Abstract
TGF-β subtypes are expressed in tissues derived from cranial neural crest cells during early mouse craniofacial development. TGF-β signaling is critical for mediating epithelial-mesenchymal interactions, including those vital for tooth morphogenesis. However, it remains unclear how TGF-β signaling contributes to the terminal differentiation of odontoblast and dentin formation during tooth morphogenesis. Towards this end, we generated mice with conditional inactivation of the Tgfbr2 gene in cranial neural crest derived cells. Odontoblast differentiation was substantially delayed in the Tgfbr2;Wnt1-Cre mutant mice at E18.5. Following kidney capsule transplantation, Tgfbr2 mutant tooth germs expressed a reduced level of Col1a1 and Dspp and exhibited defects including decreased dentin thickness and absent dentinal tubules. In addition, the expression of the intermediate filament nestin was decreased in the Tgfbr2 mutant samples. Significantly, exogenous TGF-β2 induced nestin and Dspp expression in dental pulp cells in the developing tooth organ. Our data suggest that TGF-β signaling controls odontoblast maturation and dentin formation during tooth morphogenesis.
Keywords: TGF-β, dentin, odontoblast, nestin, tooth
Introduction
Neural crest cells are multipotential progenitor cells that contribute extensively to vertebrate development and give rise to various cell and tissue types. Proliferation of CNC cells produces the discrete swellings that demarcate each branchial arch, and CNC cells contribute to the formation of mesenchymal structures in the head and neck (Echelard et al., 1994; Graham and Lumsden, 1993; Imai et al., 1996; Le Douarin et al., 1993; Noden, 1991; Trainor and Krumlauf, 2000). When odontogenesis begins, the primitive oral epithelium thickens and proliferates to project into the underlying CNC-derived ectomesenchyme. Mesenchymal cellular condensation results in the establishment of the dental papilla (Cobourne and Sharpe, 2003).
Odontoblasts are neural crest-derived cells that secrete predentin and dentin after terminal differentiation (Chai et al., 2000; Ruch, 1990). During odontogenesis, odontoblast terminal differentiation is controlled by the inner dental epithelium and is also dependent on matrix-mediated interactions (Cam et al., 1992; Ruch et al., 1995; Ruoslahti and Yamaguchi, 1991). Analysis of the expression patterns of growth factors during odontogenesis suggests that members of the TGF-β family, IGFs and FGFs contribute to odontoblast terminal differentiation (Begue-Kirn et al., 1994; Cam et al., 1992; Cassidy et al., 1997; Finkelman et al., 1990; Heikinheimo, 1994; Heikinheimo et al., 1993; Helder et al., 1998; Inage and Toda, 1996; Joseph et al., 1993; Russo et al., 1998; Thesleff and Vaahtokari, 1992; Vaahtokari et al., 1991). Within the TGF-β family, TGF-β1, TGF-β2, TGF-β3, BMP-2, BMP-4, BMP-7 and follistatin have been detected in the inner dental epithelium and in polarizing and functional odontoblasts. The complexity of events in vivo has led to the use of in vitro models to investigate and characterize the roles of these growth factors in regulating tooth morphogenesis. However, as most of these studies were carried out using cultured odontoblast cells, they were unable to address the biological significance of tissue-tissue interaction in regulating odontoblast differentiation. Furthermore, it has been difficult to identify the specific role of these growth factors during odontoblast differentiation because of their overlapping expression patterns and functional redundancy.
TGF-βs bind to two distinct receptor types, and both type II and type I receptors are required for signal transduction. In order to investigate the functional significance of TGF-β signaling in regulating odontoblast differentiation and dentin formation in vivo, we generated mutant mice in which the Tgfbr2 gene is specifically inactivated in the CNC-derived odontoblast cells. Our study demonstrates that TGF-β signaling plays an essential role in odontoblast maturation, including cytological differentiation and dentin formation.
