). Experiments not shown revealed that Chelex treatment was not required for regain of PS-direct and that treatment with Zn2+ at pH 3.7, but not at pH 4.2, was sufficient for enhancement of PS-direct. In subsequent experiments MonoQ-purified PS was treated with Zn2+ in urea or at low pH, followed by dialysis. PS-direct increased >5-fold; 50% inhibition of thrombin generation by FXa/FVa was achieved near the plasma [PS], 0.33 μM (Fig. 3B). This approached the PS-direct of immunoaffinity-purified PS. Zn2+ content of 3 different MonoQ-purified or commercial PS treated with Zn2+ at low pH or in urea was measured. Zn2+ increased from < 0.1–0.3 atoms/mol to 0.6–1.4 atoms/mol, accompanied by increased PS-direct (Table 2). When Chelex-adsorbed urea was used to treat PS in the absence of Zn2+, no increase in Zn2+ or PS-direct was observed; thus, urea treatment alone did not activate PS.
Figure 3.
PS-direct is enhanced after Zn2+ treatment under mildly denaturing conditions. A) MonoQ-purified PS with poor PS-direct was treated with 10 μM Zn2+ under the conditions indicated (see Materials and Methods). After dialysis, PS was tested for ability to inhibit generation of thrombin from prothrombin by FXa/FVa. IC50(μM) was determined in each case. PS-direct was expressed as 1/IC50; 1/IC50 ≤ 0.5 was indicated as 0.5. B) Dose response of MonoQ-purified PS with poor PS-direct, with or without treatment with 10 μM Zn2+in the presence of 6 M urea or pH 2.7 buffer, followed by dialysis as in Materials and Methods. For comparison, PS-direct of typical IAP PS is also illustrated (dashed line). This experiment was repeated 4 times (Table 2).
TABLE 2.
Zn2+ incorporation and PS-direct of several PS preparations with low PS-direct
| PS preparation | Treatment | Zn2+(mol)
|
IC50(μM)
|
1/IC50
|
|||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||
| Commercial PS-A | pH 2.7/Zn2+ | 0.1 | 0.6 | >2a | 0.40 | <0.5 | 2.5 |
| Commercial PS-B | pH 2.7/Zn2+ | <0.1 | 0.7 | >2 | 0.33 | <0.5 | 3.0 |
| MQ/Ca-purified PSb | pH 2.7/Zn2+ | 0.3 | 1.2 | ≥2 | 0.11 | ≤0.5 | 9.1 |
| Commercial PS-B | 6 M urea/Zn2+ | <0.1 | 1.4 | >2 | 0.39 | <0.5 | 2.6 |
| Commercial PS-B | 6 M chelexed urea | <0.1 | <0.1 | >2 | >2 | <0.5 | <0.5 |
Highest [PS] tested was 2 μM, which is 6-fold higher than the plasma [PS].
PS purified using 2 MonoQ (MQ) steps (see Table 1).
Reversible loss/regain of PS-direct with loss/regain of Zn2+
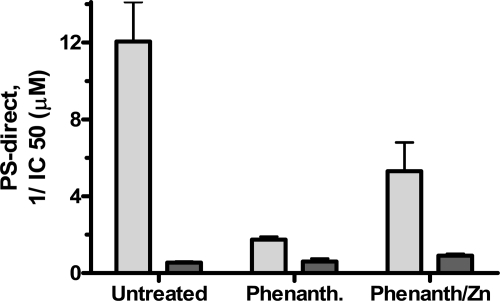
In experiments that were the reverse of those in Fig. 3, PS that contained Zn2+ and that had good PS-direct lost PS-direct when treated 10 min with the Zn2+ and Cu2+ chelator, o-phenanthroline (Fig. 4, lighter bars). Here ∼42% of PS-direct was regained on immediate dialysis against TBS-10 μM Zn2+ in the absence of low pH or urea, but not on dialysis against TBS alone. Thus, the potency of PS-direct correlated with Zn2+ content, and some of the o-phenanthroline-treated immunoaffinity-purified PS evidently remained at least temporarily in a conformation conducive to regain of Zn2+. Zn2+-deficient commercial PS was not significantly affected by o-phenanthroline or exogenous Zn2+ in the absence of low pH or urea treatment (Fig. 4, darker bars).
