Abstract
Angiogenesis is controlled by a balance between stimulators and inhibitors. We propose that the balance, as well as the general sensitivity of the endothelium to these factors, varies from individual to individual. Indeed, we have found that individual mouse strains have dramatically different responses to growth factor-induced neovascularization. Quantitative trait loci (QTLs), which influence the extent of corneal angiogenesis induced by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF2), were previously identified by our laboratory. To investigate the genetic contribution to choroidal neovascularization (CNV), a leading cause of blindness, we have undertaken a similar mapping approach to identify QTLs that influence laser-induced CNV in the BXD series of recombinant inbred mouse strains. Composite interval mapping identified new angiogenic QTLs on chromosomes 2 and 19, in addition to confirming our previous corneal neovascularization QTLs of AngVq1 and AngFq2. The new QTLs are named AngCNVq1 and AngCNVq2. The newly mapped regions contain several candidate genes involved in the angiogenic process, including thrombospondin 1, delta-like 4, BclII modifying factor, phospholipase C, beta 2, adrenergic receptor, beta 1, actin-binding LIM protein 1 and colony stimulating factor 2 receptor, alpha. Differences in these regions may control individual susceptibility to CNV.—Nakai, K., Rogers, M. S., Baba, T., Funakoshi, T., Birsner, A. E., Luyindula, D. S., D’Amato, R. J. Genetic loci that control the size of laser-induced choroidal neovascularization.
Keywords: quantitative trait locus, QTL, recombinant inbred, BXD
Angiogenesis is the process by which existing vessels give rise to new blood vessels. It is controlled by the balance between stimulatory growth factors and endogenous inhibitors. Many growth factors, including vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF2), are important in angiogenesis. Not only has angiogenesis been found to be an important factor in cancer, numerous studies have established its importance in other diseases such as macular degeneration, diabetic retinopathy, heart disease, and obesity.
Individual mouse strains have been found to vary in their response to growth factor-induced neovascularization (1). Previously, we mapped the QTLs for corneal angiogenesis induced by VEGF or FGF2 in BXD recombinant inbred mice (2, 3). We have now used the same strains to map the genetic component of the angiogenic response in the laser-induced choroidal neovascularization model.
Choroidal neovascularization (CNV) is the abnormal growth of blood vessels under the retina. This condition results when age-related macular degeneration (ARMD) progresses to the wet form (so-called because of the blood leakage that accompanies vessel growth), which is a leading cause of blindness. In previous studies of human surgical CNV samples (4) and animal models (5), VEGF was shown to be a key molecule in the development of CNV. In addition to its angiogenic property, VEGF has recently been recognized as a proinflammatory cytokine in the eye (6, 7). FGF2 is also significantly up-regulated in retinal pigment epithelial cells derived from human patients exhibiting CNV (8). Inflammation and immune activation play a role in the pathogenesis of this ARMD (9), including macrophage infiltration (5, 10,11,12) and cytokine secretion (10, 13, 14), similar to the pathological angiogenesis seen in tumors and rheumatoid arthritis. Recently, several independent studies have established a significant association of ARMD with a common coding variant in the CFH gene within the 1q31 locus (15,16,17,18,19). Other genes so far implicated in the ARMD disease process include hemicentin-1 (1q31) (20), APOE (21), fibulin-5 (22), TLR4 (23), ABCA4 (24), ACE (25), CX3CR1 (26), PLEKHA1/LOC387715 (27), and SERPING1 (28).
Laser-induced CNV is a well-established animal model of CNV that is useful for investigating the mechanisms of the corresponding human disease (29,30,31). To explore those genetic factors responsible for differential responsiveness to laser-induced choroidal neovascularization, we chose an interval mapping strategy to identify quantitative trait loci (QTLs) and, eventually, the polymorphisms in the genes responsible for the differential effects in mice.
MATERIALS AND METHODS
Mouse strains and induction of CNV
Laser-induced CNV was generated by a technique described previously with some modifications (31). Mice were anesthetized with an intraperitoneal administration of avertin (240 mg/kg). A mixture of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Mydrin P; Santen Pharmaceutical, Osaka, Japan) was applied to both eyes to dilate the pupils. A lesion was induced by a diode pumped solid state laser (0.1 s; spot size, 50 μm; power 150 mW) around the disc of the retina through a slit lamp delivery system using a Nidek photocoagulator (GYC2000, Nidek, Osaka, Japan). Laser was applied to the posterior pole of the retina, while a hand-held cover slide was used as a contact lens. Only lesions in which a subretinal bubble or focal serous detachment of the retina developed were used for the experiments. All mouse strains were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Choroidal flatmount FITC-dextran perfusion
Following euthanasia by overdose of intraperitoneally injected avertin, 20 ml of ice-cold filtered PBS followed by 1.0 ml of FITC-dextran at a concentration of 50 mg/ml were perfused via the left ventricle. Both eyes were enucleated carefully and saved for the choroidal flatmount procedure. The cornea and lens were removed, and the entire retina was carefully dissected from the eyecup. Radial cuts (average, 8) were made from the edge of the eyecup to the equator. The eyecup was flatmounted in Aquamount with the sclera facing down and the choroid facing up. Fluorescent images of choroidal flatmounts were captured using a charge-coupled device camera (DC500, Leica, Wetzlar, Switzerland). The CNV area (μm2) in choroidal flatmount was evaluated using Scion image software.
QTL analysis software
Windows QTL Cartographer ver. 2.5 (32) was used for linkage analysis. Default settings were used throughout unless otherwise noted. Composite interval mapping was performed using both forward regression with 5 and 6 control markers (default) and forward regression with backward elimination to select control markers. No appreciable difference was observed in the data resulting from the two methods of control marker selection. Significance levels for interval mapping were determined by permutation using 10,000 iterations. Likelihood ratio (LR) scores for various P values are as follows: P < 0.05 = 15.1, P < 0.01 = 18.9, and P < 0.001 = 22.7.
Genotyping and mapping
Genotypes for BXD strains and linkage map data were obtained from the Mouse Genome Database version 4.1, July 19, 2008 (MGD; http://www.informatics.jax.org/) (33), as well as from our own genotyping efforts (2, 3). Physical map positions were obtained from the NCBI annotation of Build 37 of the mouse genome (http://www.ncbi.nlm.nih.gov/genome/ guide/mouse/). For linkage analysis, data were converted to a pseudocentimorgan scale by dividing the position of the marker in mega-base pairs by 2.
Statistical analysis
CNV area was measured and presented as mean ± sd for each group. Differences are analyzed by unpaired two-tailed Student’s t test or ANOVA, and results are considered significant at a value of P < 0.05.
