Abstract
The effects of shear stress on the keratin intermediate filament (KIF) cytoskeleton of cultured human alveolar epithelial (A549) cells have been investigated. Under normal culture conditions, immunofluorescence revealed a delicate network of fine tonofibrils containing KIFs, together with many nonfilamentous, keratin-containing “particles,” mostly containing either keratin 8 (K8) or 18 (K18), but not both. Triton X-100 extracted ∼10% of the cellular keratin, and this was accompanied by a loss of the particles but not the KIFs. Shear stress dramatically reduced the soluble keratin component and transformed the fine bundles of KIFs into thicker, “wavy” tonofibrils. Both effects were accompanied by the disappearance of most keratin particles and by increased phosphorylation of K8 and K18 on serine residues 73 and 33, respectively. The particles that remained after shearing were phosphorylated and were closely associated with KIFs. We suggest that keratin particles constitute a reservoir of protein that can be recruited into KIFs under flow, creating a more robust cytoskeleton able to withstand shear forces more effectively.—Flitney, E. W., Kuczmarski, E. R., Adam, S. A., Goldman, R. D. Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments.
Keywords: phosphorylation, tonofibrils, cytoskeleton
Type I (acidic) and Type II (neutral-basic) keratins constitute the largest group of intermediate filament (IF) proteins in the cytoskeleton of eukaryotic cells (1). Keratin IFs (KIFs) are expressed in both simple and stratified epithelia as obligate heteropolymers, assembled from at least one Type I and one Type II protein (2). Different combinations of Type I and Type II proteins, known as keratin “pairs,” are coordinately expressed in epithelial cells during development (3). Simple epithelial cells, found in liver, kidney, pancreas, intestine, and lung, all express the highly conserved keratins 8 and 18 (K8 and K18), often in association with variable amounts of secondary keratins, including keratins 7, 19, 20, and 23 (4).
One of the major functions of KIFs in stratified epithelia is to enable cells to withstand mechanical stresses, for example, frictional forces or strain (3). This important role was highlighted by the discovery that mutations in the highly conserved, central α-helical rod domains of K5/14 or K1/10 cause a variety of human skin fragility diseases (5, 6). Although the functions of the keratins of simple epithelia are less well understood, they have been implicated in many other cellular processes, including cell signaling, apoptosis, cell cycle progression, gene transcription, and cytoprotection (4, 7). However, there is little evidence for a mechanical role for keratins in simple epithelia, comparable to that of the keratins in stratified epithelia.
The once commonly held view of the keratin cytoskeleton as a relatively inert entity has been overturned in light of recent experiments showing that KIFs exhibit a remarkable range of dynamic properties, at both molecular and supramolecular levels. Newly expressed mutant keratins (8) or tissue-derived keratins microinjected into epithelial cells (9, 10) readily integrate into preexisting keratin networks. Small amounts of soluble keratins (∼5%) have also been identified in some cells (11,12,13). These observations suggested that soluble keratin protein subunits exist in equilibrium with filamentous (polymerized) protein, a hypothesis since confirmed by experiments on living cells expressing GFP-keratin fusion proteins (14, 15). These studies, and related live cell imaging experiments on other IF systems showing that photobleached IFs fully recover their fluorescence on time scales ranging from a few minutes (e.g., vimentin) to several hours (e.g., keratins), provide direct evidence for the existence of a dynamic exchange between soluble and polymeric states (15,16,17,18,19,20).
Intermediate filament proteins exist in several structural forms: small, nonfilamentous aggregates, referred to as “particles” or “granules;” short, filamentous structures or “squiggles;” fully formed 10-nm IFs; and large “bundles” of IFs or tonofibrils. Particles and squiggles are highly motile structures involved in the turnover and assembly of IF networks (15, 17, 21). Both actin- and microtubule-based molecular motors have been implicated in their motion (17, 20, 22,23,24). Large-scale movements of keratin networks have also been observed. Tonofibrils have been seen to translocate from one part of a cell to another and to undergo complex bending movements, including the propagation of longitudinal waves (14). Changes in keratin organization occur during mitosis, in which orchestrated rounds of disassembly and reassembly of KIF networks have been reported for some epithelial cells (25,26,27,28). Furthermore, entire keratin networks can be made to disassemble and form discrete particles in response to drug treatment (29, 30) or as the result of mechanical interventions (31, 32).
Phosphorylation plays a major role in the organization of IFs by regulating their assembly state and/or by modifying the extent and manner of their interactions with other cell components (reviewed by ref. 33). K8 and K18 are phosphorylated on several serine residues (Ser-23, -73, and -431 on K8 and Ser-33 and -52 on K18) located in their N- and C-terminal domains (4). Phosphorylation of KIFs increases during the S and G2/M phases of the cell cycle (34), during apoptosis (35), and in response to both stress (36) and growth factor stimulation (37). K8 and K18 are targets for several protein kinases, including ERK1/2 (K8 Ser-431), JNK and p38 (K8 Ser-73), PKA (K8 Ser-23), PKC (K18 Ser-52), and cdc2 kinases (K18 Ser-33) (33). The ratio of phosphorylated to dephosphorylated IF proteins is determined by the activities of these kinases acting in concert with types 1 and 2A protein phosphatases (38,39,40,41).
Vimentin and keratin IFs can respond to the tractive force generated by a flowing liquid acting at the surface of a cell, called fluid shear stress. The nature of the response depends on the cell type and on the magnitude and duration of the forces. Moderate shear stresses (<15 dyn/cm2) are well tolerated by some cells (14), most notably by endothelial cells (42, 43). On the other hand, high shear stresses, especially when sustained for long periods, can be detrimental and may result in severe damage to IF networks. We recently reported (32) that the KIF network in primary cultures of rat type II alveolar epithelial cells suffered catastrophic collapse when cells were exposed to ∼30 dyn/cm2 for 24 h. We have now studied the morphological and biochemical responses of the KIF cytoskeleton in cultured human alveolar type II-like cells (A549 cells) exposed to more moderate shear stresses (7–15 dyn/cm2) of shorter duration (1–10 h). These comparatively mild conditions were chosen to improve the temporal resolution of our experiments, permitting us to focus on the early changes induced by fluid shear stress.
MATERIALS AND METHODS
Cell culture
A549 cells (American Type Culture Collection, Manassas, VA, USA; catalog no. CCL-185) were grown in DMEM, supplemented with 10% fetal bovine serum, 2% penicillin/streptomycin, and 23 mM HEPES buffer (pH 7.2) for 3–4 d in a humidified incubator, maintained at 37°C and in an atmosphere of 95% air/5% CO2. Culture medium was renewed on alternate days, and cells were used when fully confluent or at ∼70–80% confluence, as required.