Materials and Methods
Generation of Wnt1-Cre;R26R and Tgfbr2fl/fl;Wnt1-Cre mutant mice
The Wnt1-Cre transgenic line have been described previously (Danielian et al., 1998). Mating Tgfbr2fl/+;Wnt1-Cre with Tgfbr2fl/fl mice generated Tgfbr2fl/fl;Wnt1-Cre null allele progeny that were genotyped using PCR primers as previously described (Chytil et al., 2002). Mice with no detectable Cre transgene were used as control.
Kidney capsule transplantation
The 1st lower molar region including surrounding tissue was collected from E14.5 wild type or mutant mouse embryo. The host mouse was weighed and anesthetized using pentobarbital (0.5 mg/10 g body weight). Kidneys were exposed through incision of the skin and muscle in the back of the host mouse. The kidney capsule was opened with the fine tip of No. 5 forceps. The embryonic tissue block that contained the tooth germ was placed under the kidney capsule. Sutures were placed to close the muscular layer and skin. After operation, genotypes of each transplanted samples were confirmed by PCR. The host mouse was kept in a warm chamber until it fully recovered. The kidney capsule grafting products were harvested in some periods after the operation. All surgical procedures were performed according to IACUC guidelines.
In situ hybridization
Tissue was fixed with 4% paraformaldehyde in PBS, embedded in paraffin, serially sectioned, and mounted following standard procedures. DNA fragments of Dspp Amelogenin and Col1a1 were subcloned in pBSK, pGEM and pDrive vector plasmids, respectively. Digoxigenin (DIG)-labeled sense and antisense cRNA riboprobes were synthesized using the DIG RNA Labeling Mix (Roche Molecular Biochemicals). Paraffin-embedded sections were dewaxed and treated with proteinase K (20 μg/ml), 0.2M HCl, acetylated, and hybridized overnight with Digoxigenin labeled probes as previously described (Xu et al., 2005).
Immunohistochemistry, Immunofluorescence and Von Kossa staining
Tissue was fixed with 4% paraformaldehyde in PBS, embedded in paraffin, serially sectioned, and mounted following standard procedures. Samples were treated with mouse anti-nestin (CHEMICON International) or rabbit anti-Neurofilament M (CHEMICON International) as primary antibody. Signals were visualized with Histostain®SP for mouse primary (Zymed Laboratories) for immunohistochemistry, or with Alexa Fluor 488 or 568 (Invitrogen) as second antibody for immunofluorescence. For Von Kossa staining, the tissue was fixed with Karnovsky fixative solution, embedded in EPON, sectioned and mounted. Staining was performed according to standard procedure.
Scanning Electron Microscopy (SEM)
Dissected tooth organs were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, at 4°C for 2 hours. Specimens were washed in buffer, dehydrated in graded ethanol, and placed in perforated Beem capsules in acetone. They were then dried in a critical-point drying bomb using carbon dioxide, mounted on specimen studs, and sputter coated with gold palladium alloy in an argon atmosphere (Hummer V, Technics; pulsed for 7 minutes at 9 volts and 10 milliamps). Tooth topography was viewed with a Cambridge S4-10 scanning electron microscope operating at 10 kV.
Organ culture of wild type and Tgfbr2fl/fl;Wnt1-Cre mutant lower 1st molar tooth germ explants
Timed-pregnant mice were sacrificed on post-coital day 18.5. Dissected lower 1st molar tooth germs were cultured in serumless, chemically defined medium according to standard methods (Ito et al., 2002). We used affi-gel blue beads (BioRad) for delivery of TGF-β2. The beads were washed in PBS and then incubated for 1 hour at room temperature in 10μg/ml TGF-β2 (R&D). Control beads were incubated in 0.1% BSA.
Results
Loss of TGF-β signaling affects the terminal differentiation of odontoblasts
TGF-β signaling plays a critical role during tooth morphogenesis. To investigate whether there is a cell-autonomous requirement for TGF-β signaling during odontoblast differentiation, we generated Tgfbr2fl/fl;Wnt1-Cre mutant mice. We have previously shown that conditional inactivation of Tgfbr2 does not adversely affect the migration of CNC cells (Ito et al., 2003).