Haplotype analysis
Sequence data for relevant regions of C57BL/6J, DBA/2J, and A/J strains were obtained from dbSNP build 128. Shared haplotype blocks were identified by determining the number of polymorphic SNPs in 100-kbp regions placed every 10 kbp. Shared haplotypes were those regions in which <10 polymorphic differences/100 kbp were observed. Boundaries for haplotype blocks were identified as those SNPs that were an average of >10 kbp from adjacent polymorphic differences (i.e., those SNPs whose nearest different neighbors were >20 kbp apart).
RESULTS
We previously demonstrated substantial differences in corneal angiogenic responsiveness to FGF2 (1) and VEGF (2) between inbred mouse strains using the cornea micropocket model (34). In this paper, we describe results obtained using the laser-induced CNV model. In this model, a disruption of the retinal pigment epithelium layer, Bruch’s membrane, and choroidal vessels results from laser energy deposition in the retinal pigment. This stimulates the growth of CNV. After 14 d, animals were euthanized and perfused with FITC-dextran. Both eyes were enucleated carefully and saved for the choroidal flatmount procedure. The CNV area (μm2) in choroidal flatmount FITC-dextran perfusion was evaluated. We chose C57BL/6J as the background strain for genetic mapping and for analysis of sequence variation because the C57BL/6J genome serves as the mouse reference sequence (35).
Recombinant inbred (RI) strains allow multiple phenotypic measurements for a given genotype, increasing the rigor of QTL mapping. Two major sets of C57BL/6J-derived RI strains are available. One (AXB, BXA) is derived from a cross with A/J mice, and the other (BXD) is derived from a cross with DBA/2J mice. The laser in this model deposits energy in the pigment in the choroid, inducing a break in Bruch’s membrane and subsequent neovascularization. However, the A/J strain lacks pigment due to an albino mutation in the tyrosinase gene. As a result, ∼50% of the AXB/BXA strains derived from A/J mice are unsuitable for this model. For this reason and since the BXD strain set is larger, the BXD strain set was chosen for mapping experiments.
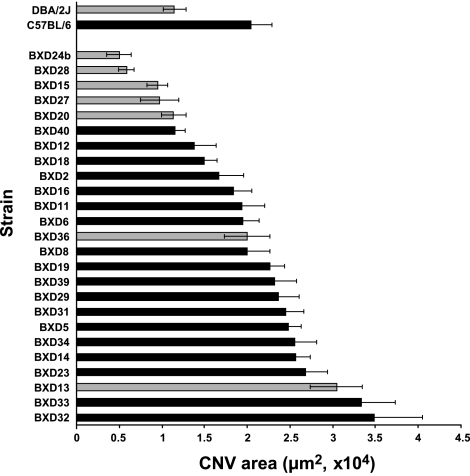
BXD strains, which represent recombinant mixtures of the parental C57BL/6J and DBA/2J strains bred to homozygosity, exhibited a broad range of CNV area from the BXD-24b value of 5010 μm2 to the BXD-32 value of 34810 μm2. The mean of the CNV area for the 27 strains was 19,400 μm2, very near that of the C57BL/6J value of 20,400 μm2 (Fig. 1).
Figure 1.
Laser induced-choroidal neovascularization response in DBA, C57BL/6J and BXD mouse strains. Bar graph plots mean response to laser-induced CNV by DBA, C57BL/6J, and BXD strains. Gray bars, brown mice; black bars, black mice. Error bars = means ± se.
To map the regions of the genome responsible for the variation in the size of laser-induced CNV, genotypes of the BXD strains at marker positions throughout the genome and the map positions of those markers were required. Marker genotypes were obtained as described (3). To facilitate evaluation of candidate regions, we present the mapping information in terms of NCBI build 37 of the mouse genome. The data were analyzed using the QTL Cartographer software package (32).
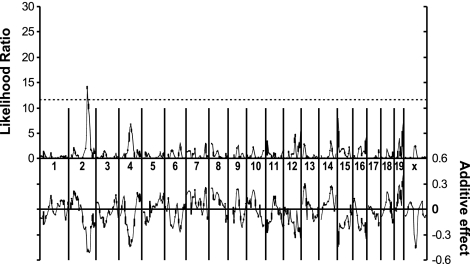
Single-marker regression analysis yielded associations between the size of laser-induced CNV and regions on chromosomes 2, 4, and 15 (P<0.01, Table 1). Markers on chromosomes 12, 17, and 19 showed somewhat weaker association with the size of laser-induced CNV (P<0.05, Table 1). Simple interval mapping (36) revealed a region of significant linkage with the CNV growth on chromosome 2 (Fig. 2). In addition, we noted linkage to the region of the brown locus on chromosome 4 (Fig. 2). In our model, the energy deposited behind the retina is dependant on absorption by pigment. Animals bearing the brown mutation have significantly less choroidal pigment, and thus the amount of energy deposited by the laser is expected to be different, resulting in quantitatively different lesions in these animals. Indeed, we could detect a large bubble in the fully pigmented C57BL/6J animals, while the less pigmented DBA/2J mice merely exhibited opacity of the choroids and retina following laser treatment. These results indicate that damage sufficient to induce angiogenesis is done to choroids of both strains, but laser energy delivered to the choroid is reduced in the brown (DBA/2J) strain compared to the fully pigmented strain (C57BL/6J), thus explaining the reduction of CNV observed in mice bearing the brown allele. To compensate for this effect, all further analysis was done using the z score of the strain in question after dividing the strains into groups according to their genotype at the brown locus.
TABLE 1.
Markers showing significant association with laser-induced choroidal neovascularization area
| Marker | Chr. | b0 (B6 area) | b1 (effect size) | Likelihood ratio | P |
|---|---|---|---|---|---|
| D2Mit62 | 2 | 1.979 | −0.311 | 4.575 | 0.042395 |
| D2Mit396 | 2 | 2.006 | −0.505 | 14.915 | 0.000706 |
| D2Mit398 | 2 | 2.006 | −0.505 | 14.915 | 0.000706 |
| D2Mit304 | 2 | 2.001 | −0.466 | 12.024 | 0.001915 |
| Tyrp1 | 4 | 1.784 | −0.421 | 7.041 | 0.013647 |
| D4Mit327 | 4 | 1.827 | −0.358 | 5.334 | 0.029457 |
| D12Mit3 | 12 | 1.961 | −0.295 | 4.184 | 0.051466 |
| D12Mit14 | 12 | 1.944 | −0.297 | 4.283 | 0.048979 |
| D12Mit8 | 12 | 2.002 | −0.341 | 5.441 | 0.028017 |
| D15Mit12 | 15 | 1.775 | −0.446 | 8.085 | 0.008767 |
| D15Mit13 | 15 | 1.775 | −0.446 | 8.1 | 0.008713 |
| D17Mit129 | 17 | 1.907 | −0.345 | 5.956 | 0.022093 |
| D19Mit1 | 19 | 1.983 | 0.336 | 5.438 | 0.028056 |
| D19Mit6 | 19 | 1.927 | 0.339 | 5.783 | 0.023912 |
Marker denotes marker tested; Chr. denotes the chromosome the marker is on; P denotes the likelihood that there is no relationship between the marker genotype and choroidal neovascularization area (by F test), when the data are fit to the simple linear regression model y = b0 + b1 x + e. The results give the estimates for b0, b1, and the F statistic for each marker. b0 is approximately the average area of C57BL/6J-allele-containing strains. b1 is an indication of the effect of substitution of the DBA/2J allele at that marker.