Flow experiments
Cells were grown (2–4 d) on microscope slides and placed in a flow chamber (Streamer; FlexCell International, Hillsborough, NC, USA) that formed part of a closed-loop perfusion system, driven by a peristaltic pump. Serum-free DMEM was withdrawn from a reservoir and passed through a bubble trap, which also eliminated flow oscillations caused by the pump before entering the chamber. The pump was adjusted to generate uniform, laminar shear stresses of 7–20 dyn/cm2. The rate of flow was incremented gradually, in steps of ∼10% of the final flow rate at intervals of ∼2 min, to minimize cell detachment. The apparatus was housed in an incubator maintained at 37°C and in an atmosphere of 95% air/5% CO2. Nonsheared (control) cells were kept under static culture conditions.
Immunofluorescence
Cells were grown for 3–4 d and routinely fixed in 3.7% formaldehyde in PBS for 10 min at room temperature. For some purposes cells were either fixed in methanol (−20°C for 5 min) instead of formaldehyde, or they were first overlain with 0.5% Triton X-100 containing buffer (TX-100 buffer: NaCl, 100 mM; sucrose, 300 mM; MgCl2, 10 mM; Triton X-100, 0.5%; PIPES, 10 mM, pH 6.8) for 5 min on ice, before being fixed in formaldehyde. Antibody staining was carried out as described elsewhere (44). Cells were examined in a Zeiss LSM510 META confocal microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA) or in a Zeiss Imager Z1 wide-field fluorescence microscope. Images were obtained using an ×100 planapochromat phase contrast objective (NA: 1.4) and processed using Adobe Photoshop imaging software (Adobe Systems, San Jose, CA, USA). Wide-field images were deconvolved using an inverse filter algorithm in Axiovision software (Carl Zeiss).
Preparation of cell extracts
Cells were grown on culture plates (100-mm diameter) or on microscope slides (6 slides, after shearing) and overlaid with ∼1 ml TX-100 buffer containing protease inhibitors (complete MINI, EDTA-free, 1 tablet/10 ml; Roche Diagnostics, Indianapolis, IN, USA) for 5 min in an ice-water bath, simulating the conditions used to preextract cells for immunofluorescence (described above). The TX-100-soluble fraction was collected, taking care not to dislodge cells from the plate/microscope slide. The TX-insoluble fraction was solubilized in ∼1 ml boiling 1× sample buffer. Both samples were centrifuged at 100,000 g for 15 min at 4°C, and the volumes of the supernatants were equalized by adding TX-100 buffer or 1× sample buffer, as appropriate. Finally, 250 μl of 3× sample buffer was added to 500 μl of TX-100 extract, and 250 μl of 1× sample buffer was added to 500 μl of the cell residue extract. Samples were then boiled for 5 min and stored at −80°C. The protein concentration (mg/ml) in each extract was measured using a reagent-detergent compatible assay kit (RC/DC Kit; Bio-Rad Laboratories, Hercules, CA, USA).
Quantitative Western blotting
Measurements of K8 and K18 in whole-cell lysates and in TX-100-soluble and -insoluble cell fractions were made by Western blotting, using recombinant human proteins for calibration purposes. SDS-polyacrylamide (7.5–12%) gels were run of cell extracts, with 6–8 known amounts of recombinant K8 or K18 loaded onto the same gel in parallel lanes. The quantities of recombinant standards used were chosen to encompass the range of each protein present in the extracts (i.e., from 5–500 ng/lane for “unknown” sample volumes of 5–10 μl). Proteins were transferred onto a nitrocellulose membrane, blocked with 7.5% nonfat milk in PBS with Tween 20 and probed with the appropriate primary and secondary antibodies. Immunoblots were developed using ECL Western blotting Detection Reagent (Amersham Biosciences, Piscataway, NJ, USA). Chemiluminescence was detected using a Kodak Image Station 440CF (Eastman Kodak, Rochester, NY, USA) adjusted optimally to ensure that the CCD detector did not saturate. Band intensities were measured using Kodak Molecular Imaging software.
The relationship between the band intensities and the quantity of protein standard applied to each lane was described by a hyperbolic function, Y = Bmax.X/[k+X], where Y is the maximum band intensity and k is the amount of protein standard giving 50% of the maximum intensity. The calibration curve obtained for each immunoblot was analyzed using a nonlinear regression algorithm, and only data sets giving regression coefficients (r2) equal to or greater than 0.98 were included in the analyses. The amounts of K8 and K18 in TX-100-soluble and -insoluble fractions were estimated by inserting the band intensity for each unknown sample into the above equation and solving for X. The mean ± se values obtained, based on 3–5 individual blots per cell extract, are expressed throughout in picomoles or micrograms per millgram protein, or as a percentage of the total cell protein.
Antibodies and reagents
pAbs that recognized K8 and K18 were produced by immunizing rabbits with proteins isolated from rat liver and purified by SDS-PAGE. Mouse mAbs to K8 (Ks 8.7) and K18 (Ks 18.04) and recombinant human K8 and K18 proteins were obtained from Research Diagnostics (Flanders, NJ, USA). Phosphoepitope-specific antibodies recognizing K8-Ser-73P (LJ4) and K18-Ser-33P (IB4) were purchased from NeoMarkers (Fremont, CA, USA). Goat secondary anti-rabbit and anti-mouse antibodies tagged with Alexa-488 or -568 fluorochromes (respectively) were supplied by Molecular Probes (Eugene, OR, USA).
Statistical analyses
Results were analyzed using GraphPad Prism statistical software (GraphPad, San Diego, CA, USA). Data sets were compared using an unpaired, 2-tailed Student’s t test after first excluding statistical outliers that differed by more than ±2 sd from the mean value. Welch’s correction was applied to data sets found to have unequal variances. Differences between mean values are considered statistically significant for values of P < 0.05. All quantities are given as means ± se.
