Abstract
Although the solute carrier 35B1 (SLC35B1) is evolutionarily conserved, its functions in metazoans remain unknown. To elucidate its function, we examined developmental roles of an SLC35B1 family gene (HUT-1: homolog of UDP-Gal transporter) in Caenorhabditis elegans. We isolated a deletion mutant of the gene and characterized phenotypes of the mutant and hut-1 RNAi-treated worms. GFP-HUT-1 reporter analysis was performed to examine gene expression patterns. We also tested whether several nucleotide sugar transporters can compensate for hut-1 deficiency. The hut-1 deletion mutant and RNAi worms showed larval growth defect and lethality with disrupted intestinal morphology. Inactivation of hut-1 induced chronic endoplasmic reticulum (ER) stress, and hut-1 showed genetic interactions with the atf-6, pek-1, and ire-1 genes involved in unfolded protein response signaling. ER ultrastructure and ER marker distribution in hut-1-deficient animals showed that HUT-1 is required for maintenance of ER structure. Reporter analysis revealed that HUT-1 is an ER protein ubiquitously expressed in tissues, including the intestine. Lethality and the ER stress phenotype of the mutant were rescued with the human hut-1 ortholog UGTrel1. These results indicate important roles for hut-1 in development and maintenance of ER homeostasis in C. elegans.
Keywords: ER stress, RNAi, deletion mutant, glycosylation, model organism
Transport of nucleotide sugars and 3′-phosphoadenosine 5′-phosphosulfate (PAPS), the donor substrates of glycosylation and sulfation, into the endoplasmic reticulum (ER) and Golgi apparatus is a critical step for synthesis of glycolipids, glycoproteins, and glycosaminoglycans. Most, but not all, glycosylation and sulfation occurs in the ER and Golgi apparatus, whereas the donor substrates are synthesized in the cytosol or nucleus. Specialized multispanning type III nucleotide sugar transporters (NSTs) mediate transport of nucleotide sugars and PAPS from the cytosol into the ER/Golgi lumen using an antiport mechanism. NSTs, classified as the drug metabolite transporter family (solute carrier family 35: SLC35) (1, 2), function as antiporters not only of nucleotide-sugar/nucleotide-monophosphate or PAPS/3′-phosphoadenosine 5′-phosphate (PAP), but also of nucleotide-sugar/nucleotide-sugar (3). Recent studies demonstrated NSTs transport substrates in a competitive or “independent and simultaneous” manner (4, 5). Although transporters of nucleotide sugars utilized in major glycosylation and sulfation pathways have been characterized, the functions of a large part of the SLC35 family remain unknown. Mutations in NST genes cause various hereditary diseases and developmental abnormalities in humans, which are due to impaired specific oligosaccharides synthesis that requires the transport of donor substrates into the lumen by each NST (6,7,8,9). For example, the Golgi GDP-fucose transporter gene is identified as the responsible gene of leukocyte adhesion deficiency/congenital disorder of glycosylation IIc (LAD II/CDG IIc), which is characterized by a decreased expression of fucose in glycoconjugates and results in leukocyte adhesion deficiency and morphological and neurological defects (10, 11). Thus, NST gene knockout will not only provide valuable insight into NST physiological function, but also serve as a useful tool for studying roles of glycosylation in development.
The NSTs classified as SLC35B1 or UDP-Gal transporter-related 1 (Ugtrel1 or Utr1) are conserved among eukaryotes. The yeast SLC35B1, HUT1 (homolog of UDP-Gal transporter), transports UDP-Gal but not UDP-Glc (12, 13). The plant SLC35B1, AtUTr1, transports both UDP-Gal and UDP-Glc (14, 15). On the other hand, human SLC35B1, hUGTrel1, is reported as a UDP-GlcA transporter (16). Interestingly, in yeast (schut1, sphut1) and in plants (AtUtr1), these genes were suggested to be involved in the protein-folding process in the ER. Reyes et al. (15) have suggested that rather than the UDP-Gal transport activity, the UDP-Glc transport activity of Arabidopsis thaliana gene (AtUtr1) would be linked to the protein-folding process by the calnexin/calreticulin cycle via glucosylation of N-glycan on unfolded proteins, the glycosylation of which is mediated by UDP-Glc:glycoprotein glucosyltransferase (UGGT). However, because of lack of UGGT activity in Saccharomyces cerevisiae (17), functions of schut1 in protein folding cannot be explained by the protein glucosylation related to scHUT1. Thus, it remains unclear as to how SLC35B1 is involved in ER functions. Furthermore, in previous genome-wide screening for sensitivity of oxidants in S. cerevisiae (18), scHut1 showed resistance to diamide that preferentially oxidizes small thiols. Recent genome-wide analyses of gene expression patterns indicate that expression of the SLC35B1 gene is increased under ER stress conditions in various organisms, such as A. thaliana (19, 20) and Caenorhabditis elegans (21), as well as mouse embryonic fibroblasts (22) and cultured human cells (23). These gene expression profiles suggest that SLC35B1 plays a critical role in ER function or protein folding throughout evolution. Although it is likely essential for viability in the protozoan parasite Leishmania (24), genes encoding SLC35B1 proteins in yeasts and plant have been shown not to be essential for viability in normal conditions (13, 15). On the other hand, no examples of genetic deficiency and gene knockout have been reported in metazoans. In this study we examined the roles of the protein encoded by Y111B2A.20 that we named hut-1 during development in the nematode C. elegans using gene knockdown techniques.
MATERIALS AND METHODS
Strains and general methods
N2 was used as the wild-type strain. Strains were maintained and cultured as described previously (25). Strains carrying the following alleles were used in the study: ire-1(ok799)II, hut-1(tm1435)III, zcIs4(hsp-4::GFP)V, pek-1(ok275)X, and atf-6(ok551)X; all were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA), except for hut-1(tm1435). The hut-1(tm1435) allele was obtained from the TMP/UV library as described previously (26) and was identified by PCR amplification with primers spanning the deleted region. The hut-1 deletion mutant was backcrossed 6 times with N2. This strain was crossed to zcIs4 males, and the hut-1(tm1435) homozygous progeny generated from the hut-1(tm1435)/+ hermaphrodites were examined. Mutant strains were confirmed by PCR and/or phenotypic analysis.
Microscopy
Differential interference contrast (DIC) and fluorescent images were obtained with a Leica DMRXA full automatic microscope (Leica Microsystems, Wetzlar, Germany) equipped with MetaMorph software (version 6.1r5; Universal Imaging, Downingtown, PA, USA). Confocal images were acquired with a Zeiss LSM510 system (Carl Zeiss, Oberkochen, Germany). Worms were anesthetized with a 10 mM sodium azide solution and placed on an 8-well printed microscope slide glass (Matsunami Glass, Kishiwada, Japan).