Figure 2.
Simple interval mapping. Whole-genome simple interval map. Top: likelihood ratio statistic or likelihood of a region being linked to laser-induced choroidal neovascularization. Bottom: predicted additive effect of a region on laser-induced choroidal neovascularization.
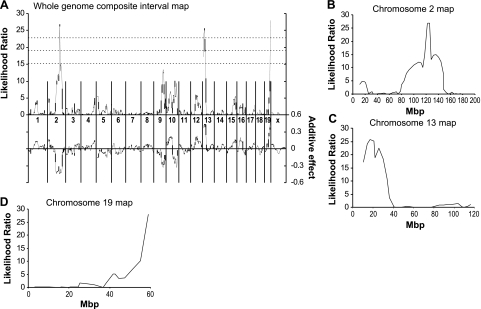
Interval mapping (36) and composite interval mapping was used as a technique to identify genetic regions responsible for the differential angiogenic responsiveness to laser-induced CNV. Composite interval mapping (37, 38) considers the effect of multiple loci. Given that marker association suggests the possibility of multiple linked QTLs and simple interval mapping is inappropriate in such situations, we performed composite interval mapping (37, 38) (CIM; Fig. 3A) with significance levels determined by permutation. This reduced the number of linked areas to 3 exhibiting highly significant linkages (Chr. 2, 13, 19; P<0.001). On chromosomes 2 and 13, the DBA/2J allele causes a decrease in CNV, while on chromosome 19, an increase is observed (Fig. 3A, bottom trace). We concluded that there are laser response-modifying polymorphisms between C57BL/6J and DBA/2J mice on chromosome 2 near 124 Mbp (95% CI from interval mapping, 117.9–148.7 Mbp) (Fig. 3B), on chromosome 13 proximal to 33.8 Mbp (Fig. 3C) and on chromosome 19 distal to 54.9 Mbp (Fig. 3D). In addition, we observed regions on chromosomes 9 and 10 that exhibited near-significant linkage. The locus on chromosome 9 corresponds to the dilute locus, which may have a similar effect as the brown locus on laser energy deposition, albeit weaker. The locus observed on chromosome 10 is consistent with the VEGF-response locus AngVq1 (2) previously mapped by our group. Similarly, the highly significant region on chromosome 13 between 0 and 32 Mbp coincides with AngFq2 (3). We have designated the new regions on chromosomes 2 and 19 AngCNVq1 and AngCNVq2, respectively, for angiogenesis due to laser-induced choroidal neovascularization QTL.
Figure 3.
Composite interval mapping. A) Whole-genome composite interval map. Top: likelihood ratio statistic or likelihood of a region being linked to laser-induced choroidal neovascularization. Bottom: predicted additive effect of a region on laser-induced choroidal neovascularization. B) Chromosome 2 map. C) Chromosome 13 map. D) Chromosome 19 map.
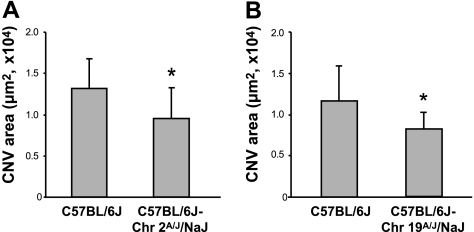
To further localize the region responsible for the decrease in the size of CNV induced by AngCNVq1, we assayed congenic mice bearing a portion of DBA chromosome 2 on an otherwise C57BL/6J background. We applied laser to C57BL/6J, B6.D2-(Mit1-rs3681655)/LusJ and B6.D2-(D2Mit405-D2Mit457)/LusJ mice (39) and compared the size of CNV. However, we did not observe a difference among these 3 strains of mice (data not shown). These results exclude the congenic regions in these strains from further consideration. Genotyping of the B6.D2-(D2Mit405-D2Mit457)/LusJ strain showed that the congenic region extends to rs13476824 in this strain and reduced the critical region for AngCNVq1 to a portion of chromosome 2 between D2Mit62 (117.9 Mbp) and rs13476824 (147.5 Mbp). Because mice congenic for the remaining part of DBA chromosome 2 were unavailable, and given that significant fractions of the A/J and DBA genomes are identical by descent, we tested C57BL/6J-Chr 2A/J/NaJ for the presence of the DBA allele of AngCNVq1. This chromosome substitution strain (40) is a C57BL/6J derivative in which chromosome 2 from the A/J strain replaces the C57BL/6J chromosome 2. We applied laser to C57BL/6J and C57BL/6J-Chr 2A/J/NaJ mice and compared the size of laser-induced CNV. As shown in Fig. 4A, we observed a 27% reduction in C57BL/6J-Chr 2A/J/NaJ mice compared to C57BL/6J mice. This result demonstrates the existence of a QTL on chromosome 2 that decreases CNV and suggests that A/J mice and DBA/2J share the allele at AngCNVq1. An alternative, but less likely possibility, is that a second, distinct, CNV-affecting QTL of similar effect size and direction is present on A/J chromosome 2.
Figure 4.
Size of choroidal neovascularlization area of consomic mice. A) We observed a 27% reduction in C57BL/6J-Chr 2A/J/NaJ mice compared to C57BL/6J mice (1.3±0.3×104 vs. 0.95±0.4×104 μm2, n=10 eyes/group). Error bars = sd. B) On chromosome 19, the C57BL/6J-Chr 19A/J/NaJ mice showed a significant decrease (30%) in CNV area (1.2±0.4×104 vs. 0.8±0.2×104 μm2, n=10 eyes/group). Error bars = means ± sd.