RESULTS
A549 cells contain numerous keratin particles
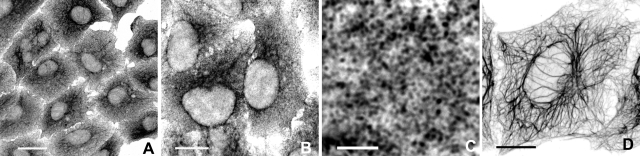
Human alveolar epithelial cells (A549; ref. 45) were maintained under normal culture conditions (i.e., no flow), then fixed in formaldehyde and processed for immunofluorescence using anti-K18 or anti-K8 pAbs. This procedure revealed a diffuse staining pattern in which very few filamentous structures could be seen (Fig. 1A, B). At higher magnification, it became clear that this appearance was due to large numbers of small, nonfilamentous, keratin-positive particles dispersed throughout the cytoplasm (Fig. 1C). This pattern of staining was unexpected, because epithelial cells usually contain conspicuous bundles of KIFs. We therefore considered the possibility that the particles might be masking a more substantive network of KIFs. This proved to be the case, because a well-developed cytoskeleton consisting of slender tonofibrils (bundles of KIFs) was seen when cells were treated briefly with TX-100 before fixation in formaldehyde (Fig. 1D). Similar images were also obtained when cells were fixed directly in methanol instead of formaldehyde or when they were fixed in methanol and then subsequently refixed in formaldehyde (not shown). Relatively few keratin particles remained in the cytoplasm following either one of these protocols, suggesting that their removal was responsible for exposing the underlying KIF network.
Figure 1.
A549 cells immunostained with anti-K18 pAb. The appearance of the keratin cytoskeleton is critically dependent on the fixation procedure. A characteristic “diffuse” staining pattern is seen when cells are fixed directly in formaldehyde (A, B). This appearance is due to the presence of large numbers of small, keratin-positive particles that occupy the interspaces between the filaments (C). In contrast, a well-developed KIF network is observed when cells are treated with a nonionic detergent (Triton X-100 buffer) before being fixed in formaldehyde (D). A similar filamentous pattern is seen in cells fixed directly in methanol (not shown). The presence of an extensive network of filamentous keratin after each of the last 2 protocols correlates with the absence of keratin particles. Confocal images recorded initially in color, then converted to gray scale and displayed in reverse contrast. Scale bars = 20 μm (A); 10 μm (B, D); 2 μm (C).
The majority of keratin particles contain either K8 or K18
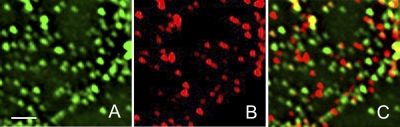
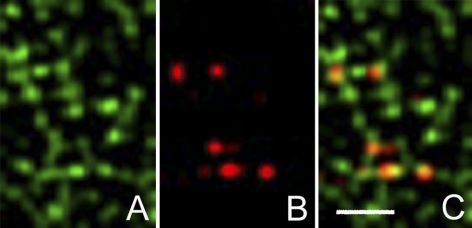
Double-label immunofluorescence analyses of formaldehyde-fixed cells revealed 3 types of keratin particle (Fig. 2). The number in each category was counted in thin areas of cytoplasm near the cell periphery, where individual particles could be clearly resolved. Fifteen areas in 4 cells were included in the analysis. Two classes of particles were identified that stained with either anti-K8 (35.1±23.7%) or anti-K18 (50.8±24.1%) antibodies, but not with both. Together they accounted for the majority (>80%) of the particles (n=1201). A third class of particle was positive for both antibodies (Fig. 2C), amounting to 13.7 ± 7.3% of the total. Statistically, there was no significant difference in the size of the subpopulations that were positive for either K8 or K18 (P=0.2), which suggests that they are present in the cell in approximately equal numbers. However, both were significantly larger than the population of particles positive for both K8 and K18. Interestingly, all 3 types of particles were abundant in lamellipodial extensions (not shown), where de novo keratin particle formation has been reported to occur (15, 21).
Figure 2.
Double immunofluorescnce of nonsheared cells fixed in formaldehyde and stained with pAb K18 and mAb K8 reveals 3 distinct populations of keratin particles. Particle counts showed that the majority (>80%) were positive for either K18 (green; A) or K8 (red; B), whereas the remainder stained for both K8 and K18 (yellow-orange; C). Region shown is located close to the cell periphery. Deconvolved images of Z stacks taken on a wide-field fluorescence microcope (Zeiss Imager Z1). Scale bars = 2 μm.
Shear stress induces the disappearance of keratin particles and enhances bundling of KIFs
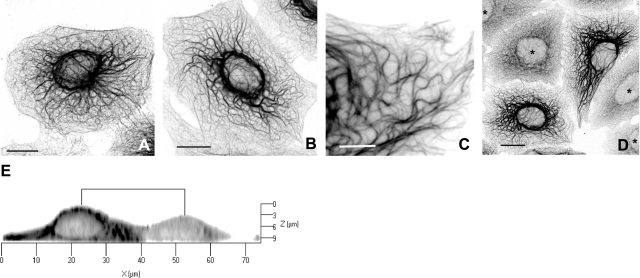
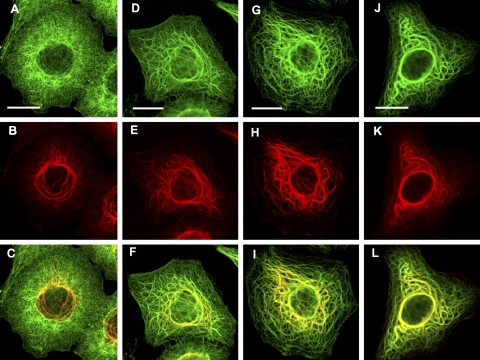
Cells were exposed to shear stresses of 7 or 15 dyn/cm2 for periods up to 10 h, fixed immediately in formaldehyde, and then stained with anti-K8 or anti-K18 pAbs. Immunofluorescence revealed considerable heterogeneity in the response of cells to flow. The keratin cytoskeleton was profoundly altered in some cells, as seen by the formation of thick, often highly convoluted (“wavy”) tonofibrils (Fig. 3A–C) and by the loss of most of the keratin particles (Fig. 3C). These changes were seen in ∼50% of the cells after exposure to a shear force of 7 dyn/cm2 for 5 h (50.5±3.5%; n=1682 cells). Interestingly, cells that responded in this fashion were interspersed with others that appeared to be completely unaffected by flow. These “nonresponders,” amounting to ∼40% of the total, were morphologically indistinguishable from nonsheared cells, yet they were frequently found in proximity to cells in which the KIF cytoskeleton was extensively reconfigured by shear stress (Fig. 3D). The remaining cells (∼10% of the total) displayed features intermediate between these 2 extremes.
Figure 3.