RNAi by feeding
RNAi experiments were performed essentially as described previously (27). The following primers were used for construction of the hut-1 RNAi vector: 5′-TGCCAAAAAACCATGAGACGCCAC-3′ and 5′-ATTTATTATGCACTTTTGGCTCAG-3′. HT115, harboring the plasmid pPD129.36 without any insert, was used as a control. RNAi feeding of wild-type, atf-6(ok551), and pek-1(ok275) strains was performed at 25°C, and RNAi feeding of ire-1(ok799) was performed at 20°C because of its lethality at 25°C. Feeding was initiated at the L4 stage, and phenotypes in the next generation were examined. Developmental stages were classified based on the morphology of gonad and vulva.
Phenotypic characterization
To score the scrawny morphology (Scr) phenotype of hut-1(tm1435), P0 L4 hut-1(tm1435)/+ animals were transferred onto NGM agar plates seeded with OP50 and allowed to lay eggs for 24 h at 20°C, followed by removal of P0 animals. Sixty hours after removing the parents, F1 wild-type adult or L4-, Scr L4-, and L1–L3-arrested animals were scored. To compare the severity of larval growth defects at 20 and 25°C, gravid hut-1(tm1435)/+;zcIs4 adult worms were allowed to lay eggs for 2 h on the NGM agar plates seeded with OP50 or HT115 producing each dsRNA. The unhatched eggs were scored as dead embryos 15.5 and 16 h after removing the parents at 25 and 20°C, respectively. The L1/L2, L3, early L4, and midlate L4 worms were scored to determine the larval arrest stage after 40 and 40.5 h at 25 and 20°C, respectively.
Reporter constructs and transgenic rescue
Construction of the reporters including the hut-1 promoter or the hut-1 promoter and genomic locus were essentially as described previously (27, 28), except that genomic DNA was amplified using KOD-plus DNA polymerase (Toyobo, Osaka, Japan) or the Expand Long Template PCR System (Roche Applied Science, Indianapolis, IN, USA). For construction of Peft-4::hut-1::egfp, Peft-4::ZK896.9 (ORF-3 in ref. 10), and Peft-4::sqv-7 (C52E12.3), cDNAs were amplified by PCR from C. elegans total cDNA. For construction of Peft-4::schut1, Peft-4::sphut1, and Peft-4::hUGTrel1, cDNAs were amplified by PCR from pG3-scHUT1, pCR-spHUT1 (gifts from Dr. Yoshifumi Jigami, National Institute of Advanced Industrial Science and Technology, Tokyo, Japan) (12), and YEp352-GAPII-hUGTrel1, respectively, using primers including restriction enzyme sites followed by cloning into NotI or NotI/BglII sites of the expression vector pFX_LVT-R03G5.1 (29). To generate Peft-4::egfp::hut-1 and Peft-4::egfp::SP12 (the signal peptidase, C34B2.10), hut-1 and SP12 cDNA were cloned into XhoI/BamHI sites of pEGFP-C2 (Clontech, Mountain View, CA, USA), and the egfp::hut-1 and egfp::SP12 fragments were amplified by PCR from the plasmids using primers including restriction enzyme sites, followed by cloning into the NotI/BglII sites of the pFX_LVT-R03G5.1 plasmid. Peft-4::mCherry::SP12 and Paman-2::aman-2::mCherry were constructed by replacing EGFP in Peft-4::egfp::SP12 and Paman-2::aman-2::egfp with mCherry, which was amplified with PCR from pRSET-mCherry (30). DNA sequence analysis was performed using the Prism 3130 Genetic analyzer (Applied Biosystems, Foster City, CA, USA). PCR primers used in this section are listed in Supplemental Table S1. Microinjections were performed as described by Mello and Fire (31). Expression constructs under the control of the eft-4 promoter or other promoters were injected at 2 or 30 ng/μl, respectively, with either the coinjection marker Ptph-1::dsred or Paman-2::aman-2::mCherry at 20 ng/μl and/or rol-6(gf) at 80 ng/μl. To determine tissue expression and subcellular localization of fluorescent protein, at least 10 animals/transgenic line and at least 2 independent extrachromosomal lines were examined for each construct.
Measurement of effect of RNAi on Phsp-4::GFP induction
Fluorescence and time of flight of 3 populations of worms fed with control or hut-1 dsRNA were measured using a COPAS Biosort (Union Biometrica, Holliston, MA, USA) at 119 h at 20°C after placing 5 L4 SJ4005 worms, following the manufacturer’s instructions. Further data analysis was performed with Excel software (Microsoft, Redmond, WA, USA).
Electron transmission microscopy
Electron transmission microscopy analysis was conducted by Hanaichi Ultrastructure Research Institute Co. (Okazaki, Japan). For RNAi by feeding, young L4 worms were fed with bacteria-producing hut-1 or control dsRNA for 72 h at 25°C, and the second generation was examined. Dissected young L4 animals were fixed with 4% paraformaldehyde and 1% glutaraldehyde in 100 mM cacodylic acids buffer for 1 h at room temperature, followed by further fixation with 2% paraformaldehyde and 2% glutaraldehyde in 100 mM cacodylic acids buffer for 1 d and an overnight wash in 100 mM cacodylic acids buffer at 4°C. Postfixation was carried out in 2% OsO4 in 100 mM cacodylic acid buffer for 24 h, followed by dehydration and infiltration with a resin Q651 (Mitsui Chemicals, Tokyo, Japan). Ultrathin sections of intestinal area were analyzed using an electron microscope (JEM-2000EX; Jeol, Akishima, Japan).
RESULTS
Identification of a C. elegans SLC35B1 gene, hut-1
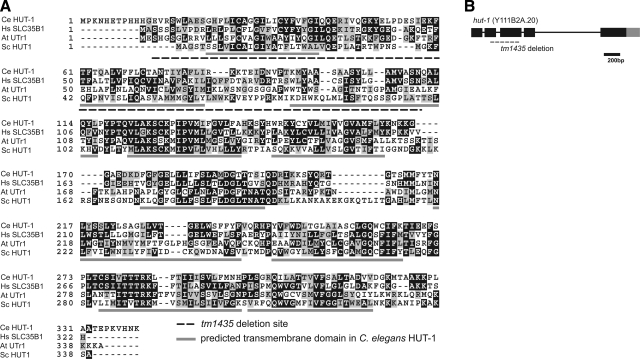
Phylogenetic tree analysis of SLC35 family proteins revealed that the SLC35B nucleotide sugar transporter family contains 4 subfamilies: SLC35B1 (Ugtrel1), SLC35B2 (PAPST1), SLC35B3 (PAPST2), and SLC35B4 (UDP-Xyl/GlcNAc transporter) (Supplemental Fig. S1). Only the SLC35B1 subfamily is conserved among eukaryotes from plants, budding/fission yeast to human. The C. elegans HUT-1 amino acid sequence was 42.4, 41.5, and 23.2% identical to the human, Drosophila, and S. cerevisiae ortholog, respectively. Only 1 orthologous gene is found in the C. elegans genome in each clade: SLC35B1 to SLC35B4 (hut-1/Y111B2A.20, pst-1/M03F8.2, pst-2/F54E7.1 and F15B10.1, F54E7.1 and F15B10.1 are also described as ORF-9 and ORF-11, respectively in ref. 10; see Supplemental Fig. S1). Transmembrane regions were predicted using the web tool PolyPhobius (http://phobius.sbc.su.se/poly.html) (32, 33). Both C. elegans HUT-1 and human Ugtrel1 possess 10 transmembrane domains, corresponding to the topology of other SLC35 family members, whose N and C termini are located in the cytosol.