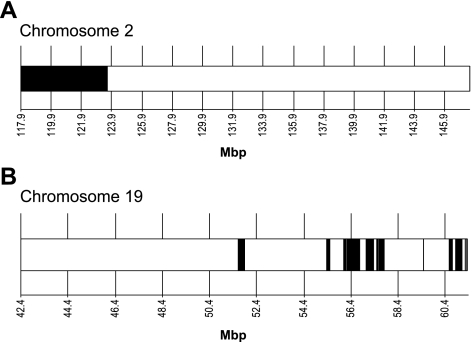
Assuming that A/J and DBA/2J share an AngCNVq1 allele, we next sought to further narrow the region of interest by haplotype analysis. As all three of these strains have been largely sequenced, it is possible to identify regions of the genome where these strains share haplotype (are identical by descent) (41,42,43). In this region, we identified the region in which A/J shares haplotype with DBA/2J that differs from the C57BL/6J haplotype (Fig. 5A). This region contains 118 of the 328 genes in the critical region (Supplemental Table 1).
Figure 5.
Haplotype analysis of AngCNVq1 on chromosome 2 and AngCNVq2 on chromosome 19. A) There is a single region on chromosome 2 (black) in which A/J shares a haplotype with DBA/2J that differs from the C57BL/6J haplotype. B) There are multiple regions on chromosome 19 (white) in which DBA/2J shares haplotype with either C57BL/6J or A/J mice. These regions are less likely to contain the polymorphism that causes AngCNVq2.
We next sought to ascertain whether A/J mice share the DBA/2J allele of AngCNVq2 on chromosome 19. To do this, we applied laser to C57BL/6J and C57BL/6J-Chr 19A/J/NaJ mice and compared the size of laser-induced CNV. If A/J mice harbor the DBA/2J allele of AngCNVq2, we expected a significant increase in CNV areas (Fig. 3A, bottom trace). As shown in Fig. 4B, we did observe a significant difference between these mice, however, the C57BL/6J-Chr 19A/J/NaJ mice showed a significant decrease in CNV area. It is possible that epistatic interactions between this locus and another DBA/2J locus could alter the direction of this QTL, for example, if the chromosome 19 region altered sensitivity to both angiogenic stimulators and inhibitors and the epistatic region altered the balance of stimulators and inhibitors. However, an underpowered multiple interval mapping analysis failed to identify any candidates for such a region and we consider this unlikely. Thus, we conclude that A/J mice do not harbor the DBA/2J allele of AngCNVq2. As a result of the C57BL/6J-Chr 19A/J/NaJ phenotype, we deprioritize regions in which DBA/2J shares haplotype with either C57BL/6J or A/J mice. This leaves 11 regions on chromosome 19 (Fig. 5B) containing 22 of the 167 genes in the original critical region (Supplemental Table 2).
DISCUSSION
Polymorphisms in several genes, such as VEGF, HIF 1 alpha (44), and collagen XVIII/endostatin (45, 46), that regulate angiogenesis have been shown to affect tumor incidence and tumor growth rate. Outside of oncology, VEGF polymorphisms have been linked to a wide variety of angiogenesis-dependent diseases, including arthritis, atherosclerosis, cardiovascular disease, Alzheimer’s disease, Behcet disease, endometriosis, diabetic retinopathy, retinopathy of prematurity, kidney stones, psoriasis, and sarcoidosis. VEGF receptor 2 polymorphisms have been shown to be associated with coronary artery lesions in Kawasaki disease patients (47). In addition, polymorphisms in angiopoietins have been shown as a candidate for a rheumatoid arthritis susceptibility allele (48). Lastly, polymorphisms in thrombospondins have also been associated with cardiovascular disease (49).
Our results demonstrate that a significant fraction of the variance in the mean angiogenic response to laser-induced CNV among BXD strains of mice can be explained by 3 major genetic differences. Because all alleles in RI strains are homozygous, the design of these experiments does not allow for the determination of dominance. One of these loci overlaps with those for FGF-induced corneal angiogenesis (AngFq2). This implies a generalized role of these QTLs in controlling VEGF and bFGF responsiveness throughout the body. It is not surprising that some of the QTLs are shared since VEGF and FGF are each important in the formation of both corneal and choroidal neovasculariation (4, 5, 8).
Candidate genes known to be located near the AngCNVq1 peak on chromosome 2 include thrombospondin 1 (Thbs1), a potent angiogenesis inhibitor (50), delta-like 4 (Dll4), a regulator of endothelial cell sprouting (51), BclII modifying factor (Bmf), an apoptosis-suppressing oncogene (52), and phospholipase C, beta 2 (Plcb2), major signaling enzymes involved in cell migration (53). Haplotype analysis of AngCNVq1 revealed 118 genes in the implicated regions, of which there are 44 genes with annotated amino acid altering SNPs (cSNPs). Thbs1, the gene for thrombospondin 1 exhibits several point mutations that could lead to a change in amino acid sequence. Plcb2, the gene for phopholipase C isoform beta-2, exhibits 1 amino acid change (K1148E). Also, Dll4 exhibits 2 amino acid changes (Q418L, S626G). One or more of these mutations, or polymorphisms in gene regulatory regions may be the cause of the decreased CNV observed in animals from both the DBA/2J and A/J crosses. We are testing these possibilities.
Candidate genes known to be located near the AngCNVq2 peak on chromosome 19 include the adrenergic receptor, beta 1 (Adrb1), which plays an important role in the carcinogenesis of solid tumors (54); actin-binding LIM protein 1 (Ablim1), known as a tumor suppressor protein (55); and colony stimulating factor 2 receptor, alpha (Csf2ra), the high-affinity receptor for granulocyte macrophage colony-stimulating factor (GM-CSF) receptor a subunit (56). However, of these, only Ablim1 cannot be excluded by shared haplotype with DBA/2J or C57BL/6J mouse strains. Among the 22 genes that result from haplotype analysis of the AngCNVq2 region, only Nrap (K179R, L419P), Ablim1 (K17T, S353F), and Sfxn4 (T93S) contain annotated cSNPs. Nrap is unlikely to affect CNV because it is a muscle-specific protein (57). Ablim1 is actin-binding LIM protein 1, known as a tumor suppressor protein (55). Sfxn4 is unlikely to have an effect on CNV because it is largely restricted to kidney, brain, and heart (58).
Several published QTLs whose phenotypic effect could be due to altered angiogenesis coincide with the AngCNVq1 loci (Table 2). These include several QTLs for overall body size, such as Bwq5 (59), which affects body weight at 129P3/J and C57BL/6ByJ cross. In addition, several QTLs whose phenotypic effect could be due to altered angiogenesis coincide with the AngCNVq2 loci that we have identified (Table 2). These include several for overall body size, such as Bw21 (60) and Obwq5 (61). The requirement for new vasculature to support additional tissue mass may explain the correlation between these traits and the size of laser-induced CNV.