Shear stress transformed the thin KIF bundles found in control cells (see Fig. 1) into much thicker tonofibrils in a shear time-dependent manner. A–D) Cells were exposed to flow generating a shear force of 15 dyn/cm2 for 2 h (A), 4 h (B), 7 h (C), or 6 h (D). The thickened KIF bundles become progressively more convoluted (wavy) with increasing shear times. Shearing also resulted in the disappearance of most of the keratin particles from the interfilamentary spaces (C). Not all cells responded to shear stress in this way. KIF networks in 2 cells shown in D were extensively reconfigured by flow, whereas several others in proximity (asterisks) were entirely unaffected and retained all of the features of nonsheared cells (cf. Fig. 1). E) Vertical section through 2 adjacent cells reconstructed from a Z stack of confocal images. Cells were sheared for 4 h at 15 dyn/cm2 and stained with K18 pAb. Note that the height of the cell that responded to flow (left) is considerably greater (∼1.5×) than the one that did not (right). This difference was seen consistently when responder cells were compared to nonresponders. Reverse-contrast confocal (monochrome) images of sheared cells fixed in formaldehyde and immmunostained with pAb K18. Scale bars = 10 μm (A, B, D); 5 μm (C).
Shear forces acting on cells under flow are determined in large measure by the surface topography of cells (46,47,48). We therefore compared cells that responded to shear with those that did not in order to determine whether any variation in their overall surface geometry could explain their different responses. Three-dimensional reconstructions made from Z stacks of confocal images (Fig. 3E) revealed a systematic difference between the 2 types of cells. The peak heights h of cells that responded to flow were invariably greater than their immediate neighbors that did not, ranging from ∼9 to 14 μm (mean=∼11±0.2 mm; n=45) for the responders to 5.5–8.5 μm (mean=7.0±0.1 μm; n=54) for the nonresponders. Statistically, the difference between the 2 means (Δh∼4 μm) is highly significant (P<0.0001).
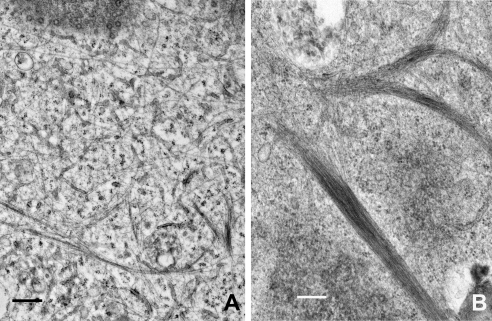
The increased filament bundling seen after shearing cells was also evident at the ultrastructural level. Electron microscopic observations of nonsheared cells typically showed individual KIFs scattered throughout the cytoplasm, together with some loosely packed arrays (Fig. 4A), most likely corresponding to the finer bundles of KIFs seen by immunofluorescence. In marked contrast, densely packed, much thicker bundles of KIFs were present in many cells following shear stress, and individual filaments were not readily seen (Fig. 4B).
Figure 4.
TEM images comparing nonsheared (control) and sheared cells. Individual or loosely packed bundles of IFs can be seen scattered thoughout the cytoplasm in nonsheared cells (A). Shearing (5 dyn/cm2, 4 h) results in greater filament bundling and reduced numbers of individual IFs (B), consistent with the appearance of immunostained cells. Cells fixed in glutaraldehyde, embedded in Epon, and stained with uranyl acetate and lead citrate. Scale bars = 500 nm.
Phosphorylation of K8 and K18 is up-regulated by shear stress
Shear stress resulted in an overall increase in the phosphorylation of both K8 and K18 on serine residues 73 and 33, respectively. Phosphoepitope-specific mAbs were used to detect K8-Ser-73P (mAb LJ4) or K18-Ser-33P (mAb IB4) in both nonsheared and sheared cells by immunofluorescence. A small fraction of the particles were found to be phosphorylated in nonsheared cells (Fig. 5). Analysis of deconvolved images showed that virtually all the particles that were positive for K8-Ser-73P colocalized with anti-K8 (98.5±1.2%, n=6 cells), and nearly all of the particles that were positive for K18-Ser-33P colocalized with anti-K18 (97.3±1.4%, n=6 cells). On the other hand, only ∼10% of K8 and K18-positive particles colocalized with particles that were positive for K8-Ser-73P (11.2±3.2%) or K18-Ser-33P (10.6±1.8%, n=6 cells), respectively. These results show, first, that approximately equal numbers of K8- and K18-containing particles are phosphorylated on serine residues 73 or 33 in nonsheared cells; and second, that phosphorylated particles comprise ∼10% of the total. In addition to staining this subpopulation of keratin particles, both mAbs highlighted fine tonofibrils located in the immediate vicinity of the nucleus of nonsheared cells (Fig. 6B). There was little or no fluorescence associated with more distal tonofibrils.
Figure 5.
Keratin particles in nonsheared cells immunostained with K18 pAb (green; A) and phosphoepitope-specific mAb K18-Ser-33P (clone IB4; red; B). Several particles that stain with anti-K18 are also positive for mAb K18-Ser-33P (yellow-orange; C), indicating that a proportion of the particles are phosphorylated in nonsheared cells. A similar result (not shown) was obtained when nonsheared cells were immunostained with anti-K8 pAb and ani-K8-Ser-73P (clone LJ4). Quantitative analyses using Axiovision colocalization software showed that ∼10% of K18 and K8 particles were phosphorylated at these sites. Deconvolved images of Z stacks taken on a wide-field fluorescence microcope (Zeiss Imager Z1). Scale bar = 1 μm.
Figure 6.
Double immunofluorescence of control and sheared cells fixed in formaldehyde and immunostained with pAb K18 (green; top row) together with a phosphoepitope-specific mAb (IB4 clone) recognizing K18-Ser-33P (red; middle row). Experimental conditions were as follows: A–C) control (nonsheared) cells; D–F) 7 dyn/cm2 for 5 h; G–I) 7 dyn/cm2 for 10 h; J–L) 20 dyn/cm2 for 5 h. Phosphorylation is restricted to a few fine keratin fibrils located in the vicinity of the nucleus in nonsheared cells (A–C). The increased phosphorylation of K18-Ser-33 and the bundling of KIFs seen after exposing cells to flow increases with the duration of shearing (compare D–F with G–I) and with the shear force (compare D–F with J–L). The characteristic waviness of enlarged KIF bundles generated under flow is illustrated in J–L. Confocal images. Scale bars = 10 μm.
Shear stresses (7 or 15 dyn/cm2 for periods up to 10 h) resulted in a marked increase in the phosphorylation of both serine residues, as seen by immunofluorescence and by Western blotting of cell extracts (below). When cells were exposed to modest shear forces of short duration (e.g., 7 dyn/cm2; ∼1 h) the increase was confined to KIFs that were closest to the nucleus. However, when either the shear force or the duration of shearing was increased, the fluorescence appeared to advance outward from the nucleus, highlighting progressively more of the KIF network (compare Fig. 6E, H), ultimately affecting tonofibrils close to the cell periphery (Fig. 6K). Thus, phosphorylation of K18 on serine 33 showed a dependence on both the magnitude and duration of the shear stress. Similar results were obtained when cells were stained for K8-Ser-73P (not shown).