Figure 4.
PS-direct is lost or regained with loss or regain of Zn2+. PS that contained Zn2+ and had good PS-direct (lighter bars) and Zn2+-deficient PS with weak PS-direct (darker bars) were each treated for 10 min with 10 mM of the Zn2+-chelator, o-phenanthroline. Half of each treated PS was then dialyzed against TBS (middle set of bars), and the other half of each was dialyzed against TBS-10 μM Zn2+ (last set of bars). Each was then dialyzed twice against TBS. PS antigen level was determined, and several concentrations were tested for ability to inhibit generation of thrombin from prothrombin by FXa/FVa. Reciprocal of IC50 is indicated. Data from 2 experiments performed on different days were combined.
Effect of Zn2+ content on PS binding to FXa and TFPI
In experiments such as those in Fig. 3B, Zn2+ occupancy of PS was essential for efficient inhibition of prothrombinase activity. Binding of PS to FXa and to TFPI was also examined, particularly because PS was recently reported to enhance the activity of TFPI during inhibition of extrinsic FXase (8). FXa bound to immobilized Zn2+-containing PS with high affinity (Kdapp=13±3 nM) (Fig. 5A), which agreed well with our previous fluid phase binding data (Kdapp=17 nM) (6). In comparison, Zn2+-deficient PS bound poorly in the same experiments (Kdapp=92±10 nM; P<0.0001) (Fig. 5A). In SPR experiments, FXa also bound with high affinity to Zn2+-containing PS (Kdapp=9–13 nM), and with much lower affinity to Zn2+-deficient commercial PS (Kdapp=110–240 nM) (Fig. 5B). Binding of prothrombin to Zn2+-containing PS was too weak to calculate Kd.
Figure 5.
Binding of FXa to PS. A) Binding of FXa to Zn2+-containing PS (squares) or to Zn2+-deficient commercial PS (circles) immobilized on a plate at 2 μg/ml. Results were confirmed in 2 separate experiments, and results for Zn2+-containing PS confirm our earlier report (6). B) SPR binding experiments. See Materials and Methods for details. Peak binding from 5–6 sensograms obtained using different concentrations of FXa binding to Zn2+-containing PS (squares) or to commercial Zn2+-deficient PS (circles). Weak binding of prothrombin (FII, triangle) to Zn2+-containing PS is used as a control; consistent results for several higher concentrations of FII are not shown. Global fits of sensograms corresponding to these data are shown in Table 3.
In parallel experiments, TFPI bound to PS immobilized on microtiter plates with high affinity, regardless of Zn2+ content (Kdapp=8.8±1.2 vs. 9.0±1.4 nM; P=0.9) (Fig. 6A). TFPI also bound to PS with high affinity in SPR experiments, regardless of Zn2+ occupancy (Kdapp=21–28 nM) (Fig. 6B and Table 3). The association rate (kon) for FXa binding to Zn2+-containing PS was ∼6-fold higher than that for TFPI binding.
Figure 6.
Binding of TFPI to PS. A) Binding of TFPI to Zn2+-containing PS (squares) or to Zn2+-deficient commercial PS (circles) immobilized on a plate at 2 μg/ml. Results were confirmed in a separate experiment. B) SPR binding experiments. See Materials and Methods for details. Peak binding of 2 different concentrations of TFPI to Zn2+-containing PS and 3 different concentrations of TFPI to commercial Zn2+-deficient PS. Data for the 2 PS were indistinguishable and were combined in one graph. Global fit of sensograms corresponding to these data is shown in Table 3.
TABLE 3.
Global fit of SPR binding data
| PS type | FXa binding | TFPI binding |
|---|---|---|
| Zn2+-containing | 1.8 × 10−2/1.9 × 106 = 9.3 × 10−9 | 6.9 × 10−3/3.2 × 105 = 2.1 × 10−8 |
| Zn2+-deficient | 2.6 × 10−1/2.4 × 106 = 1.1 × 10−7 | 1.8 × 10−2/6.5 × 105 = 2.8 × 10−8 |
Calculated as koff (s−1)/kon (M−1s−1) = Kd (M). Calculations were made from simultaneous fit of 5 or more sensograms using Biacore software.