TABLE 2.
Published QTLs coincident with laser-induced CNV angiogenesis QTLs
| CNV QTL | Correlate | Effect of correlated QTL | Parental strains |
|---|---|---|---|
| AngCNVq1 | Bdln3 | Body length (48) | 129P3/J × C57BL/6ByJ |
| AngCNVq1 | Bgeq2 | Body growth early (75) | LG/J × SM/J |
| AngCNVq1 | Bts1 | Bladder tumor susceptibility (52) | C57BL/6 J × NON male mice |
| AngCNVq1 | Bw22 | Body weight (49) | DBA/2 × AKR |
| AngCNVq1 | Bw25 | Body weight (49) | DBA/2 × AKR |
| AngCNVq1 | Bwq5 | Body weight (48) | 129P3/J ×C57BL/6ByJ |
| AngCNVq1 | Erars1 | Erosive arthritis susceptibility (53) | C57BL/6J × DBA/2J |
| AngCNVq1 | Lbm2 | Lean body mass (76) | MRL/MPJ × SJL/J |
| AngCNVq1 | Lgth2 | Body length(77) | MRL/MPJ × SJL/J |
| AngCNVq1 | Obq10 | Obesity (78) | NZO × SM |
| AngCNVq1 | Obq3 | Obesity (79) | AKR/J × C57L/J |
| AngCNVq1 | Org1 | Organ weight (80) | LG/J × SM/J |
| AngCNVq1 | Pcyts1 | Plasmacytoma susceptibility (81) | BALB/cAn × C57BL/6J |
| AngCNVq1 | Sluc2 | Susceptibility to lung cancer (51) | O20 × C57BL/10 |
| AngCNVq1 | wtmq1 | Weight module (82) | C57BL/6J × C3H/HeJ |
| AngCNVq2 | Afw8 | Abdominal fat weight (83) | Tally Ho mice × C57BL/6J |
| AngCNVq2 | Bglq13 | Body growth late (75) | LG/J × SM/J |
| AngCNVq2 | Bw21 | Body weight (49) | DBA/2 × AKR |
| AngCNVq2 | Obwq5 | Obesity and body weight (50) | SM/J × NZB/BINJ strains |
| AngCNVq2 | Pgia12 | Proteoglycan induced arthritis (54) | BALB/c × DBA/2 |
| AngCNVq2 | Wtmq9 | Weight module (82) | C57BL/6J × C3H/HeJ |
The necessity for angiogenesis may explain the correspondence between some tumor-related QTLs and our laser-induced angiogenesis QTLs. For example, a lung tumor susceptibility gene, Sluc2 (62), is coincident with laser-induced CNV angiogenesis-related genes. More interesting is the coincidence between AngCNVq1 and Bts1 (63), a locus associated with increased tumor invasiveness in F1 hybrids between B6 and NON (BNF1) mice. Tumors in these mice show peritoneal dissemination, implying that an angiogenesis-related effect may be responsible for increased tumor invasiveness. Also AngCNVq1 is colocated with Erars1 (64) and AngCNVq2 with Pgia12 (65), a QTL for arthritis. We note that in addition to tumor growth, angiogenesis has been shown to be important in several other pathogenic processes, including atherosclerosis and arthritis (66).
Angiogenesis has been demonstrated in many normal and pathological processes. Examples of the former include organ development and regeneration (67), fat deposition (68), hair growth (69), and cyclic changes in the female reproductive tract (70). The latter group includes cancer, macular degeneration, diabetic retinopathy, psoriasis, atherosclerosis, arthritis (66), and endometriosis (71). Further studies are needed to test the relevance of polymorphisms controlling angiogenic responsiveness in these other processes. While it is possible that genetic loci that influence laser-induced CNV may not alter blood vessel development in other tissues, we believe many of the QTLs will have systemic effects. We have already demonstrated a correlation between corneal neovascularization and endothelial migration from aortic rings among different strains of mice (1). We have also demonstrated a correlation between corneal neovascularization and circulating endothelial precursor cell levels among different strains of mice (72). Thus, characterization of novel angiogenesis-regulating genes detected by the QTL mapping of the laser-induced CNV phenotype may broadly identify additional systemic therapeutic targets for antiangiogenic agents.
It is clear that ARMD is a complex genetic disorder (73, 74). A large genome-wide scan study identified four potential ARMD loci (1q31, 2q14.3, 10q26, and 17q25) (74). More recently, in a family study by Klein et al. (75), with 124 families (1669 individuals), including three or more members with ARMD, they added further confirmatory evidence that susceptibility loci lie on 1q, 3p, 9q, and 10q. Furthermore, they identified new loci, including a locus on 6q. It is important to note that these studies are primarily designed to identify alleles that confer risk of developing ARMD, rather than those that increase progression of the disease to blindness.
Human chromosomal regions syntenic to the three highly significant QTLs are 2p, 15q, 20p, 20q (AngCNVq1), 10q (AngCNVq2), and 1q, 6p 7p, 7q, 10p (AngFq2). One of these QTLs found in our study is in mouse locus syntenic to the ARMD-associated human locus on 10q. The other QTLs represent loci of genes that previously were not implicated in ARMD and may serve as candidates for study in ARMD once the responsible gene is identified in mice.
These experiments reveal the important effect that host genetics has on angiogenesis, and therefore on angiogenesis-dependent pathologies. We expect that identification of the specific genes that underlie responsiveness to laser-induced CNV will have prognostic value, as the functional relevance of individual polymorphisms is determined and therapeutic value, as compounds that modify their activity are discovered. There is the possibility that specific and novel antiangiogenic therapies can be developed, which when implemented at early stages of disease development would lower the angiogenic responsiveness of individuals, resulting in increased health and, in the case of life-threatening illness such as cancer, increase survival.
Supplementary Material
Acknowledgments
We thank Kristin Johnson for help in the preparation of digital art. Portions of this work were funded by a grant from the National Eye Institute (RO1 EY12726).