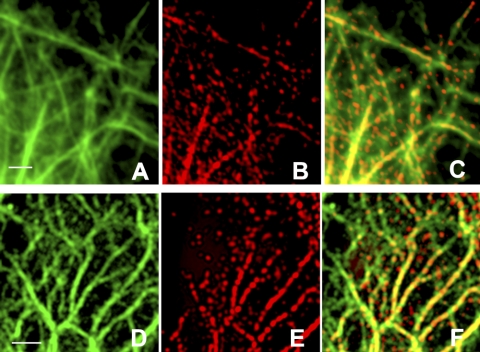
Very few phosphorylated particles that remained after shearing were observed lying freely in the interspaces between KIFs. Instead, most were found in close association with fine tonofibrils. This was most clearly seen in areas near the cell periphery, where their association with tonofibrils created distinctive linear arrays. For example, Fig. 7 shows cells that were exposed to 15 dyn/cm2 for 3 h and then double-stained with pAb K18 (green, 7A, D) and either anti-K18-Ser-33P mAb (red, 7B) or anti-K8-Ser-73P mAb (red, 7E). These images of the cell periphery were taken at high magnification and show phosphorylated keratin particles closely associated with bundles of KIFs, creating a discontinuous (“punctate”) staining pattern in which regions that appear orange/yellow alternate with segments of green fluorescence.
Figure 7.
Sheared cells (15 dyn/cm2for 3 h) stained with pAb K18 (green) and either K18-Ser-33P (A–C; red) or K8-Ser-73P (D–F; red). Most phosphorylated particles are closely associated with keratin filaments, generating a discontinuous (or punctate) staining pattern. Images such as these suggest that phosphorylated keratin particles become attached to and are subsequently incorporated into keratin filaments under flow. Deconvolved images of Z stacks taken on a wide-field (Zeiss Imager Z1) fluorescence microscope. Scale bars = 2 μm.
A soluble pool of keratin is present in A549 cells
We showed earlier that permeabilizing cells with TX-100-containing buffer before formaldehyde fixation exposed a well-developed network of filaments (Fig. 1D) and that this coincided with the disappearance of most of the keratin-positive particles. This observation suggested that the particles might represent a more soluble form of nonpolymerized keratin. To address this question, we measured K8 and K18 levels in whole-cell lysates and in both TX-100-soluble and -insoluble cell fractions by quantitative Western blotting. Immunoblots were made using known amounts of purified recombinant human K8 and K18 as internal standards (see Materials and Methods).
Measurements of K8 and K18 in whole-cell lysates made from nonsheared cells showed that the 2 proteins were expressed in equimolar amounts. However, K8 proved to be more susceptible to proteolysis than K18 during extraction with TX-100 buffer (Fig. 8A). A comparison of the total quantity of K8 (soluble+insoluble fractions) recovered from TX-100-extracted cells (105±14.9 pmol/mg protein) with that present in whole-cell lysates (216±47.4 pmol/mg protein) showed that almost half was degraded during the extraction procedure. On the other hand, K18 was more resistant to proteolysis and survived virtually intact. The effects of shear stress were therefore confined to measurements of K18.
Figure 8.
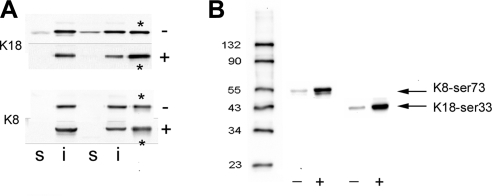
Western blots of control and sheared cells. A) Immunoblots of TX-100-soluble and -insoluble fractions from control (−) and sheared (+) cells probed with anti-K18 and anti-K8 pAbs. Blots from 2 experiments are illustrated. s, TX-100-soluble fraction; i, TX-100-insoluble fraction. Note that TX-100 buffer extracted K18 from nonsheared cells (−), but this was reduced to below the detection limit after shearing the cells (+). In this experiment, K8 was below the limit of detection in both nonsheared and sheared TX-100 extracts. Asterisks indicate lanes containing recombinant protein standards. B) Immunoblots of native IF preparations comparing the degree of phosphorylation of K8-Ser-73 and K18-Ser-33 in control (−) and sheared (+) cells. Samples were run simultaneously on the same gel, and the resulting blot cut in 2 for immunostaining with the different antibodies. Shearing (7 dyn/cm2 for 6 h) increased the band intensities corresponding to the phosphoproteins by ∼11–13×.
The pool of soluble K18 is reduced by shear stress: loss of keratin particles and increased KIF bundling
Shear stress resulted in a significant reduction in the soluble K18 fraction (Fig. 8A). On average, the TX-100-soluble component from nonsheared cells was found to contain 40.5±10.9 pmol/mg K18 (n=9 experiments), and this decreased to 7.3±2.4 pmol/mg soluble K18 (n=7 experiments) after shearing (7 dyn/cm2 for 5 h), a reduction of >80% (P<0.02). Immunofluorescence of cells simultaneously exposed to the same flow conditions again confirmed that the decreased solubility of K18 was accompanied by the loss of most of the keratin particles and by enhanced KIF bundling in ∼50–60% of the cells (not shown).
K18-Ser-33P is found only in the TX-100-insoluble keratin fraction in both sheared and nonsheared cells
Immunofluorescence localization of K8-Ser-73P and K18-Ser-33P suggested that phosphorylation of both keratins increased substantially after shearing. Phosphorylation of these residues was also confirmed by immunoblotting. In the experiment illustrated in Fig. 8B, cells were exposed to 7 dyn/cm2 for 6 h. Immunoblots of native IFs were then made using the appropriate phosphoepitope-specific mAbs. The intensities of the bands corresponding with K8-Ser-73P and K18-Ser-33P were seen to increase after shearing, by ∼13- and 11-fold, respectively. Interestingly, both phosphoproteins were found in the TX-100-insoluble fraction but not in the TX-100-soluble extracts, from either sheared or nonsheared cells (not shown). This is consistent with the small number of phosphorylated particles detected by immunofluorescence using these same antibodies.