Suggested conformation difference in SHBG-like region of PS associated with Zn2+ content
The location of Zn2+ in PS is uncertain at this point. In most non-Zn2+-finger Zn2+ metalloproteins, such as carboxypeptidases, Zn2+ is coordinated with 2 His and 1 Glu/Asp residue (26,27,28). It is unlikely that a Zn2+ site could reside in the N-terminal 40% of PS that is homologous to other vitamin K-dependent proteins, because only one His residue is located in that part of the molecule (in EGF2), and at present there is no indication that EGF2 is in the close vicinity of the C-terminal portion of PS. Ten His residues are located in the SHBG-like region in the C terminus of PS; thus, the Zn2+ site is likely to reside in this region. In earlier studies, we reported that peptide PSP14, representing PS residues 621–635 in the SHBG-like region, mimicked the PS ability to inhibit prothrombinase activity (25). Antibodies against this peptide also blocked inhibition of prothrombinase by PS (25). The ability of anti-PSP14 antibodies to recognize Zn2+-containing vs. Zn2+-deficient PS was tested (Fig. 7). Anti-PSP14 antibodies only weakly recognized commercial (ERL) PS that was shown to be nearly devoid of Zn2+, but they recognized the same batch of commercial PS after it was activated with urea and Zn2+ and was shown to have incorporated Zn2+. The antibodies also recognized with similar efficiency immunoaffinity-purified PS that contained Zn2+. These data suggest that PS that contains Zn2+ has a different conformation than PS that is devoid of Zn2+.
Figure 7.
Antibodies against PS residues 621–635 (PSP14) preferentially recognize PS that contains Zn2+. Several PS preparations were coated to the wells of a microtiter plate at the variable concentrations indicated on the x axis. Immunoaffinity-purified antibodies against PSP14 were incubated in the wells, and bound antibodies were detected with biotinylated secondary antibodies as described in Materials and Methods. ERL PS, untreated Zn2+-deficient commercial PS; urea/Zn2+ ERL PS, same PS after treatment with Zn2+ in urea as in Fig. 3; IAP PS, Zn2+-containing immunoaffinity-purified PS.
Lack of correlation of Zn2+ content with multimeric forms of PS
Our previous studies showed that the multimeric forms and monomeric forms in immunoaffinity-purified PS had similar PS-direct, that monomeric unpurified recombinant PS in conditioned medium could reconstitute PSdP to the level of PS-direct of normal plasma, and that PS in plasma exists naturally in monomeric, dimeric, and higher-order forms with similar PS-direct (10, 11). Here Zn2+-containing PS and Zn2+-deficient PS were examined for multimeric forms on native-PAGE. The Zn2+-deficient conventionally purified commercial PS used in the present studies was largely monomeric. When activated with Zn2+ in urea, it remained largely monomeric, and when activated with Zn2+ at low pH, multimers were induced (Fig. 8). The two types of activated preparations had similar PS-direct (Fig. 3B). PS partially purified conventionally using hexyl agarose and without use of anion exchange resins or mild denaturing methods had PS-direct, contained 0.65 atoms Zn2+/mol, and was primarily monomeric. Both PS preparations that contain substantial proportions of multimers and PS preparations that contain 0–5% multimeric forms may contain Zn2+ and have PS-direct, or they may not. Thus, Zn2+ content and PS-direct do not depend on or affect multimeric forms.
Figure 8.
Zn2+ content and PS-direct do not correlate with multimeric forms of PS. Native-PAGE was used to examine monomeric and multimeric forms of PS. Commercial PS that was >95% monomeric and Zn2+-deficient was untreated (lanes 2, 6); activated with Zn2+ in 6 M urea (lane 1) or with Zn2+ at pH 2.7 (lanes 4, 8); or taken through dialysis steps without Zn2+ treatment (lanes 3, 7, from 2 different experiments). PS partially purified by conventional chromatography steps not including MonoQ contained Zn2+ and had PS-direct (lane 5). Monomers (M), dimers (D), and trimers (T) indicated at right (9). Zn (+), Zn2+ content ≥ 0.7 atoms/mol PS; PS-direct (+), PS-direct ≥ 2.6 (IC50≤0.39 μM). Two experiments from different days are shown.