Disruption of hut-1 induces ER stress and temperature-sensitive larval growth defects
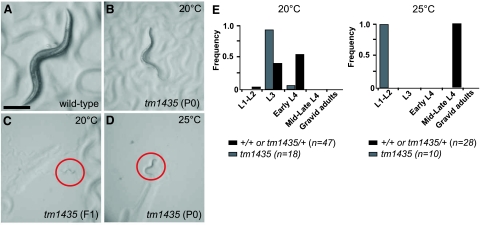
To investigate the role of the hut-1 gene in the nematode, a hut-1 deletion mutant allele (tm1435) was isolated from trimethylpsoralen/ultraviolet-treated deletion libraries. Because the reading frame contains a 413-bp deletion in the exon 2 and exon 3 region resulting in a frameshift, tm1435 is predicted to be a null allele of hut-1 (Fig. 1A, B). No differences were observed between tm1435/+ and wild-type animals in regard to apparent morphology and larval growth at 15, 20, and 25°C. In contrast, tm1435 homozygotes segregated from tm1435 heterozygous hermaphrodites exhibited slow larval growth, with clear and scrawny body morphology at 15 and 20°C. Approximately one-quarter of the progeny from the heterozygous worms began to show slowed development at L3 and L4 stages with scrawny body morphology (Table 1). These individuals developed to the adult stage ∼2 d later than wild-type animals and produced small numbers of progeny (<10 progeny/worm) (Fig. 2A, B); worms were confirmed as homozygous for the null allele of hut-1 by single-worm PCR (n>20, data not shown). The second generation of tm1435 homozygotes hatched and died at L1 stage (Fig. 2C). The differences in phenotype between P0 and F1 suggest the presence of a maternal effect on tm1435 homozygous larval development. Adult hermaphrodites of tm1435 homozygote successfully mated with wild-type males and produced normal progeny, which suggests that zygotic hut-1 is sufficient for larval development.
Figure 1.
HUT-1 protein and hut-1 gene. A) Alignment of SLC35B1 of C. elegans (Ce), Homo sapiens (Hs), Arabidopsis thaliana (At), and Saccharomyces cerevisiae (Sc). Identical amino acids are shaded in black; similar amino acids are shaded in gray. Transmembrane segments as predicted by the Phobius web server are underlined in gray. The deletion region in tm1435 is shown by a dashed underline. B) Structure of the hut-1 gene. Exons are indicated by boxes. Black and gray boxes are translated and untranslated regions, respectively. Dashed line indicates the deletion in tm1435.
TABLE 1.
Phenotypes of tm1435 progenies
| P0 genotype | n | Normal L4-adult (%) | Scr L4 (%) | Arrested L1–L3 (%) |
|---|---|---|---|---|
|
tm1435/+
|
P0 = 6,F1 = 1535 | 75 ± 5 | 18 ± 5 | 6 ± 2 |
| +/+ | P0 = 1,F1 = 212 | 100 | 0 | 0 |
Wild-type and tm1435/+ worms were grown at 20°C and scored for growth status as described in Materials and Methods. Parental genotypes of tm1435/+ were confirmed by single-worm PCR. Values are means ± sd.
Figure 2.
Temperature-sensitive growth defect of the hut-1 deletion mutant. A–D) Dissecting microscope images (×60) of a wild-type gravid adult grown at 25°C (A), a first-generation tm1435 homozygote adult grown at 20°C (B), a second-generation tm1435 homozygote L1 larvae grown at 20°C (C), and a tm1435 homozygote-arrested L2 larvae grown at 25°C (D). Red circles indicate worms in C and D. Scale bar = 200 μm. E) Growth of wild-type (population containing +/+ or tm1435/+ animals, black) and tm1435 homozygote (gray) at 20 and 25°C.
By contrast, tm1435 homozygotes from heterozygous hermaphrodites exhibited a developmental arrest and lethality at the L2 stage at 25°C (Fig. 2D), which was earlier than at 15 and 20°C. This temperature is permissive for wild-type worms but causes weak heat stress. Because the heat stress could induce the production of misfolded proteins, the sensitivity to heat stress in hut-1-defective animals is consistent with previous studies in yeasts and plants, indicating the involvement of HUT1 or AtUTr1 in protein folding in the ER. Thus, we next examined the effect of hut-1 gene inactivation on the ER stress response. HSP-4 is a C. elegans homolog of the ER-resident chaperon BiP (GRP78), and its translation is up-regulated by ER stress, such as high temperature, dithiothreitol, and tunicamycin treatment. The transgenic strain hsp-4::gfp(zcIs4)V (34), which expresses GFP under the control of hsp-4 promoter, was crossed with the tm1435 mutant. At 20°C wild-type and tm1435/+ animals exhibited relatively low Phsp-4::GFP activity (Fig. 3A, left; and not shown). In contrast, tm1435 homozygotes persistently showed high Phsp-4::GFP expression (Fig. 3A, middle), which suggests that inactivation of hut-1 chronically induces ER stress.
Figure 3.
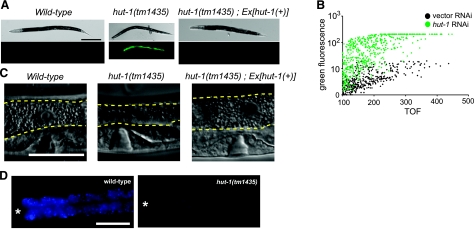
Effect of hut-1 knockout and knockdown on the expression of Phsp-4::GFP and intestinal morphology. A) Knockout or knockdown of hut-1 induces Phsp-4::GFP reporter. DIC (top) and fluorescence (bottom) images of wild-type L4 (left), hut-1(tm1435) L4 (middle), and adult of hut-1(tm1435) rescued with hut-1 genomic DNA (right), which are expressing gfp under the control of hsp-4 promoter. B) Effect of control RNAi and hut-1 RNAi on Phsp-4::GFP analyzed by worm sorter COPAS Biosort. The fluorescence and time-of-flight (TOF) are expressed in arbitrary units. C, D) Intestinal phenotypes of various worms. C) DIC images of the intestines of wild-type, hut-1(tm1435), and rescued hut-1(tm1435) larvae. Intestines are outlined in dashed yellow lines. Scale bar = 50 μm. D) Intestinal granules visualized by DAPI filter in wild-type and hut-1(tm1435) larvae. Asterisks indicate position of the pharyngeal terminal bulb. Scale bars = 200 μm (A); 50 μm (C, D).