References
- Rohan R M, Fernandez A, Udagawa T, Yuan J, D'Amato R J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- Rogers M S, Rohan R M, Birsner A E, D'Amato R J. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. FASEB J. 2003;17:2112–2114. doi: 10.1096/fj.03-0246fje. [DOI] [PubMed] [Google Scholar]
- Rogers M S, Rohan R M, Birsner A E, D'Amato R J. Genetic loci that control the angiogenic response to basic fibroblast growth factor. FASEB J. 2004;18:1050–1059. doi: 10.1096/fj.03-1241com. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere P V, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- Ishibashi T, Hata Y, Yoshikawa H, Nakagawa K, Sueishi K, Inomata H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1997;235:159–167. doi: 10.1007/BF00941723. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell S E, Moromizato Y, Aiello L P, Ogura Y, Adamis A P. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–1739. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo K G, Amano S, Hida T, Oguchi Y, Adamis A P. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Martin G, Schlunck G, Hansen L L, Agostini H T. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2004;242:321–326. doi: 10.1007/s00417-003-0838-y. [DOI] [PubMed] [Google Scholar]
- Penfold P L, Madigan M C, Gillies M C, Provis J M. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–1898. [PubMed] [Google Scholar]
- Tsutsumi C, Sonoda K H, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo I F, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati B K, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Krzystolik M G, Afshari M A, Adamis A P, Gaudreault J, Gragoudas E S, Michaud N A, Li W, Connolly E, O'Neill C A, Miller J W. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Amin R, Puklin J E, Frank R N. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35:3178–3188. [PubMed] [Google Scholar]
- Edwards A O, Ritter R, 3rd, Abel K J, Manning A, Panhuysen C, Farrer L A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Hageman G S, Anderson D H, Johnson L V, Hancox L S, Taiber A J, Hardisty L I, Hageman J L, Stockman H A, Borchardt J D, Gehrs K M, Smith R J, Silvestri G, Russell S R, Klaver C C, Barbazetto I, Chang S, Yannuzzi L A, Barile G R, Merriam J C, Smith R T, Olsh A K, Bergeron J, Zernant J, Merriam J E, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J L, Hauser M A, Schmidt S, Scott W K, Olson L M, Gallins P, Spencer K L, Kwan S Y, Noureddine M, Gilbert J R, Schnetz-Boutaud N, Agarwal A, Postel E A, Pericak-Vance M A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Klein R J, Zeiss C, Chew E Y, Tsai J Y, Sackler R S, Haynes C, Henning A K, SanGiovanni J P, Mane S M, Mayne S T, Bracken M B, Ferris F L, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareparsi S, Branham K E, Li M, Shah S, Klein R J, Ott J, Hoh J, Abecasis G R, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D W, Klein M L, Humpert A J, Luzier C W, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V, Doyle T M, Martin T M, Weleber R G, Francis P J, Acott T S. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12:3315–3323. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Saunders A M, De La Paz M A, Postel E A, Heinis R M, Agarwal A, Scott W K, Gilbert J R, McDowell J G, Bazyk A, Gass J D, Haines J L, Pericak-Vance M A. Association of the apolipoprotein E gene with age-related macular degeneration: possible effect modification by family history, age, and gender. Mol Vis. 2000;6:287–293. [PubMed] [Google Scholar]
- Stone E M, Braun T A, Russell S R, Kuehn M H, Lotery A J, Moore P A, Eastman C G, Casavant T L, Sheffield V C. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Buraczynska M, Branham K E, Shah S, Eng D, Li M, Pawar H, Yashar B M, Moroi S E, Lichter P R, Petty H R, Richards J E, Abecasis G R, Elner V M, Swaroop A. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet. 2000;67:487–491. doi: 10.1086/303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi H K, Reznik J, Castellon R, Atilano S R, Ong J M, Udar N, Tavis J H, Aoki A M, Nesburn A B, Boyer D S, Small K W, Brown D J, Kenney M C. Alu DNA polymorphism in ACE gene is protective for age-related macular degeneration. Biochem Biophys Res Commun. 2002;295:668–672. doi: 10.1016/s0006-291x(02)00728-3. [DOI] [PubMed] [Google Scholar]
- Tuo J, Smith B C, Bojanowski C M, Meleth A D, Gery I, Csaky K G, Chew E Y, Chan C C. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsdottir J, Conley Y P, Weeks D E, Mah T S, Ferrell R E, Gorin M B. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S, Jomary C, Mullins R, Cree A, Chen X, Macleod A, Jones S, Collins A, Stone E, Lotery A. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008;372:1828–1834. doi: 10.1016/S0140-6736(08)61348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S J. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc. 1979;77:707–745. [PMC free article] [PubMed] [Google Scholar]
- Dobi E T, Puliafito C A, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch Ophthalmol. 1989;107:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna J D, Ozaki H, Okamoto N, Derevjanik N L, Vinores S A, Basilico C, Campochiaro P A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z B, Weir B S, Basten C J. Zmap—a QTL cartographer. Smith C, Gavora J S, Benkel B, Chesnais J, Fairfull W, Gibson J P, Kennedy B W, Burnside E B, editors. Guelph, ON, Canada: Organizing Committee, 65th World Congress on Genetics Applied to Livestock Production; 1994:65–66. [Google Scholar]