Lack of correlation of Zn2+ content with APC cofactor activity
APC cofactor assay of the various purified PS preparations did not correlate with Zn2+ content or with PS-direct. Zn2+-deficient commercial PS had cofactor activity of ≥100%; Zn2+-deficient PS purified using MonoQ steps had 89% cofactor activity; and several immunoaffinity-purified PS had cofactor activity of 84 to ≥ 100%. Conditions for denaturation with pH 2.7, as used to incorporate Zn2+ into PS with poor PS-direct in Table 2 and Fig. 3, are suggested to be relatively mild for PS, because immunoaffinity-purified PS prepared with pH 2.7 elution had good APC cofactor activity.
DISCUSSION
Controversy regarding PS-direct rooted in loss of essential Zn2+ ion
Here we extend our finding that immunoaffinity-purified PS approximates the PS-direct of PS in plasma, whereas MonoQ-purified and commercial PS have weak activity in plasma (10), and we show that PS contains Zn2+ that is essential for PS-direct. Whether monomeric or multimeric, PS inhibits prothrombinase activity and plasma clotting assays in a manner dependent on its Zn2+ content. The unrecognized loss of essential Zn2+ during certain conventional purification procedures has contributed to controversy about whether PS inhibits prothrombinase activity to a meaningful degree.
Not all conventional methods were deleterious in our studies, however. Vitamin K-dependent factors are typically enriched from plasma in a first purification step by adsorption to barium citrate. They are then eluted with either high concentrations of EDTA (≥200 mM) or with ammonium sulfate. The former elution method probably results in loss of most divalent metal ions. The latter elution method was used to prepare the affinity-purified and hexyl agarose-purified PS analyzed in this study and did not result in loss of Zn2+. Even when ammonium sulfate elution was used, MonoQ chromatography in the presence of 1–4 mM EDTA resulted in loss of Zn2+ from PS in these studies, with consequent loss of PS-direct. Possibly other divalent ions in other vitamin K-dependent factors or in other proteins are lost by these particular conventional methods. Although Ca2+ and Mg2+ might be readily replaced from traces of the ions in buffers, Zn2+ in PS is lost and is not replaced from buffers, or from Zn2+ added to buffers. Here 1 mM EDTA alone did not cause loss of Zn2+ from PS, because immunoaffinity chromatography in the presence of 1 mM EDTA did not result in loss of Zn2+. Possibly MonoQ resin attracted the acidic residue coordinated with Zn2+, resulting in weaker binding of the Zn2+ and its subsequent chelation by EDTA. The common use of MonoQ chromatography, at least when EDTA was used, may have obscured the roles of divalent metal ions in other proteins.
Stoichiometry for Zn2+ and Mg2+ in PS
Measurements indicated that PS naturally contains 1 Zn2+/molecule, but since a mean of 1.4 atoms of Zn2+ was obtained for immunoaffinity-purified PS, we cannot exclude that there may be 2 Zn2+/molecule. (This mean would be lower if hexyl agarose-purified PS were included.) We used dialysis against citrate, followed by dialyses without citrate to minimize loosely bound Zn2+, as was reported for carboxypeptidases (29).
Our studies revealed that PS contains 1 atom of Mg2+, although the function of Mg2+ was not revealed. In the crystal structures of the gla domains of FVIIa and FIX, Mg2+ affected conformation, and these domains contained 3 Mg2+ and 5 Ca2+ (30, 31), not the 8 Ca2+ that were assumed to be present. Mg2+ also accelerated activation of FIX and FX (31, 32). Those studies and ours challenge the assumption that all metal ions in vitamin K-dependent proteins are Ca2+.
Immunoaffinity-purified and hexyl agarose-purified PS contained ∼8 atoms of Ca2+ per 1 atom each of Zn2+ and Mg2+ per molecule of PS, but we cannot exclude that other preparations might have different metal ion profiles.