Pronounced intestinal abnormality was observed in the tm1435 homozygous mutant, in which the intestine contained fewer gut granules than in wild-type animals (Fig. 3C). Furthermore, the intensity and number of autofluorescent intestinal vesicles were reduced in the tm1435 homozygous mutant (Fig. 3D).
To confirm the phenotype of hut-1 gene inactivation, we also performed hut-1 RNA interference (RNAi) experiments using the feeding method. To monitor the effect of hut-1 gene inactivation, Phut-1::hut-1::egfp and Peft-4::egfp::hut-1(cDNA) transgenic worms that express full-length HUT-1-EGFP fusion protein under the control of the hut-1 and ubiquitous eft-4 promoters, respectively, were fed hut-1 dsRNA. Substantial reduction of fluorescence in hut-1 RNAi-treated animals was observed (Supplemental Fig. S2 and Fig. 5A, B), indicating that hut-1 was successfully knocked down in the RNAi-treated animals. At 25°C, hut-1 RNAi treated animals slowly developed into semisterile adults with a scrawny and clear body morphology, as observed in the tm1435 mutant. Their intestine also contained fewer gut granules as in hut-1(tm1435). In contrast, at 20°C hut-1 RNAi-treated animals developed into semifertile adults, with mild changes in growth and body morphology, which would reflect the relative inefficiency of RNAi under this condition. However, treatment with hut-1 RNAi led to strong induction of Phsp-4::GFP in hsp-4::gfp(zcIs4) worms even at 20°C; by comparison, control RNAi did not induce GFP fluorescence (Fig. 3A, right). In contrast, hut-1 RNAi treatment did not lead to activation of a small heat shock protein hsp-16.2 (Supplemental Fig. S3). Taken together, these results suggest that hut-1 is essential for larval development and its inactivation chronically induces ER stress.
Figure 5.
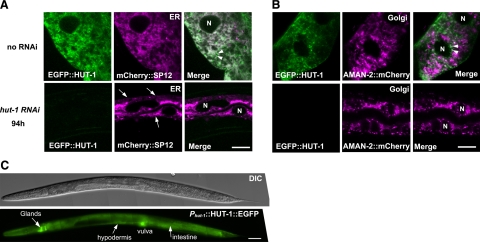
HUT-1 localizes to the ER and part of the Golgi in the intestine and is required for normal ER morphology. A, B) Confocal micrographs of intestinal cells expressing EGFP::HUT-1 and the ER marker mCHERRY::SP12 (A) and the Golgi apparatus marker AMAN-2::mCHERRY (B). Nuclei are indicated by “N.” A) Top: EGFP::HUT-1 colocalized with the ER marker as well as distributed as small particles adjacent to the ER. Some of these particles are indicated by arrowheads. Bottom: knockdown of hut-1 by RNAi led to aggregation and resulted in abnormal ER network morphology resembling thin ropes or strings (arrows). B) Top: EGFP::HUT-1 partially colocalized with the Golgi apparatus marker (arrowheads). EGFP::HUT-1 tended to be detected as small particles when coexpressed with AMAN-2::mCHERRY for unknown reason. Bottom: no apparent alteration in the Golgi apparatus marker was observed with hut-1 RNAi. C) Representative DIC and fluorescent images of HUT-1::EGFP under the control of hut-1 promoter show expression in an L4 larva. Scale bars = 10 μm (A); 50 μm (B, C).
The human SLC35B1 gene (UGTrel1) can rescue abnormalities of hut-1-deficient worms
To compare the functional properties of Ce HUT-1 and the yeast and human ortholog, we performed rescue experiments. First, we examined transgenic rescue using hut-1(+) genomic DNA. Introduction of the PCR product containing the hut-1 promoter, coding sequence, and 3′ UTR into tm1435 animals rescued the ER stress phenotype and larval lethality with intestinal abnormality (Fig. 3C, right; Table 2), confirming that the mutant defects in tm1435 animals are due to disruption of tm1435. Next, we examined complementation of tm1435 by ubiquitous expression of schut1, sphut1, or hUGTrel1. We confirmed that these proteins tagged with EGFP were expressed in the transgenic worms (data not shown). As shown in Table 2, expression of hUGTrel1, but not schut1 and sphut1, in tm1435 animals rescued the ER stress phenotype and larval lethality with intestinal abnormality. In addition, worms expressing hUGTrel1 showed resistance to the ER stress phenotype caused by hut-1 RNAi (Supplemental Fig. S4). These results indicate that functions of hUGTrel1 and worm HUT-1 are similar in both organisms and also suggest the possibility that hUGTrel1 may also be physiologically important in mammals. That yeast HUT1 orthologs exhibited no rescue activity indicates that they may not function properly when ectopically expressed in C. elegans, although we cannot exclude the possibility that their biological activities are different.
TABLE 2.
Rescue experiment
| Line | Obtained transgenic lines | Rescued | Not rescued | Rescuing ER stress of F1 |
|---|---|---|---|---|
| Phut-1::egfpa | 3 | 0 | 3 | − |
| PCR product of hut-1 | 3 | 3 | 0 | + |
| Phut-1::hut-1::egfpa | 2 | 2 | 0 | + |
| Peft-4::hut-1::Venusa | 5 | 5 | 0 | + |
| Peft-4::egfp::hut-1a | 5 | 3 | 2 | − |
| Peft-4::hUGTrel1 | 2 | 2 | 0 | − |
| Peft-4::schut1 | 3 | 0 | 3 | − |
| Peft-4::sphut1 | 4 | 0 | 4 | − |
| Peft-4::ZK896.9 | 2 | 0 | 2 | − |
| Peft-4::sqv-7 | 2 | 0 | 2 | − |
DNA was injected to 20–30 tm1435/+;zcIs4 hermaphrodite adults, and at least 50 F1 tm1435 heterozygotes, which produced tm1435 homozygous progeny strongly expressing Phsp-4::GFP, were screened. Obtained transgenic lines were cultured for 3 generations by blindly picking 8 individuals at each generation. Transgenic rescue was determined by the emergence of non-Scr tm1435 homozygous adults.
Fluorescence of Phsp-4::GFP can be easily distinguished from that of Phut-1::egfp, Phut-1::hut-1::egfp, Peft-4::hut-1::Venus, and Peft-4::egfp::hut-1 because their expression patterns are different.