DISCUSSION
Moderate fluid shear forces acting at the surface of cultured lung epithelial cells have a profound effect on the organization of the keratin cytoskeleton. The loss of keratin-containing particles and the redistribution of keratins from the soluble to the insoluble (polymerized) state, along with the emergence of a more robust keratin cytoskeleton, suggest a possible mechanism that could account for some of the effects of shear stress. We postulate that under static (no-flow) conditions the pool of soluble keratin is in equilibrium with filamentous protein and that exposing cells to moderate shear stresses perturbs the balance in favor of the polymeric state, causing more soluble protein subunits to become integrated into KIFs. These changes correlate with shear stress-induced phosphorylation of both K8 and K18, pointing to a possible role for phosphorylation in the restructuring process. In addition, electron microscopy clearly revealed increased numbers of thick KIF bundles (tonofibrils) and far fewer individual KIFs after flow, suggesting that shear also promotes the lateral aggregation (“bundling”) of preexisting filaments.
Keratin particles and the soluble keratin fraction
The hypothesis outlined above suggests that the keratin particles are related to the TX-100-soluble component and that they become incorporated into the KIF network in response to shear stresses. The evidence in support of this is circumstantial but nonetheless compelling. First, we showed that keratin particles were not preserved in nonsheared cells when they were processed in such a way as to render cell membranes permeable, either by preextracting cells with the TX-100-containing buffer before fixing them in formaldehyde or by fixing cells in methanol instead of formaldehyde. Both protocols revealed an extensive network of KIFs that was largely obscured in cells fixed directly in formaldehyde (Fig. 1). Furthermore, Western blots revealed that both proteins were present in the TX-100-soluble fraction. These observations demonstrate that the particles are more soluble than the filaments. Second, exposing cells to shear stress significantly reduced the amount of soluble keratins (>80%) that could be extracted with the TX-100 buffer (Fig. 8A) and simultaneously reduced the number of keratin particles in the interspaces between KIFs (Fig. 3C). The reduced solubility of keratin seen after exposing cells to flow therefore coincided with the transition from a predominantly diffuse staining pattern to a more filamentous one. Taken together, these findings suggest that the keratin-containing particles are the principal components of the pool of soluble keratin.
The molecular architecture of the particles has yet to be worked out. Several studies have shown that keratin monomers rapidly assemble in vitro to form short, full-width, filamentous structures, referred to as unit-length filaments (ULFs) ∼50–60 nm in length (49,50,51). Simple keratins form ULFs extremely rapidly and subsequently anneal longitudinally to form mature IFs. This raises the question: are the particles seen in the present study the cellular equivalents of ULFs? We cannot exclude this possibility altogether, although we consider it unlikely, because mature KIFs are known to be heterpolymers, formed from one type I and one type II protein, whereas the majority of the particles seen here contained only one type of keratin. Unfortunately, the dimensions of the particles as seen by fluorescence microscopy does not help in elucidating their molecular composition, because the apparent size of structures at or below the limit of resolution of the microscope is governed by the point-spread function of the optical system; consequently, fluorescent images of particles below a certain size become independent of their real size.
Variability in the response of the KIF cytoskeleton to shear stress
We were surprised to find that some cells were profoundly altered by shear stress, whereas others, even their immediate neighbors, appeared to be entirely unaffected (Fig. 3D). This intriguing result is most likely caused by the nonuniform distribution of shear forces acting at cell surfaces. Barbee et al. (46, 47) used atomic force microscopy to map the surface topography of endothelial cell monolayers. When these maps were subjected to computational fluid dynamics to simulate the pattern of flow over their surfaces, it emerged that shear stress increased linearly with cell height: the greatest stresses therefore act at the highest point of a cell, namely, the region over the nucleus (47). This was confirmed later by direct measurements of vimentin IF displacements under flow in endothelial cells expressing GFP-vimentin (43).
Our reconstructed images of sheared cells showed that the mean height of flow-responsive cells was ∼1.6× greater than that of nonresponders and that their surface incline (slope), from the periphery to the region above the nucleus, was also significantly greater (Fig. 3E). A quantitative description of the distribution of shear stresses acting on A549 cells is not available, absent the necessary high-resolution topographic data. Nevertheless, it is safe to conclude that some cells will experience shear forces that are significantly less than others even when they are exposed to the same nominal shear force. The complete lack of an effect of flow in the case of some cells could therefore be explained if a critical threshold level of shear stress, essential for eliciting a response in the KIF network, is not reached. The possibility that other, as yet unidentified, heterogeneties in the cell population could also contribute to the variability in the response to flow is not precluded by this hypothesis. However, it is worth emphasizing that clear differences in cell surface topography (cell height) were found in every case in which neighboring responders were compared to nonresponders.
Keratin particles appear to be rapidly incorporated into KIF networks in response to shear stress: a possible role for keratin phosphorylation?
Most keratin particles in nonsheared cells contained either K8 or K18: <15% contained both proteins. Chang et al. (19) showed that a similarly small proportion of messenger RNP particles contained both K8 and K18 mRNAs in spreading HeLa cells. Taken together, these findings suggest that the majority of mRNAs for K8 and K18 are translated independently of one another at different locations and that the newly synthesized polypeptide chains are then maintained as separate nonfilamentous precursor particles, possibly in association with molecular “chaperones,” until such time as they are required for assembly into KIFs.
Western blots showed that shear stress resulted in a substantial overall increase (∼12-fold) in the phosphorylation of K8-Ser-73 and K18-Ser-33 and that this was almost entirely associated with the TX-100-insoluble (filamentous) component. Double-label immunofluorescence, using phosphoepitope-specific antibodies recognizing K8-Ser-73P or K18-Ser-33P together with K18 pAb, revealed a marked nonuniformity in the spatial distribution of both phosphoproteins after shearing. This was seen most clearly in cells exposed to relatively low shear stresses: under such conditions fine tonofibrils found near the cell periphery exhibited a characteristic discontinuous (punctate) staining pattern (Fig. 7), in contrast to those located more centrally, which stained more uniformly. We suggest that this pattern arises within individual cells as a direct consequence of the nonuniform distribution of shear forces acting at their surfaces, discussed above. The phosphorylation of both serine residues was found to depend on the magnitude of the applied stress, and so we can anticipate that the process will proceed most rapidly in the immediate vicinity of the nucleus, where shear forces are greatest (47), and least rapidly near the cell periphery. The possibility that the punctate staining pattern could represent local differences in the susceptibility of serine residues to protein kinases should also be considered, although this could not by itself account for the large decrease in soluble keratins associated with flow. Based on these considerations, we think that the punctate staining pattern probably reflects an early stage in the process whereby phosphorylated particles become integrated into the keratin network.