Reversible loss/gain of PS-direct with loss/gain of Zn2+
Zn2+ and PS-direct could be restored to Zn2+-deficient PS only by incubation with Zn2+ at pH 2.7–3.7 or in urea. This suggests that the conformation of MonoQ-purified, Zn2+-deficient PS was unfavorable for insertion of Zn2+ unless mild denaturation was employed. Possibly a different metal, such as Ca2+, occupying the natural Zn2+ site in inactive PS blocks Zn2+ binding unless inactive PS is partially denatured. PS prepared by novel conventional methods that did not include MonoQ, low pH, or urea contained Zn2+ and had good PS-direct (Table 1 and Fig. 8), further supporting the conclusion that plasma PS naturally contains Zn2+.
The Zn2+- and Cu2+-selective chelator, o-phenanthroline, caused loss of PS-direct for PS that contained Zn2+. Most of the PS-direct was restored by subsequent dialysis in Zn2+. Either Zn2+ was restored to PS, or o-phenanthroline bound to the Zn2+-containing PS was lost on dialysis. Based on many purifications, Zn2+ content clearly correlated with PS-direct.
Mechanism by which Zn2+ content affects PS-direct
Zn2+ content of PS appears to affect PS-direct largely via binding to FXa. The Kdapp of 9–13 nM for Zn2+-containing PS binding to FXa obtained in SPR experiments agreed well with our previously published value of 18 nM (6). The affinity of Zn2+-deficient PS for FXa was ∼16-fold less. In a novel finding, PS bound TFPI with similar affinity as it bound FXa, but Zn2+ was not required for PS-TFPI interaction.
Anticoagulant cofactor activity of PS for APC was not affected by purification method, pH 2.7 treatment, or by Zn2+ content. This suggests that commonly used types of immununoaffinity-purification procedures as well as the procedures used to incorporate Zn2+ into PS are relatively mild for PS. It also suggests that some features important for PS-direct differ from the features important for APC cofactor activity, as previously reported (33). The N terminus through EGF1 of PS is thought to contribute most strongly to cofactor activity of PS (19, 34, 35), which suggests that the Zn2+ site may not be located in the N-terminal part of the molecule.
Although Zn2+ content of PS correlates well with PS-direct, there may be other molecular features of PS that affect PS-direct. It is unlikely that the S7 monoclonal antibody used in affinity purification selects for a particular form of PS, since PS prepared with a different monoclonal antibody had similar PS-direct.
Possible Zn2+-dependent conformation difference in PS
Conformation differences were suggested to exist between PS that contains Zn2+ and PS that has lost Zn2+, based on recognition of the former and not the latter by antibodies to PS residues 621–635. Data suggest either that part of residues 621–635 is fully exposed only when PS contains Zn2+, or that these residues exhibit Zn2+-dependent, conformation-specific epitopes. Conformation differences are also suggested by the need for mild denaturing conditions to restore Zn2+ to Zn2+-deficient PS.
Potential location of Zn2+ site in PS
Several pieces of information suggest that the Zn2+ site is located in the SHBG-like region of PS, possibly at the interface of the two LG domains. First, except for Zn2+-finger proteins, the most important residues involved in Zn2+-binding proteins are typically 2 His and 1 Glu/Asp residues, and there is only 1 His residue in PS that is located outside the SHBG region. Examining the model of Villoutreix (22), several His residues and two negatively charged residues are located in proximity at the interface of the two LG domains: His433, His617, His623, Asp292, and Glu294. Second, residues 621–635, including His623, are located at the interface of the LG domains, and antibodies against peptide 621–635 preferentially recognize PS that contains Zn2+. Third, APC cofactor activity of PS is unaffected by Zn2+ content of PS, and cofactor activity is largely independent of the SHBG region of PS.
Based on the crystal structure of the homologous LG-1 domain of SHBG (22), Villoutreix et al. (23) modeled a Ca2+ binding site in the LG-1 domain in the SHBG-like region of PS, consisting of Glu294, Asp292, and Arg404 (23). Thus, we speculate that either Ca2+ or Zn2+ could bind at the interface of the LG domains in PS. In addition to the proposed His residues, 7 other His residues are located in the SHBG-like region and cannot be ruled out at this time. Zn2+ is located at domain interfaces in over 53 proteins (36). A Zn2+ binding site was identified in SHBG (37), and 2 Zn2+ sites were identified in FVIIa (31, 38), but these are not at domain interfaces, and homologous Zn2+-binding residues are not present in PS.