- Blake J A, Richardson J E, Bult C J, Kadin J A, Eppig J T. The nouse genome database (MGD): the model organism database for the laboratory mouse. Nucleic Acids Res. 2002;30:113–115. doi: 10.1093/nar/30.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M S, Birsner A E, D'Amato R J. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- Waterston R H, Lindblad-Toh K, Birney E, Rogers J, Abril J F, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis S E, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent M R, Brown D G, Brown S D, Bult C, Burton J, Butler J, Campbell R D, Carninci P, Cawley S, Chiaromonte F, Chinwalla A T, Church D M, Clamp M, Clee C, Collins F S, Cook L L, Copley R R, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty K D, Deri J, Dermitzakis E T, Dewey C, Dickens N J, Diekhans M, Dodge S, Dubchak I, Dunn D M, Eddy S R, Elnitski L, Emes R D, Eswara P, Eyras E, Felsenfeld A, Fewell G A, Flicek P, Foley K, Frankel W N, Fulton L A, Fulton R S, Furey T S, Gage D, Gibbs R A, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves T A, Green E D, Gregory S, Guigó R, Guyer M, Hardison R C, Haussler D, Hayashizaki Y, Hillier L W, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe D B, Johnson L S, Jones M, Jones T A, Joy A, Kamal M, Karlsson E K, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent W J, Kirby A, Kolbe D L, Korf I, Kucherlapati R S, Kulbokas E J, Kulp D, Landers T, Leger J P, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott D R, Mardis E R, Matthews L, Mauceli E, Mayer J H, McCarthy M, McCombie W R, McLaren S, McLay K, McPherson J D, Meldrim J, Meredith B, Mesirov J P, Miller W, Miner T L, Mongin E, Montgomery K T, Morgan M, Mott R, Mullikin J C, Muzny D M, Nash W E, Nelson J O, Nhan M N, Nicol R, Ning Z, Nusbaum C, O'Connor M J, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin K H, Peterson J, Pevzner P, Plumb R, Pohl C S, Poliakov A, Ponce T C, Ponting C P, Potter S, Quail M, Reymond A, Roe B A, Roskin K M, Rubin E M, Rust A G, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz M S, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer J B, Slater G, Smit A, Smith D R, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson J P, Von Niederhausern A C, Wade C M, Wall M, Weber R J, Weiss R B, Wendl M C, West A P, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson R K, Winter E, Worley K C, Wyman D, Yang S, Yang S P, Zdobnov E M, Zody M C, Lander E S. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Lander E S, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z B. Precision mapping of quantitative trait loci. Genetics. 1994;136:1457–1468. doi: 10.1093/genetics/136.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z B. Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc Natl Acad Sci U S A. 1993;90:10972–10976. doi: 10.1073/pnas.90.23.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R C, Schadt E E, Smith D J, Hsieh E W, Cervino A C, van Nas A, Rosales M, Doss S, Meng H, Allayee H, Lusis A J. A genome-wide set of congenic mouse strains derived from DBA/2J on a C57BL/6J background. Genomics. 2005;86:259–270. doi: 10.1016/j.ygeno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nadeau J H, Singer J B, Matin A, Lander E S. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Wade C M, Kulbokas E J, 3rd, Kirby A W, Zody M C, Mullikin J C, Lander E S, Lindblad-Toh K, Daly M J. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- Yang H, Bell T A, Churchill G A, Pardo-Manuel de Villena F. On the subspecific origin of the laboratory mouse. Nat Genet. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- Frazer K A, Eskin E, Kang H M, Bogue M A, Hinds D A, Beilharz E J, Gupta R V, Montgomery J, Morenzoni M M, Nilsen G B, Pethiyagoda C L, Stuve L L, Johnson F M, Daly M J, Wade C M, Cox D R. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Yoshiga K, Eguchi H, Kaneyasu M, Ukon K, Kumazaki T, Oue N, Yasui W, Imai K, Nakachi K, Poellinger L, Nishiyama M. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis. 2003;24:1779–1783. doi: 10.1093/carcin/bgg132. [DOI] [PubMed] [Google Scholar]
- Iughetti P, Suzuki O, Godoi P H, Alves V A, Sertie A L, Zorick T, Soares F, Camargo A, Moreira E S, di Loreto C, Moreira-Filho C A, Simpson A, Oliva G, Passos-Bueno M R. A polymorphism in endostatin, an angiogenesis inhibitor, predisposes for the development of prostatic adenocarcinoma. Cancer Res. 2001;61:7375–7378. [PubMed] [Google Scholar]
- Balasubramanian S P, Cross S S, Globe J, Cox A, Brown N J, Reed M W. Endostatin gene variation and protein levels in breast cancer susceptibility and severity. BMC Cancer. 2007;7:107. doi: 10.1186/1471-2407-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyazono H, Ohno T, Khajoee V, Ihara K, Kusuhara K, Kinukawa N, Mizuno Y, Hara T. Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res. 2004;56:953–959. doi: 10.1203/01.PDR.0000145280.26284.B9. [DOI] [PubMed] [Google Scholar]
- Shiozawa S, Komai K, Kawasaki H, Sato M, Nakatsukasa M, Nakashima T, Okayama R, Sakai C. [The molecular genetics of rheumatoid arthritis disease gene] Nippon Rinsho. 2002;60:2269–2275. [PubMed] [Google Scholar]
- Topol E J, McCarthy J, Gabriel S, Moliterno D J, Rogers W J, Newby L K, Freedman M, Metivier J, Cannata R, O'Donnell C J, Kottke-Marchant K, Murugesan G, Plow E F, Stenina O, Daley G Q. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–2644. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- Ren B, Yee K O, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Lobov I B, Renard R A, Papadopoulos N, Gale N W, Thurston G, Yancopoulos G D, Wiegand S J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X B, Masago R, Kong L, Zhang B X, Masago S, Vela-Roch N, Katz M S, Yeh C K, Zhang G H, Talal N, Dang H. G-protein signaling abnormalities mediated by CD95 in salivary epithelial cells. Cell Death Differ. 2000;7:1119–1126. doi: 10.1038/sj.cdd.4400745. [DOI] [PubMed] [Google Scholar]
- Suh P G, Hwang J I, Ryu S H, Donowitz M, Kim J H. The roles of PDZ-containing proteins in PLC-beta-mediated signaling. Biochem Biophys Res Commun. 2001;288:1–7. doi: 10.1006/bbrc.2001.5710. [DOI] [PubMed] [Google Scholar]
- Wong H P, Yu L, Lam E K, Tai E K, Wu W K, Cho C H. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci. 2007;97:279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- Kim A C, Peters L L, Knoll J H, Van Huffel C, Ciciotte S L, Kleyn P W, Chishti A H. Limatin (LIMAB1), an actin-binding LIM protein, maps to mouse chromosome 19 and human chromosome 10q25, a region frequently deleted in human cancers. Genomics. 1997;46:291–293. doi: 10.1006/geno.1997.5029. [DOI] [PubMed] [Google Scholar]
- Brannan C I, Disteche C M, Park L S, Copeland N G, Jenkins N A. Autosomal telomere exchange results in the rapid amplification and dispersion of Csf2ra genes in wild-derived mice. Mamm Genome. 2001;12:882–886. doi: 10.1007/s00335-001-2084-0. [DOI] [PubMed] [Google Scholar]
- Carroll S, Lu S, Herrera A H, Horowits R. N-RAP scaffolds I-Z-I assembly during myofibrillogenesis in cultured chick cardiomyocytes. J Cell Sci. 2004;117:105–114. doi: 10.1242/jcs.00847. [DOI] [PubMed] [Google Scholar]
- Zheng H, Ji C, Zou X, Wu M, Jin Z, Yin G, Li J, Feng C, Cheng H, Gu S, Xie Y, Mao Y. Molecular cloning and characterization of a novel human putative transmembrane protein homologous to mouse sideroflexin associated with sideroblastic anemia. DNA Seq. 2003;14:369–373. doi: 10.1080/10425170310001605491. [DOI] [PubMed] [Google Scholar]