A strikingly similar association between keratin particles and KIFs was seen by Miller et al. (10), who microinjected cells with biotin-tagged type I keratin. The injected protein rapidly formed discrete “spots” that first became attached to tonofibrils, then later became fully incorporated into the preexisting KIF network. Unfortunately it was not possible to determine the phosphorylation status of individual keratin spots at the time these experiments were carried out. However, in the present study, the availability of phosphoepitope-specific mAbs for serine residues on K8 and K18 has enabled us to show that the keratin particles associated with the tonofibrils are phosphorylated after shear stress, unlike most (∼90%) of the particles in nonsheared cells.
Phosphorylation is an important posttranslational modification often associated with increased solubility of IF protein and is generally taken as indicative of filament disassembly, for example, during mitosis (52) and in response to certain forms of drug treatment (29, 30). However, this correlation is by no means an invariant one: for example, microinjection of protein kinase A into rat fibroblasts resulted in vimentin phosphorylation and major alterations in the subcellular distribution of IFs, but with no increase in vimentin solubility (53). The present study points to a role for phosphorylation in reinforcing the keratin cytoskeleton in response to shear stress, and this was clearly associated with a large reduction in the pool of soluble keratins. The results suggest that shear stress serves to activate transduction pathways that stimulate protein kinases and/or inhibit protein phosphatases, leading to increased phosphorylation of K8/K18 particles. In this context keratin phosphorylation can be viewed as an important permissive step that enables keratin particles to associate with and later become assimilated into preexisting KIFs.
Functional implications of shear stress-induced keratin reorganization
The morphological changes to the keratin cytoskeleton resulting from shear stress would be expected to modify the micromechanical properties of the cytoskeleton. The recent study by Sivaramakrishnan et al. (54), using particle tracking microrheology to measure the viscoeleastic properties of the KIF network before and after exposing cells to flow, supports this conclusion. The results obtained with nonsheared cells revealed an inverse relationship between the bulk storage modulus (G′), a measure of cytoplasmic stiffness, and the KIF network mesh size. Thus, G′ decreased ∼3.5-fold from 335 dyn/cm2 in the perinuclear region to 95 dyn/cm2 at the cell periphery, whereas the mesh size increased 15-fold, from 0.02 μm2 in the nuclear region to 0.3 μm2 in the peripheral region. Subjecting cells to shear forces comparable to those used in the present study (15 dyn/cm2 for 4 h) resulted in a more uniform distribution of KIFs. The effect was most pronounced at the cell periphery, where the mean mesh size decreased 7.5-fold, from 0.3 to 0.04 μm2. This was associated with a 2.2-fold increase in G′, from 95 to 209 dyn/cm2. The increased stiffness associated with the reconfigured KIF network can therefore be viewed as a protective mechanism that enables cells to better resist the potentially damaging effects of external mechanical forces.
Acknowledgments
This work has been supported by a grant from the NIH (GM36806).
References
- Schweizer J, Bowden P E, Coulombe P A, Langbein L, Lane E B, Magin T M, Maltais L, Omary M B, Parry D A, Rogers M A, Wright M W. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P M, Idler W W, Zimmerman S B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976;108:547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Owens D W, Lane E B. The quest for the function of simple epithelial keratins. Bioessays. 2003;25:748–758. doi: 10.1002/bies.10316. [DOI] [PubMed] [Google Scholar]
- Coulombe P A, Hutton M E, Letai A, Hebert A, Paller A S, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Lane E B, Rugg E L, Navsaria H, Leigh I M, Heagerty A H, Ishida-Yamamoto A, Eady R A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356:244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Oshima R G. Apoptosis and keratin intermediate filaments. Cell Death Differ. 2002;9:486–492. doi: 10.1038/sj.cdd.4400988. [DOI] [PubMed] [Google Scholar]
- Albers K, Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J Cell Biol. 1989;108:1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R K, Khuon S, Goldman R D. Dynamics of keratin assembly: exogenous type I keratin rapidly associates with type II keratin in vivo. J Cell Biol. 1993;122:123–135. doi: 10.1083/jcb.122.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R K, Vikstrom K, Goldman R D. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991;113:843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall L, Karsenti E. Soluble cytokeratins in Xenopus laevis oocytes and eggs. Biol Cell (Paris) 1987;61:33–38. doi: 10.1111/j.1768-322x.1987.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Franke W W, Winter S, Schmid E, Sollner P, Hammerling G, Achtstatter T. Monoclonal cytokeratin antibody recognizing a heterotypic complex: immunological probing of conformational states of cytoskeletal proteins in filaments and in solution. Exp Cell Res. 1987;173:17–37. doi: 10.1016/0014-4827(87)90328-4. [DOI] [PubMed] [Google Scholar]
- Chou C F, Riopel C L, Rott L S, Omary M B. A significant soluble keratin fraction in ‘simple’ epithelial cells. Lack of an apparent phosphorylation and glycosylation role in keratin solubility. J Cell Sci. 1993;105:433–444. doi: 10.1242/jcs.105.2.433. [DOI] [PubMed] [Google Scholar]
- Yoon K H, Yoon M, Moir R D, Khuon S, Flitney F W, Goldman R D. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J Cell Biol. 2001;153:503–516. doi: 10.1083/jcb.153.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R, Woll S, Strnad P, Leube R E. Identification of novel principles of keratin filament network turnover in living cells. Mol Biol Cell. 2004;15:2436–2448. doi: 10.1091/mbc.E03-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C L, Martys J L, Mikhailov A, Gundersen G G, Liem R K. Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J Cell Sci. 1998;111:1767–1778. doi: 10.1242/jcs.111.13.1767. [DOI] [PubMed] [Google Scholar]
- Prahlad V, Yoon M, Moir R D, Vale R D, Goldman R D. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand B T, Loomis P, Yoon M, Goldman R D. Rapid transport of neural intermediate filament protein. J Cell Sci. 2003;116:2345–2359. doi: 10.1242/jcs.00526. [DOI] [PubMed] [Google Scholar]