Forced expression of NSTs for UDP-GlcA, UDP-Glc, UDP-Gal, UDP-GlcNAc, and UDP-GalNAc does not rescue abnormalities of hut-1-deficient worms
The Ce HUT-1 may function as a UDP-Gal and/or UDP-Glc and/or UDP-GlcA transporter, because its orthologs are shown to be UDP-Gal/UDP-Glc or UDP-GlcA transporters in plants and yeasts or in humans. In C. elegans, NSTs encoded by ZK896.9 and sqv-7 show transporter activities for UDP-Glc/Gal/GlcNAc/GalNAc (35) and UDP-GlcA/GalNAc/Gal (4), respectively. If HUT-1 function as a UDP-Gal, UDP-Glc, or UDP-GlcA transporter is crucial in larval development, hut-1 deficiency could be complemented by forced expression of these NSTs. To test this possibility, we performed rescue experiments by expressing these genes under control of the eft-4 promoter. When worm hut-1::Venus expression was driven by the eft-4 promoter, both viability and ER stress induction in hut-1(tm1435) were restored, and the phenotype was similar to that of the wild type (Table 2). In contrast, neither expression of ZK896.9 nor sqv-7 exhibited any rescue activities in tm1435 animals (Table 2). The results suggest that HUT-1 has a unique role that is distinct from transport of UDP-Gal, UDP-Glc, and UDP-GlcA.
Abnormal ER morphology in hut-1-inactivated animals
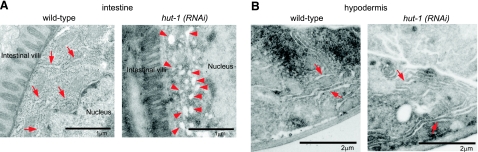
To examine whether hut-1 gene inactivation affects organelle structures, we performed electron microscopy analysis of cross sections of control and hut-1 RNAi-treated worms. Flattened and elongated vesicles that are likely to be ER (36) were observed in the intestine of wild-type worms but not in hut-1 RNAi-treated worms (Fig. 4A, left). Instead, intestinal cells of hut-1 RNAi larvae contained multiple round vesicles (Fig. 4A, right), which were not observed in the intestine of control animals. No obvious abnormality was detected in the hypodermis (Fig. 4B). These observations support the idea that hut-1 inactivation could affect the ER structure specifically in the intestine. To confirm this possibility, we examined distribution patterns of the ER marker (mCHERRY::SP12) and Golgi apparatus marker (α-mannosidase II: AMAN-2::mCHERRY) (36) that were ectopically expressed by the eft-4 promoter. In wild-type animals, mCherry::SP12 signals were seen as a reticular network (Fig. 5A, top) (36). In hut-1 RNAi animals, however, the reticular networks became somewhat agglutinated and formed thin rope structures (Fig. 5A, bottom). In contrast, the distribution pattern of the Golgi marker (AMAN-2::mCHERRY) was not altered by hut-1 knockdown (Fig. 5B), which suggests that the abnormal structure observed by electron microscopy is the ER. These data indicate that hut-1 inactivation could induce abnormal ER morphology.
Figure 4.
Abnormal cytological features of intestinal cells in hut-1 RNAi. Transmission electron micrographs of transverse sections of intestinal and hypodermal cells in control RNAi and hut-1 RNAi-treated L4 animals. A) Left: in control RNAi animals, flattened and elongated vesicles (arrows) were distributed throughout intestinal cell. Right: in hut-1 RNAi animals, many round vesicles (red arrowheads), which were not observed in control, were distributed throughout intestinal cell. B) Flattened and elongated vesicles (arrows) were observed in the hypodermis of both control and hut-1 RNAi worms.
Subcellular localization and expression pattern of hut-1
More than half of the SLC35B1 orthologs of other species contain a C-terminal ER retention signal (1). Subcellular localization of the worm HUT-1 has not been described to date. The WoLF PSORT program (http://wolfpsort.org) predicted worm HUT-1 to be an ER protein containing KVHN in the C terminus as an ER membrane retention signal (37). We next examined the subcellular localization of HUT-1 by generating an N-terminal EGFP fusion (EGFP::HUT-1); however, worms possessing Phut-1::egfp::hut-1 did not express EGFP strong enough to assess its localization. Peft-4::egfp::hut-1 is functional, although its rescuing activity is somewhat weaker than Phut-1::hut-1::egfp (Table 2, see below). Intestinal cells are one of the largest cells in C. elegans, which allows us to analyze the distribution pattern of fluorescent proteins in detail. EGFP::HUT-1 colocalized with the ER marker (Fig. 5A), although it only partially colocalized with the Golgi marker (Fig. 5B). The EGFP::HUT-1 signal was also detected as small particles that associated with, but did not merge with, mCHERRY::SP12. These data suggest that HUT-1 localizes to the ER, part of the Golgi apparatus, and possibly to an ER-Golgi intermediate compartment.
To determine the spatial and temporal expression of hut-1, we next expressed Phut-1::hut-1::egfp (the hut-1-coding sequence fused to egfp under the control of the hut-1 promoter). Because the PCR product containing the 1-kb sequence from the predicted initiation codon was sufficient to rescue various phenotypes of the hut-1 mutant as described above, the same 1-kb region was used as the hut-1 promoter. Our previous data that the hut-1::egfp construct rescued the phenotypes of hut-1-deficient worms (tm1435) implied that HUT-1::GFP is functional and its expression patterns would reflect endogenous HUT-1 localization (Table 2). Consistent with the observed phenotypes in hut-1 RNAi-treated worms and hut-1(tm1435), Phut-1::hut-1::egfp was expressed in the intestine during larval development (Fig. 5C). In addition, its expression was observed in a variety of tissue types, including the uterus, epidermis (including vulval epithelial cells), pharyngeal muscles, glands, somatic gonads, and coelomocytes (Fig. 5C and Supplemental Fig. S5, and data not shown). Expression levels were relatively low in these tissues and organs.
Depletion of UPR signaling molecules, pek-1, atf-6, and ire-1, enhance the growth defect of hut-1 knockdown animals
ER stress induces the unfolded protein response (UPR) consisting of 3 signaling pathways mediated by the ER stress receptors, ATF-6, IRE-1, and PEK-1, to reduce ER stress (38). Induction of hut-1 by ER stress was previously reported to be affected by the ire-1/xbp-1 signaling pathway in C. elegans (Supplemental Table 1 in ref. 19). To examine the relationship between HUT-1 and UPR signaling, we examined the effect of hut-1 RNAi treatment in atf-6(ok551), ire-1(ok799), and pek-1(ok275) worms (Fig. 6). As described in a previous paper, atf-6(ok551), ire-1(ok799), and pek-1(ok275) are likely null mutants (39).
Figure 6.
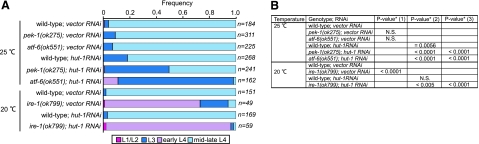
Mutants of genes involved in UPR are sensitive to hut-1 knockdown. A) Developmental stages of mutant animals treated with hut-1 RNAi or vector RNAi. See also Materials and Methods. B) To evaluate differences of growth rate, statistic significances were tested with 2-tailed Mann-Whitney U test. Data were compared with 1) wild-type; vector RNAi, 2) vector RNAi in corresponding genotypes, and 3) hut-1 RNAi in wild-type. N.S., no significant difference.