To examine the status of odontoblast functional differentiation in Tgfbr2fl/fl;Wnt1-Cre mutant mice, we assayed the expression of markers using in situ hybridization. Type I collagen and dentin phosphoprotein (Dpp) are the major organic components in dentin and predentin, respectively. Fully differentiated odontoblasts express type I collagen (Col1a1) and dentin sialophosphoprotein (Dspp), which have been used extensively as differentiation markers for odontoblasts. In wild type mice at E18.5, Col1a1 expression was observed in the odontoblast layer and osteogenic region (Fig. 1A), and Dspp was present in the odontoblast and ameloblast layer (Fig. 1C). In Tgfbr2fl/fl;Wnt1-Cre mutant mice, however, Col1a1 expression in the odontoblast layer was dramatically reduced (Fig. 1B), and Dspp expression was not detectable (Fig. 1D). Interestingly, expression of Amelogenin, an ameloblast differentiation marker, was also reduced in the Tgfbr2fl/fl;Wnt1-Cre sample (Fig. 1E,F). To determine when Tgfbr2 was expressed in dental pulp cells, we performed in situ hybridization analysis. Tgfbr2 expression was first detectable in CNC-derived dental pulp cells at E16.5 (Fig. 1G) and in odontoblast cells at E18.5 (Fig. 1H). Our data suggest that TGF-β signaling plays an important role in tooth development at late embryonic stages.
Fig. 1. Abnormal odontoblastic and ameloblastic differentiation in lower 1st molar teeth of E18.5 Tgfbr2fl/fl;Wnt1-Cre mice.
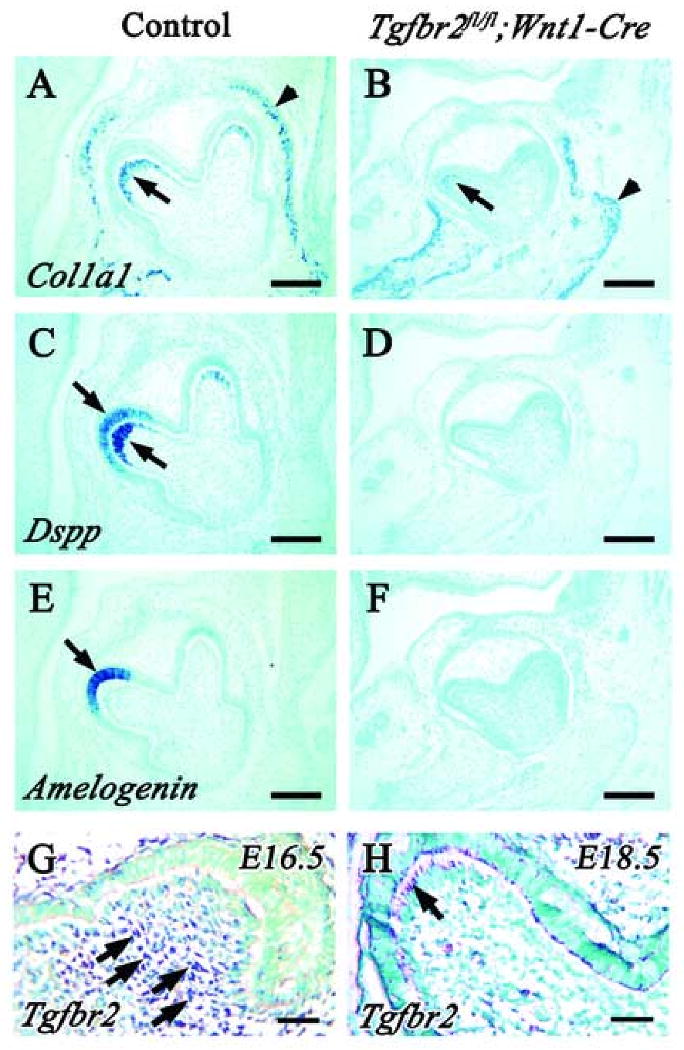
In situ hybridization of Dspp, Col1a1 and Amelogenin using wild type (A,C,E) and Tgfbr2fl/fl;Wnt1-Cre (B,D,F) samples at E18.5. (A,B) Col1a1 is expressed in osteoblasts (arrowhead) in both wild type and Tgfbr2;wnt1-Cre mutant mice, but expression in Tgfbr2; Wnt1-Cre mice is dramatically reduced compared to wild type. (C,D) Dspp is expressed in odontoblasts and ameloblasts in wild type (arrow). In contrast, Dspp expression is not observed in Tgfbr2;wnt1-Cre mice. (E,F) Amelogenin expression is detected in ameloblasts in wild type mice (arrow), but is not detectable in Tgfbr2; wnt1-Cre mice. (G,H) In situ hybridization of Tgfbr2 using wild type 1st lower molar at E16.5 (G) and E18.5 (H). Tgfbr2 is expressed in dental mesenchymal cells at E16.5 (arrow) (G), and odontoblast cells at E18.5 (arrow) (H). Scale bars: 100 μm in A-F, 30 μm in G and F.