TFPI-independent and -dependent modes of PS anticoagulant activity
Hackeng et al. (8) recently reported that PS down-regulates coagulation by enhancing the ability of TFPI to inhibit the extrinsic TF/FVIIa pathway. Together with the present data, this suggests that there are TFPI-dependent and TFPI-independent effects of PS on coagulation. In this study, we focused on the TFPI-independent anticoagulant effects of PS. Nevertheless, we demonstrated for the first time that PS binds TFPI with high affinity, although this interaction was independent of Zn2+ content in PS.
CONCLUSIONS
Discovery here of Zn 2+ in plasma-derived PS and the requirement for Zn2+ for PS-direct establishes an entirely new perspective from which to investigate PS-direct in future studies, and resolves some existing controversies. Zn2+ is not required for binding of PS to TFPI or for APC-cofactor activity of PS. Thus, there are significant Zn2+-dependent and Zn2+-independent effects of PS on blood coagulation.
Acknowledgments
We gratefully acknowledge the crucial technical assistance of Lacthu Tonnu. We thank Prof. Emeritus Samuel I. Rapaport, Dr. L. Vijay Mohan Rao, and Dr. Bonnie Warn-Cramer (formerly of the University of California at San Diego School of Medicine, San Diego, CA, USA) for graciously providing anti-TFPI antibodies. We are grateful to Dr. Bruno Villoutreix, who provided refined coordinates of his molecular model of the PS SHBG region; Dr. Subramanian Yegneswaran and Dr. Laurent Mosnier, who assisted in examining the model; Dr. Brian Grinnell (Eli Lily, Indianapolis, IN, USA), who donated a sample of PS; Dr. M. G. Finn, who provided instrumentation and advice; and Dr. E. Hohenester, who provided helpful comments. A preliminary report of this work was presented at the 2007 Congress of the International Society on Thrombosis and Haemostasis. Support was from National Institutes of Health grants HL70002, HL 0888375, and HL21544 and the Stein Endowment Fund.
References
- Schwarz H P, Fischer M, Hopmeier P, Batard M A, Griffin J H. Plasma PS deficiency in familial thrombotic disease. Blood. 1984;64:1297–1300. [PubMed] [Google Scholar]
- Mahmoodi B K, Brouwer J L, Veeger N J, van der Meer J. Hereditary deficiency of protein C or PS confers increased risk of arterial thromboembolic events at a young age. Circulation. 2008;118:1659–1657. doi: 10.1161/CIRCULATIONAHA.108.780759. [DOI] [PubMed] [Google Scholar]
- Mahasandana C, Suvatte V, Marlar R A, Manco-Johnson M J, Jacobson L J, Hathaway W E. Neonatal purpura fulminans associated with homozygous PS deficiency [Letter] Lancet. 1990;335:61–62. doi: 10.1016/0140-6736(90)90201-f. [DOI] [PubMed] [Google Scholar]
- Walker F J. Regulation of APC by a new protein: a possible function for bovine PS. J Biol Chem. 1980;255:5521–5524. [PubMed] [Google Scholar]
- Heeb M J, Mesters R M, Tans G, Rosing J, Griffin J H. Binding of PS to FVa associated with inhibition of prothrombinase that is independent of APC. J Biol Chem. 1993;268:2872–2877. [PubMed] [Google Scholar]
- Heeb M J, Rosing J, Bakker H M, Fernández J A, Tans G, Griffin J H. PS binds to and inhibits FXa. Proc Natl Acad Sci U S A. 1994;91:2728–2732. doi: 10.1073/pnas.91.7.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackeng T M, van't Veer C, Meijers J C M, Bouma B N. Human PS inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with FVa and FXa. J Biol Chem. 1994;269:21051–21058. [PubMed] [Google Scholar]