We found little difference between growth of wild-type, atf-6(ok551), and pek-1(ok275) treated with control RNAi at 25°C. RNAi knockdown of hut-1 significantly reduced growth of wild-type, atf-6(ok551), and pek-1(ok275). Interestingly, enhancements of hut-1 RNAi effects were found in atf-6(ok551) and pek-1(ok275) worms. In contrast, ire-1(ok799) exhibited larval growth defects even at 20°C. At 20°C hut-1 RNAi treatment had no effect on growth of wild-type worms. However, significant enhancement of hut-1 RNAi activity in ire-1(ok799) worms was found. Thus, hut-1 shows synthetic growth defects with atf-6, pek-1, and ire-1, although the degree of abnormality differed in each combination. These results suggest that growth inhibition caused by hut-1 inactivation is weakened by endogenous UPR mediated by ATF-6, PEK-1, and IRE-1/XBP-1 in wild-type animals, and that HUT-1 is required for maintenance of ER homeostasis.
DISCUSSION
This study demonstrated that the C. elegans SLC35B1 gene (hut-1: UDP-Gal transporter-related gene) is crucial for larval development and ER function. SLC35B1 can be substituted by the human HUT-1 ortholog but not by the worm UDP-Glc or UDP-GlcA transporter genes. Although substrate specificity of a NST cannot be deduced from its phylogeny or primary structure (40, 41), the physiological function of SLC35B1 is likely to be conserved through evolution, as described in the introduction. Our study demonstrated that the SLC35B1 subfamily has a conserved function in maintenance of ER homeostasis in metazoans.
Several lines of evidence support the view that HUT-1 has a cellular function other than nucleotide sugar/PAPS transport. In higher eukaryotes, the major donor substrates for glycosylation and sulfation are UDP-Gal, UDP-Glc, UDP-GalNAc, UDP-GlcA, UDP-GlcNAc, UDP-Xyl, GDP-Man, GDP-Fuc, PAPS, and CMP-Sia. The NSTs comprised at least 18 families. Some NSTs have transport activity for multiple nucleotide sugars and are often functionally redundant, which implies that some NSTs could transport small molecules that are distinct from nucleotide sugars and PAPS or have roles other than as a transporter.
C. elegans contains high amounts of glucose-containing mucin-type O-glycan (42). If HUT-1 transports UDP-Glc into the ER/Golgi lumen, depletion of HUT-1 would alter the total glycoprotein profile. However, the total glycoprotein profile as examined by lectin blotting with UEA-I, DBA, WGA, PNA, GS-I, GS-II, BPA, MPA, SBA, and UDA showed no obvious difference between wild-type and hut-1 RNAi-treated worms; the exception was ConA, which reflected ER protein overproduction by the UPR (unpublished results). In agreement with this observation, a previous report described that N- and O-glycosylation of proteins is unaltered in scHut1 and sphut1+ mutants in yeast (13). AtUtr1 has been reported to be involved in ER quality control of N-glycosylated proteins by supplying UDP-Glc, a substrate of UGGT, to the calnexin/calreticulin cycle (15). However, null mutations of cnx-1 and crt-1, which encode the C. elegans homologs of calnexin and calreticulin, respectively, exhibit a milder effect on viability than that of hut-1 (43, 44). In addition, cnx-1 and crt-1 mutants exhibit a morphological defect at higher temperatures, which is clearly distinct from the hut-1 phenotype. Furthermore, our genome-wide RNAi survey of glycosyltransferase genes revealed that Phsp-4::GFP induction in hut-1 RNAi-treated worms was stronger than treatment with any RNAi tested in our laboratory, including not only genes encoding UGGT (F26H9.8 and F48E3.3) and α1,3-glucosidase II (F40F9.6), but also the glycosyltransferases (C14A4.3, C08B11.8, ZC513.5, C08H9.3, and T24D1.4) involved in synthesis of the N-glycosylation donor in the ER lumen (unpublished results) (45). In addition, ubiquitous expression of the ZK896.9 protein that has UDP-Glc transporter activity did not rescue the hut-1 null mutant. Thus, it is highly possible that C. elegans HUT-1 does not function as a UDP-Glc transporter coupled with the calnexin/calreticulin cycle in C. elegans.
A previous study showed that hUGTrel1 can transport UDP-GlcA in proteoliposomes (16). However, ubiquitous expression of SQV-7, a UDP-GlcA transporter, failed to rescue the hut-1 mutant. This result argues against the idea that depletion of UDP-GlcA in the ER/Golgi lumen causes a growth defect in hut-1 deficiency. As HUT-1 is similar to two identified PAPS transporters (46, 47), HUT-1 may also be involved in the Golgi sulfation reaction. However, the phenotype of the hut-1 knockout/knockdown worm is also obviously distinct from that of pps-1, a gene encoding the single C. elegans PAPS synthase ortholog (27), which suggests that hut-1 is not involved in the sulfation reaction. Although we cannot exclude the possibility that HUT-1 is the only transporter that is able to transport nucleotide sugars and/or PAPS to a physiologically important subcellular compartment, we showed evidence suggesting that Ce HUT-1 has unique roles in development and ER function other than in major glycosylation reactions. Results of our in vitro experiment also support this finding. To determine the substrates of HUT-1, we expressed the hut-1 gene in yeast and examined the uptake for nucleotide sugars and PAPS. However, none was incorporated into microsomes, whereas recombinant HUT-1 was efficiently expressed (Supplemental Fig. S6).
ER stress is known to trigger phospholipid synthesis to cope with the accumulation of unfolded proteins in the ER (48). HUT-1 could be involved in this process by supplying nucleotide sugar-like molecules that are required for production of membrane components. One supporting piece of evidence is from a genome-wide yeast 2-hybrid study of Drosophila that indicated that the hut-1 fly ortholog interacts with lace, a homolog of the LCB2 subunit of serine palmitoyltransferase (49,50,51). A recent study demonstrated that chronic palmitate induced ER stress and impaired ER morphology (52). Thus, HUT-1 and lipid synthesis may possibly be linked via a direct protein-protein interaction. In any case, a study to determine the interaction partners and real substrates for HUT-1 will provide further understanding of its role in ER homeostasis as well as undiscovered functions of other NSTs. Alternatively, HUT-1 may be involved in nucleotide diphosphate (NDP) metabolism by transport of NDPs (53, 54) because accumulation of the NDPs, by-products yielded by glycosyltransferases, are toxic for the ER, and RNAi of genes required for its metabolism induce the ER stress phenotype in C. elegans.