- Chang L, Shav-Tal Y, Trcek T, Singer R H, Goldman R D. Assembling an intermediate filament network by dynamic cotranslation. J Cell Biol. 2006;172:747–758. doi: 10.1083/jcb.200511033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y H, Flitney F W, Chang L, Mendez M, Grin B, Goldman R D. The motility and dynamic properties of intermediate filaments and their constituent proteins. Exp Cell Res. 2007;313:2236–2243. doi: 10.1016/j.yexcr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Windoffer R, Kolsch A, Woll S, Leube R E. Focal adhesions are hotspots for keratin filament precursor formation. J Cell Biol. 2006;173:341–348. doi: 10.1083/jcb.200511124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand B T, Mikami A, Vallee R B, Goldman R D. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol. 2002;157:795–806. doi: 10.1083/jcb.200202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liovic M, Mogensen M M, Prescott A R, Lane E B. Observation of keratin particles showing fast bidirectional movement colocalized with microtubules. J Cell Sci. 2003;116:1417–1427. doi: 10.1242/jcs.00363. [DOI] [PubMed] [Google Scholar]
- Woll S, Windoffer R, Leube R E. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur J Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Kupfer H, Eshhar Z, Geiger B. Reorganization of arrays of prekeratin filaments during mitosis. Immunofluorescence microscopy with multiclonal and monoclonal prekeratin antibodies. Exp Cell Res. 1981;134:281–290. doi: 10.1016/0014-4827(81)90427-4. [DOI] [PubMed] [Google Scholar]
- Lane E B, Goodman S L, Trejdosiewicz L K. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1:1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosevear E R, McReynolds M, Goldman R D. Dynamic properties of intermediate filaments: disassembly and reassembly during mitosis in baby hamster kidney cells. Cell Motil Cytoskelet. 1990;17:150–166. doi: 10.1002/cm.970170303. [DOI] [PubMed] [Google Scholar]
- Windoffer R, Leube R E. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J Cell Sci. 1999;112:4521–4534. doi: 10.1242/jcs.112.24.4521. [DOI] [PubMed] [Google Scholar]
- Strnad P, Windoffer R, Leube R E. Induction of rapid and reversible cytokeratin filament network remodeling by inhibition of tyrosine phosphatases. J Cell Sci. 2002;115:4133–4148. doi: 10.1242/jcs.00096. [DOI] [PubMed] [Google Scholar]
- Woll S, Windoffer R, Leube R E. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol. 2007;177:795–807. doi: 10.1083/jcb.200703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D, Andrews P D, James J, Lane E B. Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J Cell Sci. 2004;117:5233–5243. doi: 10.1242/jcs.01407. [DOI] [PubMed] [Google Scholar]
- Ridge K M, Linz L, Flitney F W, Kuczmarski E R, Chou Y H, Omary M B, Sznajder J I, Goldman R D. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem. 2005;280:30400–30405. doi: 10.1074/jbc.M504239200. [DOI] [PubMed] [Google Scholar]
- Omary M B, Ku N O, Tao G Z, Toivola D M, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Chou C F, Omary M B. Mitotic arrest with anti-microtubule agents or okadaic acid is associated with increased glycoprotein terminal GlcNAc’s. J Cell Sci. 1994;107:1833–1843. doi: 10.1242/jcs.107.7.1833. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Merrison W, Dinsdale D, Cohen G M. Active caspases and cleaved cytokeratins are sequestered into cytoplasmic inclusions in TRAIL-induced apoptosis. J Cell Biol. 2000;148:1239–1254. doi: 10.1083/jcb.148.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Ku N O, Omary M B. Stress, apoptosis, and mitosis induce phosphorylation of human keratin 8 at Ser-73 in tissues and cultured cells. J Biol Chem. 1997;272:17565–17573. doi: 10.1074/jbc.272.28.17565. [DOI] [PubMed] [Google Scholar]
- Stumptner C, Omary M B, Fickert P, Denk H, Zatloukal K. Hepatocyte cytokeratins are hyperphosphorylated at multiple sites in human alcoholic hepatitis and in a mallory body mouse model. Am J Pathol. 2000;156:77–90. doi: 10.1016/S0002-9440(10)64708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J E, Brautigan D L, Vallee R, Olmsted J, Fujiki H, Goldman R D. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992;89:11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zhou X, Liao J, Omary M B. Pervanadate-mediated tyrosine phosphorylation of keratins 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci. 1999;112:2081–2090. doi: 10.1242/jcs.112.13.2081. [DOI] [PubMed] [Google Scholar]
- Toivola D M, Goldman R D, Garrod D R, Eriksson J E. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J Cell Sci. 1997;110(Pt.:23–33. doi: 10.1242/jcs.110.1.23. [DOI] [PubMed] [Google Scholar]
- Tao G Z, Toivola D M, Zhou Q, Strnad P, Xu B, Michie S A, Omary M B. Protein phosphatase-2A associates with and dephosphorylates keratin 8 after hyposmotic stress in a site- and cell-specific manner. J Cell Sci. 2006;119:1425–1432. doi: 10.1242/jcs.02861. [DOI] [PubMed] [Google Scholar]
- Helmke B P, Goldman R D, Davies P F. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- Helmke B P, Thakker D B, Goldman R D, Davies P F. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney E W, Goldman R D. Fluorescence-based methods for studying intermediate filaments. Methods Cell Biol. 2004;78:297–319. doi: 10.1016/s0091-679x(04)78011-5. [DOI] [PubMed] [Google Scholar]
- Giard D J, Aaronson S A, Todaro G J, Arnstein P, Kersey J H, Dosik H, Parks W P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Barbee K A, Davies P F, Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ Res. 1994;74:163–171. doi: 10.1161/01.res.74.1.163. [DOI] [PubMed] [Google Scholar]
- Barbee K A, Mundel T, Lal R, Davies P F. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol. 1995;268:H1765–H1772. doi: 10.1152/ajpheart.1995.268.4.H1765. [DOI] [PubMed] [Google Scholar]
- Davies P F, Mundel T, Barbee K A. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech. 1995;28:1553–1560. doi: 10.1016/0021-9290(95)00102-6. [DOI] [PubMed] [Google Scholar]
- Eichner R, Rew P, Engel A, Aebi U. Human epidermal keratin filaments: studies on their structure and assembly. Ann N Y Acad Sci. 1985;455:381–402. doi: 10.1111/j.1749-6632.1985.tb50424.x. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Haner M, Brettel M, Ku N O, Aebi U. Characterization of distinct early assembly units of different intermediate filament proteins. J Mol Biol. 1999;286:1403–1420. doi: 10.1006/jmbi.1999.2528. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Wedig T, Porter R M, Lane E B, Aebi U. Characterization of early assembly intermediates of recombinant human keratins. J Struct Biol. 2002;137:82–96. doi: 10.1006/jsbi.2002.4466. [DOI] [PubMed] [Google Scholar]
- Chou Y H, Rosevear E, Goldman R D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989;86:1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N J, Fernandez A, Feramisco J R, Welch W J. Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the cAMP-dependent protein kinase. J Cell Biol. 1989;108:2409–2422. doi: 10.1083/jcb.108.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, DeGiulio J V, Lorand L, Goldman R D, Ridge K M. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci U S A. 2008;105:889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]