- Hackeng T, Sere K M, Tans G, Rosing J. PS stimulates inhibition of the tissue factor pathway by TFPI. Proc Natl Acad Sci U S A. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sere K, Janssen M, Willems G, Tans G, Rosing J, Hackeng T M. Purified PS contains multimeric forms with increased APC-independent anticoagulant activity. Biochemistry. 2001;40:8852–8860. doi: 10.1021/bi002500a. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Schuck P, Xu X. PS multimers and monomers each have direct anticoagulant activity. J Thromb Haemost. 2006;4:385–391. doi: 10.1111/j.1538-7836.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Radtke K-P, Fernandez J A, Tonnu L. Plasma contains PS monomers and multimers with similar direct anticoagulant activity. J Thromb Haemost. 2006;4:2215–2222. doi: 10.1111/j.1538-7836.2006.02117.x. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Marzec U, Gruber A, Hanson S R. In vivo antithrombotic activity of protein S that is independent of activated protein C [Abstract] J Thromb Haemost. 2007;5:O-W-094. doi: 10.1160/TH11-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen R R, Tans G, Van Oerle R, Hamulyak K, Rosing J, Hackeng T M. The APC-independent anticoagulant activity of PS in plasma is decreased by elevated prothrombin levels due to the prothrombin G20210A mutation. Blood. 2003;102:1686–1692. doi: 10.1182/blood-2003-02-0620. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Koenen R R, Fernandez J A, Hackeng T M. Direct anticoagulant activity of PS-C4b binding protein complex in Heerlen heterozygotes and normals. J Thromb Haemost. 2004;2:1766–1773. doi: 10.1111/j.1538-7836.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- Van Wijnen M, van't Veer C, Meijers J C, Bertina R M, Bouma B N. A plasma coagulation assay for an APC-independent anticoagulant activity of PS. J Thromb Haemost. 1998;80:930–935. [PubMed] [Google Scholar]
- Grinnell B W, Walls J D, Marks C, Glasebrook A L, Berg D T, Yan S B, Bang N U. Gamma-carboxylated isoforms of recombinant human PS with different biologic properties. Blood. 1990;76:2546–2554. [PubMed] [Google Scholar]
- Stenflo J A. A new vitamin K-dependent protein: purification from bovine plasma and preliminary characterization. J Biol Chem. 1976;251:355–363. [PubMed] [Google Scholar]
- Bajaj S P, Rapaport S I, Meki S L, Brown S F. A procedure for isolation of human protein C and PS as by-products of the purification of factors VII, IV, X and prothrombin. Prep Biochem. 1983;13:191–214. doi: 10.1080/00327488308064248. [DOI] [PubMed] [Google Scholar]
- Stenflo J, Stenberg Y, Muranyi A. Calcium-binding EGF-like modules in coagulation proteinases: function of the calcium ion in module interactions. Biochim Biophys Acta. 2000;1477:51–63. doi: 10.1016/s0167-4838(99)00262-9. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B, Hildebrand B, Linse S. Novel type of very high affinity calcium-binding sites in β-hydroxy-asparagine-containing epidermal growth factor-like domains in vitamin K-dependent PS. J Biol Chem. 1990;265:18481–18489. [PubMed] [Google Scholar]
- Stenberg Y, Drakenberg T, Dahlback B, Stenflo J. Characterization of recombinant epidermal growth factor (EGF)-like modules from vitamin-K-dependent PS expressed in Spodoptera cells: the cofactor activity depends on the N-terminal EGF module in human PS. Eur J Biochem. 1998;251:558–564. doi: 10.1046/j.1432-1327.1998.2510558.x. [DOI] [PubMed] [Google Scholar]
- Grishkovskaya I, Avvakumov G V, Sklenar G, Dales D, Hammond G L, Muller Y A. Crystal structure of human SHBG: steroid transport by a laminin G-like domain. EMBO J. 2000;19:504–512. doi: 10.1093/emboj/19.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villoutreix B O, Dahlback B, Borgel D, Gandrille S, Muller Y A. Three-dimensional model of the SHBG-like region of anticoagulant PS: new structure-function insights. Proteins. 2001;43:203–216. [PubMed] [Google Scholar]