In C. elegans, perturbations of genes involved in ER quality control also induce abnormality in larval development, which is similar to the phenotypes caused by hut-1 gene inactivation. For example, the hut-1 deletion mutant partially phenocopied the defects of the p97-Npl-4-Ufd-1 complex, which are thought to be essential for ER-associated degradation, and its disruption can strongly induce Phsp-4::GFP expression (data not shown, and refs. 55, 56). These facts support the idea that some sort of ER function controls normal intestinal development and larval growth. HUT-1 would have a critical role in such a contribution of the ER to developmental process. In agreement with this finding, hut-1 RNAi exhibited a synergistic effect on growth defects in the worms with an inactivated UPR. Although it remains to be determined how important the hUGTrel1 protein is in human development, hUGTrel1 is able to compensate for hut-1 genetic inactivation in C. elegans, indicating that hUGTrel1 may be related to the genetic disorders of the ER functions, such as type II diabetes (57, 58). A knockout mouse will provide useful information about the pathophysiologic role of hUGTrel1.
Supplementary Material
Acknowledgments
We thank Dr. Yoshifumi Jigami and Dr. Takehiko Yoko-o (National Institute of Advanced Industrial Science and Technology, Tokyo, Japan) for providing yeast hut1 genes; Dr. Roger Tsien (University of California, San Diego, CA, USA) for providing the mCherry gene; and the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA; supported by the National Institutes of Health–National Center for Research Resources) for providing C. elegans strains. This work was supported by a grant-in-aid for JSPS fellows (to K.D.) and a grant-in-aid for young scientists (B) (to S.M.) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. The study is also supported by the Core Research for Evolutional Science and Technology Program of the Japan Science and Technology Corp. (to K.N.) and for Scientific Research on Priority Areas 14082207 (to K.N.) from MEXT, Japan.
References
- Martinez-Duncker I, Mollicone R, Codogno P, Oriol R. The nucleotide-sugar transporter family: a phylogenetic approach. Biochimie (Paris) 2003;85:245–260. doi: 10.1016/s0300-9084(03)00046-4. [DOI] [PubMed] [Google Scholar]
- Ishida N, Kawakita M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35) Pflügers Arch. 2004;447:768–775. doi: 10.1007/s00424-003-1093-0. [DOI] [PubMed] [Google Scholar]
- Muraoka M, Miki T, Ishida N, Hara T, Kawakita M. Variety of nucleotide sugar transporters with respect to the interaction with nucleoside mono- and diphosphates. J Biol Chem. 2007;282:24615–24622. doi: 10.1074/jbc.M611358200. [DOI] [PubMed] [Google Scholar]
- Berninsone P, Hwang H Y, Zemtseva I, Horvitz H R, Hirschberg C B. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc Natl Acad Sci U S A. 2001;98:3738–3743. doi: 10.1073/pnas.061593098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffaro C E, Hirschberg C B, Berninsone P M. Independent and simultaneous translocation of two substrates by a nucleotide sugar transporter. Proc Natl Acad Sci U S A. 2006;103:16176–16181. doi: 10.1073/pnas.0608159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffaro C E, Hirschberg C B. Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc Chem Res. 2006;39:805–812. doi: 10.1021/ar0400239. [DOI] [PubMed] [Google Scholar]
- Handford M, Rodriguez-Furlan C, Orellana A. Nucleotide-sugar transporters: structure, function and roles in vivo. Braz J Med Biol Res. 2006;39:1149–1158. doi: 10.1590/s0100-879x2006000900002. [DOI] [PubMed] [Google Scholar]
- Ishida N, Kuba T, Aoki K, Miyatake S, Kawakita M, Sanai Y. Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics. 2005;85:106–116. doi: 10.1016/j.ygeno.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hiraoka S, Furuichi T, Nishimura G, Shibata S, Yanagishita M, Rimoin D L, Superti-Furga A, Nikkels P G, Ogawa M, Katsuyama K, Toyoda H, Kinoshita-Toyoda A, Ishida N, Isono K, Sanai Y, Cohn D H, Koseki H, Ikegawa S. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med. 2007;13:1363–1367. doi: 10.1038/nm1655. [DOI] [PubMed] [Google Scholar]
- Luhn K, Wild M K, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- Lubke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, Korner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28:73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- Kainuma M, Chiba Y, Takeuchi M, Jigami Y. Overexpression of HUT1 gene stimulates in vivo galactosylation by enhancing UDP-galactose transport activity in Saccharomyces cerevisiae. Yeast. 2001;18:533–541. doi: 10.1002/yea.708. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Nakayama K, Yokota A, Tachikawa H, Takahashi N, Jigami Y. Hut1 proteins identified in Saccharomyces cerevisiae and Schizosaccharomyces pombe are functional homologues involved in the protein-folding process at the endoplasmic reticulum. Yeast. 2001;18:543–554. doi: 10.1002/yea.707. [DOI] [PubMed] [Google Scholar]
- Norambuena L, Marchant L, Berninsone P, Hirschberg C B, Silva H, Orellana A. Transport of UDP-galactose in plants. Identification and functional characterization of AtUTr1, an Arabidopsis thaliana UDP-galactos/UDP-glucose transporter. J Biol Chem. 2002;277:32923–32929. doi: 10.1074/jbc.M204081200. [DOI] [PubMed] [Google Scholar]
- Reyes F, Marchant L, Norambuena L, Nilo R, Silva H, Orellana A. AtUTr1, a UDP-glucose/UDP-galactose transporter from Arabidopsis thaliana, is located in the endoplasmic reticulum and up-regulated by the unfolded protein response. J Biol Chem. 2006;281:9145–9151. doi: 10.1074/jbc.M512210200. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Sleeman J E, Coughtrie M W H, Burchell B. Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem J. 2006;400:281–289. doi: 10.1042/BJ20060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A J. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- Thorpe G W, Fong C S, Alic N, Higgins V J, Dawes I W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci U S A. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I M, Chrispeels M J. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell. 2003;15:561–576. doi: 10.1105/tpc.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamauchi S, Nakatani H, Nakano C, Urade R. Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 2005;272:3461–3476. doi: 10.1111/j.1742-4658.2005.04770.x. [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis R E, Sakaki K, Kaufman R J. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 2005;1:e37. doi: 10.1371/journal.pgen.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H P, Zhang Y, Zeng H, Novoa I, Lu P D, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl D F, Bell J C, Hettmann T, Leiden J M, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Murray J I, Whitfield M L, Trinklein N D, Myers R M, Brown P O, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capul A A, Barron T, Dobson D E, Turco S J, Beverley S M. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J Biol Chem. 2007;282:14006–14017. doi: 10.1074/jbc.M610869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengyo-Ando K, Mitani S. Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem Biophys Res Commun. 2000;269:64–69. doi: 10.1006/bbrc.2000.2260. [DOI] [PubMed] [Google Scholar]