- Reed D R, Li X, McDaniel A H, Lu K, Li S, Tordoff M G, Price R A, Bachmanov A A. Loci on chromosomes 2, 4, 9, and 16 for body weight, body length, and adiposity identified in a genome scan of an F2 intercross between the 129P3/J and C57BL/6ByJ mouse strains. Mamm Genome. 2003;14:302–313. doi: 10.1007/s00335-002-2170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J D, Bhasin J M, Baglione J, Settle M, Xu Y, Barnard J. Atherosclerosis susceptibility loci identified from a strain intercross of apolipoprotein E-deficient mice via a high-density genome scan. Arterioscler Thromb Vasc Biol. 2006;26:597–603. doi: 10.1161/01.ATV.0000201044.33220.5c. [DOI] [PubMed] [Google Scholar]
- Stylianou I M, Korstanje R, Li R, Sheehan S, Paigen B, Churchill G A. Quantitative trait locus analysis for obesity reveals multiple networks of interacting loci. Mamm Genome. 2006;17:22–36. doi: 10.1007/s00335-005-0091-2. [DOI] [PubMed] [Google Scholar]
- Tripodis N, Hart A A, Fijneman R J, Demant P. Complexity of lung cancer modifiers: mapping of thirty genes and twenty-five interactions in half of the mouse genome. J Natl Cancer Inst. 2001;93:1484–1491. doi: 10.1093/jnci/93.19.1484. [DOI] [PubMed] [Google Scholar]
- Higashi S, Murai T, Mori S, Kamoto T, Yoshitomi M, Arakawa Y, Makino S, Fukushima S, Yoshida O, Hiai H. Host genes affecting survival period of chemically induced bladder cancer in mice. J Cancer Res Clin Oncol. 1998;124:670–676. doi: 10.1007/s004320050230. [DOI] [PubMed] [Google Scholar]
- Mountz J D, Yang P, Wu Q, Zhou J, Tousson A, Fitzgerald A, Allen J, Wang X, Cartner S, Grizzle W E, Yi N, Lu L, Williams R W, Hsu H C. Genetic segregation of spontaneous erosive arthritis and generalized autoimmune disease in the BXD2 recombinant inbred strain of mice. Scand J Immunol. 2005;61:128–138. doi: 10.1111/j.0300-9475.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- Otto J M, Cs-Szabo G, Gallagher J, Velins S, Mikecz K, Buzas E I, Enders J T, Li Y, Olsen B R, Glant T T. Identification of multiple loci linked to inflammation and autoantibody production by a genome scan of a murine model of rheumatoid arthritis. Arthritis Rheum. 1999;42:2524–2531. doi: 10.1002/1529-0131(199912)42:12<2524::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cherrington J M, Strawn L M, Shawver L K. New paradigms for the treatment of cancer: the role of anti-angiogenesis agents. Adv Cancer Res. 2000;79:1–38. doi: 10.1016/s0065-230x(00)79001-4. [DOI] [PubMed] [Google Scholar]
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- Rupnick M A, Panigrahy D, Zhang C Y, Dallabrida S M, Lowell B B, Langer R, Folkman M J. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown L F, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S K. Angiogenesis and reproduction. BJOG. 2001;108:777–783. doi: 10.1111/j.1471-0528.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6:45–55. doi: 10.1093/humupd/6.1.45. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers M S, Cervi D, Foutz T, Rawn K, Voskas D, Dumont D J, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin D J, D'Amato R J, Kerbel R S. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Gorin M B, Breitner J C, De Jong P T, Hageman G S, Klaver C C, Kuehn M H, Seddon J M. The genetics of age-related macular degeneration. Mol Vis. 1999;5:29. [PubMed] [Google Scholar]
- Weeks D E, Conley Y P, Tsai H J, Mah T S, Schmidt S, Postel E A, Agarwal A, Haines J L, Pericak-Vance M A, Rosenfeld P J, Paul T O, Eller A W, Morse L S, Dailey J P, Ferrell R E, Gorin M B. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am J Hum Genet. 2004;75:174–189. doi: 10.1086/422476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S, Francis P J, Schultz D W, Schain M B, Haynes C, Majewski J, Ott J, Acott T, Weleber R G, Klein M L. Expanded genome scan in extended families with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5453–5459. doi: 10.1167/iovs.06-0655. [DOI] [PubMed] [Google Scholar]
- Cheverud J M, Routman E J, Duarte F A, van Swinderen B, Cothran K, Perel C. Quantitative trait loci for murine growth. Genetics. 1996;142:1305–1319. doi: 10.1093/genetics/142.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinde G L, Li X, Gu W, Davidson H, Hamilton-Ulland M, Wergedal J, Mohan S, Baylink D J. Quantitative trait loci (QTL) for lean body mass and body length in MRL/MPJ and SJL/J F(2) mice. Funct Integr Genomics. 2002;2:98–104. doi: 10.1007/s10142-002-0053-7. [DOI] [PubMed] [Google Scholar]
- Masinde G L, Li X, Gu W, Hamilton-Ulland M, Mohan S, Baylink D J. Quantitative trait loci that harbor genes regulating muscle size in (MRL/MPJ x SJL/J) F(2) mice. Funct Integr Genomics. 2002;2:120–125. doi: 10.1007/s10142-002-0067-1. [DOI] [PubMed] [Google Scholar]
- Taylor B A, Wnek C, Schroeder D, Phillips S J. Multiple obesity QTLs identified in an intercross between the NZO (New Zealand obese) and the SM (small) mouse strains. Mamm Genome. 2001;12:95–103. doi: 10.1007/s003350010254. [DOI] [PubMed] [Google Scholar]
- Taylor B A, Phillips S J. Obesity QTLs on mouse chromosomes 2 and 17. Genomics. 1997;43:249–257. doi: 10.1006/geno.1997.4835. [DOI] [PubMed] [Google Scholar]
- Kenney-Hunt J P, Vaughn T T, Pletscher L S, Peripato A, Routman E, Cothran K, Durand D, Norgard E, Perel C, Cheverud J M. Quantitative trait loci for body size components in mice. Mamm Genome. 2006;17:526–537. doi: 10.1007/s00335-005-0160-6. [DOI] [PubMed] [Google Scholar]
- Symons R C, Daly M J, Fridlyand J, Speed T P, Cook W D, Gerondakis S, Harris A W, Foote S J. Multiple genetic loci modify susceptibility to plasmacytoma-related morbidity in E(mu)-v-abl transgenic mice. Proc Natl Acad Sci U S A. 2002;99:11299–11304. doi: 10.1073/pnas.162566999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazalpour A, Doss S, Zhang B, Wang S, Plaisier C, Castellanos R, Brozell A, Schadt E E, Drake T A, Lusis A J, Horvath S. Integrating genetic and network analysis to characterize genes related to mouse weight. PLoS Genet. 2006;2:e130. doi: 10.1371/journal.pgen.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A C, Olver W I, Donnelly C J, May M E, Naggert J K, Shaffer D J, Roopenian D C. Searching QTL by gene expression: analysis of diabesity. BMC Genet. 2005;6:12. doi: 10.1186/1471-2156-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.