- Sasaki T, Knyazev P G, Cheburkin Y, Gohring W, Tisi D, Ullrich A, Timpl R, Hohenester E. Crystal structure of a C-terminal fragment of growth arrest-specific protein Gas6. Receptor tyrosine kinase activation by laminin G-like domains. J Biol Chem. 2002;277:44164–44170. doi: 10.1074/jbc.M207340200. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Kojima Y, Tans G, Rosing J, Griffin J H. C-terminal residues 621-635 of PS are essential for binding to FVa. J Biol Chem. 1999;274:36187–36192. doi: 10.1074/jbc.274.51.36187. [DOI] [PubMed] [Google Scholar]
- Jensen F, Bukrinsky T, Bjerrum J, Larsen S. Three high-resolution crystal structures of cadmium-substituted carboxypeptidase A provide insight into the enzymatic function. J Biol Inorg Chem. 2002;7:490–499. doi: 10.1007/s00775-001-0324-0. [DOI] [PubMed] [Google Scholar]
- Maret W. Zinc coordination environments in proteins determine zinc functions. J Trace Elem Med Biol. 2005;19:7–12. doi: 10.1016/j.jtemb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Marx P F, Bouma B N, Meijers J C M. Role of zinc ions in activation and inactivation of thrombin-activatable fibrinolysis inhibitor. Biochemistry. 2002;41:1211–1216. doi: 10.1021/bi0115683. [DOI] [PubMed] [Google Scholar]
- Vallee B L, Rupley J A, Coombs T L, Neurath H. The role of zinc in carboxypeptidase. J Biol Chem. 1960;235:64–69. [Google Scholar]
- Shikamoto Y, Morita T, Fujimoto Z, Mizuno H. Crystal structure of Mg2+- and Ca2+-bound Gla domain of FIX complexed with binding protein. J Biol Chem. 2003;278:24090–24094. doi: 10.1074/jbc.M300650200. [DOI] [PubMed] [Google Scholar]
- Bajaj S P, Schmidt A E, Agah S, Bajaj M U, Padmanabhan K. High resolution structures of p-aminobenzamidine-and benzamidine-VIIa/soluble tissue factor. J Biol Chem. 2006;281:24873–24888. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]
- Sekiya F, Yoshida M, Yamashita T, Morita T. Magnesium(II) is a crucial constituent of the blood coagulation cascade: potentiation of coagulant activities of FIX by Mg2+ ions. J Biol Chem. 1996;271:8541–8544. doi: 10.1074/jbc.271.15.8541. [DOI] [PubMed] [Google Scholar]
- Heeb M J, Griffin J H. APC-dependent and -independent anticoagulant activities of PS have different structural requirements. Blood Cells Mol Dis. 2002;29:190–199. doi: 10.1006/bcmd.2002.0558. [DOI] [PubMed] [Google Scholar]
- Van Wijnen M, Stam J G, Chang G T, Meijers J C, Reitsma P H, Bertina R M, Bouma B N. Characterization of mini-PS, a recombinant variant of PS that lacks the SHBG-like domain. Biochem J. 1998;330:389–396. doi: 10.1042/bj3300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegneswaran S, Hackeng T M, Dawson P E, Griffin J H. The thrombin-sensitive region of PS mediates phospholipid-dependent interaction with FXa. J Biol Chem. 2008;283:33046–33052. doi: 10.1074/jbc.M806527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini A, Andreini C, Menchetti S, Rosato A, Frasconi P. Predicting zinc binding at the proteome level. BMC Bioinformatics. 2007;8:39–51. doi: 10.1186/1471-2105-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvakumov G V, Grishkovskaya I, Muller Y A, Hammond G L. Crystal structure of human SHBG in complex with 2-methoxyestradiol reveals the molecular basis for high affinity interaction with C-2 derivatives of estradiol. J Biol Chem. 2002;277:45219–45225. doi: 10.1074/jbc.M207762200. [DOI] [PubMed] [Google Scholar]
- Petersen L C, Olsen O H, Nielsen L S, Freskgard P-O, Persson E. Binding of Zn2+ to a Ca2+ loop allosterically attenuates the activity of FVIIa and reduces its affinity for tissue factor. Prot Sci. 2000;9:859–866. doi: 10.1110/ps.9.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]