- Dejima K, Seko A, Yamashita K, Gengyo-Ando K, Mitani S, Izumikawa T, Kitagawa H, Sugahara K, Mizuguchi S, Nomura K. Essential roles of 3′-phosphoadenosine 5′-phosphosulfate synthase in embryonic and larval development of the nematode Caenorhabditis elegans. J Biol Chem. 2006;281:11431–11440. doi: 10.1074/jbc.M601509200. [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando K, Yoshina S, Inoue H, Mitani S. An efficient transgenic system by TA cloning vectors and RNAi for C. elegans. Biochem Biophys Res Commun. 2006;349:1345–1350. doi: 10.1016/j.bbrc.2006.08.183. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Izumikawa T, Mizuguchi S, Dejima K, Nomura K H, Egusa N, Taniguchi F, Tamura J, Gengyo-Ando K, Mitani S, Nomura K, Sugahara K. Expression of rib-1, a Caenorhabditis elegans homolog of the human tumor suppressor EXT genes, is indispensable for heparan sulfate synthesis and embryonic morphogenesis. J Biol Chem. 2007;282:8533–8544. doi: 10.1074/jbc.M611107200. [DOI] [PubMed] [Google Scholar]
- Shaner N C, Campbell R E, Steinbach P A, Giepmans B N G, Palmer A E, Tsien R Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer E L L. An HMM posterior decoder for sequence feature prediction that includes homology information. Bioinformatics. 2005;21:i251–i257. doi: 10.1093/bioinformatics/bti1014. [DOI] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer E L L. Advantages of combined transmembrane topology and signal peptide prediction: the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till J H, Hubbard S R, Harding H P, Clark S G, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Caffaro C E, Luhn K, Bakker H, Vestweber D, Samuelson J, Berninsone P, Hirschberg C B. A single Caenorhabditis elegans Golgi apparatus-type transporter of UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and UDP-N-acetylgalactosamine. Biochemistry. 2008;47:4337–4344. doi: 10.1021/bi702468g. [DOI] [PubMed] [Google Scholar]
- Rolls M M, Hall D H, Victor M, Stelzer E H K, Rapoport T A. Targeting of rough endoplasmic reticulum membrane proteins and ribosomes in invertebrate neurons. Mol Biol Cell. 2002;13:1778–1791. doi: 10.1091/mbc.01-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C J, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Shim J, Umemura T, Nothstein E, Rongo C. The unfolded protein response regulates glutamate receptor export from the endoplasmic reticulum. Mol Biol Cell. 2004;15:4818–4828. doi: 10.1091/mbc.E04-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninsone P, Eckhardt M, Gerardy-Schahn R, Hirschberg C B. Functional expression of the murine Golgi CMP-sialic acid transporter in Saccharomyces cerevisiae. J Biol Chem. 1997;272:12616–12619. doi: 10.1074/jbc.272.19.12616. [DOI] [PubMed] [Google Scholar]
- Ma D, Russell D G, Beverley S M, Turco S J. Golgi GDP-mannose uptake requires Leishmania LPG2: a member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem. 1997;272:3799–3805. [PubMed] [Google Scholar]
- Guerardel Y, Balanzino L, Maes E, Leroy Y, Coddeville B, Oriol R, Strecker G. The nematode Caenorhabditis elegans synthesizes unusual O-linked glycans: identification of glucose-substituted mucin-type O-glycans and short chondroitin-like oligosaccharides. Biochem J. 2001;357:167–182. doi: 10.1042/0264-6021:3570167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B J, Lee D G, Yu J R, Jung S K, Choi K, Lee J, Lee J, Kim Y S, Lee J I, Kwon J Y, Lee J, Singson A, Song W K, Eom S H, Park C S, Kim D H, Bandyopadhyay J, Ahnn J. Calreticulin, a calcium-binding molecular chaperone, is required for stress response and fertility in Caenorhabditis elegans. Mol Biol Cell. 2001;12:2835–2845. doi: 10.1091/mbc.12.9.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Lee T H, Park B-J, Chang J-W, Yu J-R, Koo H-S, Park H, Yoo Y J, Ahnn J. Caenorhabditis elegans calnexin is N-glycosylated and required for stress response. Biochem Biophys Res Commun. 2005;338:1018–1030. doi: 10.1016/j.bbrc.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Berninsone P M. Carbohydrates and glycosylation. WormBook. 2006;18:1–22. doi: 10.1895/wormbook.1.125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama S, Suda T, Ueda R, Suzuki M, Okubo R, Kikuchi N, Chiba Y, Goto S, Toyoda H, Saigo K, Watanabe M, Narimatsu H, Jigami Y, Nishihara S. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J Biol Chem. 2003;278:25958–25963. doi: 10.1074/jbc.M302439200. [DOI] [PubMed] [Google Scholar]
- Kamiyama S, Sasaki N, Goda E, Ui-Tei K, Saigo K, Narimatsu H, Jigami Y, Kannagi R, Irimura T, Nishihara S. Molecular cloning and characterization of a novel 3′-phosphoadenosine 5′-phosphosulfate transporter, PAPST2. J Biol Chem. 2006;281:10945–10953. doi: 10.1074/jbc.M508991200. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman R J. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Nagiec M M, Baltisberger J A, Wells G B, Lester R L, Dickson R C. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc Natl Acad Sci U S A. 1994;91:7899–7902. doi: 10.1073/pnas.91.17.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi-Yamada T, Gotoh T, Sugimura I, Tateno M, Nishida Y, Onuki T, Date H. De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Mol Cell Biol. 1999;19:7276–7286. doi: 10.1128/mcb.19.10.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giot L, Bader J S, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao Y L, Ooi C E, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, Burgess S, McDaniel L, Stimpson E, Spriggs F, Williams J, Neurath K, Ioime N, Agee M, Voss E, Furtak K, Renzulli R, Aanensen N, Carrolla S, Bickelhaupt E, Lazovatsky Y, DaSilva A, Zhong J, Stanyon C A, Finley R L, Jr, White K P, Braverman M, Jarvie T, Gold S, Leach M, Knight J, Shimkets R A, McKenna M P, Chant J, Rothberg J M. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- Uccelletti D, O'Callaghan C, Berninsone P, Zemtseva I, Abeijon C, Hirschberg C B. ire-1-dependent transcriptional up-regulation of a lumenal uridine diphosphatase from Caenorhabditis elegans. J Biol Chem. 2004;279:27390–27398. doi: 10.1074/jbc.M402624200. [DOI] [PubMed] [Google Scholar]
- Uccelletti D, Pascoli A, Farina F, Alberti A, Mancini P, Hirschberg C B, Palleschi A C. APY-1, a novel Caenorhabditis elegans apyrase involved in unfolded protein response signalling and stress responses. Mol Biol Cell. 2008;19:1337–1345. doi: 10.1091/mbc.E07-06-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa Y, Yamanaka K, Ogura T. ER E3 ubiquitin ligase HRD-1 and its specific partner chaperone BiP play important roles in ERAD and developmental growth in Caenorhabditis elegans. Genes Cells. 2007;12:1063–1073. doi: 10.1111/j.1365-2443.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- Mouysset J, Kahler C, Hoppe T. A conserved role of Caenorhabditis elegans CDC-48 in ER-associated protein degradation. J Struct Biol. 2006;156:41–49. doi: 10.1016/j.jsb.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi N N, Ozdelen E, Tuncman G, Gorgun C, Glimcher L H, Hotamisligil G S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Lin J H, Walter P, Yen T S B. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.