Abnormal dentin formation in Tgfbr2fl/fl;Wnt1-Cre mice
Unfortunately, Tgfbr2fl/fl;Wnt1-Cre mutant mice die at birth because of cleft palate defect (Ito et al., 2003), so we cannot observe tooth development at postnatal stages to investigate the functional significance of TGF-β signaling in regulating odontoblast differentiation and dentin formation. Therefore, we transplanted the lower 1st molar tooth germ under a kidney capsule to observe postnatal dentin formation. After four weeks cultivation under the kidney capsule, the mineralized tooth was well formed, with normal cusp and root formation in wild type (Fig. 2A,B). In contrast, Tgfbr2fl/fl;Wnt1-Cre teeth appeared translucent. Tgfbr2fl/fl;Wnt1-Cre crowns were smaller than those of wild type and root formation was abnormal. We observed well-organized enamel and dentin, including a predentin layer, in wild type sections (Fig. 2C,E). Although we observed well-organized enamel in the Tgfbr2fl/fl;Wnt1-Cre mutant, we discovered that the dentin consisted of a very thin monolayer (Fig. 2D,F). Using Von Kossa staining, we found that the mineralization of the enamel and the dentin was normal in the Tgfbr2fl/fl;Wnt1-Cre sample as compared to the control. However, we rarely observed non-mineralized dentin (pre-dentin) in Tgfbr2fl/fl;Wnt1-Cre mutant sample (Fig. 2G,H).
Fig. 2. Tooth development defect in Tgfbr2fl/fl;Wnt1-Cre mice after kidney capsule transplantation.

Wild type and Tgfbr2fl/fl;Wnt1-Cre lower 1st molar tooth germ were cultivated for four weeks under host kidney capsules. Normal cusp patterning is detectable in both wild type (left side) and Tgfbr2fl/fl;Wnt1-Cre (right side) from a lateral (A) and top view (B). However, the shape of the crown and roots in the mutant sample is different than wild type (A), and the mutant sample appears translucent (B). (C-H) H&E and Von Kossa staining of wild type and Tgfbr2fl/fl;Wnt1-Cre tooth germ sections. In the wild type samples, the enamel and dentin are well organized (C) and well mineralized (G). The dentin is composed of two layers (E). In the Tgfbr2fl/fl;Wnt1-Cre samples, the enamel is well formed (D), but the dentin consist of a thin monolayer (F). Both the enamel and dentin are well mineralized, although predentin is rarely observed (H). Dotted-line boxes in C and D are enlarged and shown in E and F, respectively. e, enamel; d, dentin; pd, predentin; scale bars: 1 mm in A and B; 100 μm in C and D; 50 μm in E-H.
Given that both enamel and dentin were formed in Tgfbr2fl/fl;Wnt1-Cre mutants, we investigated the differentiation status of odontoblasts and ameloblasts at a postnatal stage two weeks after kidney transplantation. Col1a1 and Dspp expression persisted in wild type at this stage (Fig. 3C,E,G). In Tgfbr2fl/fl;Wnt1-Cre samples, the expression of Col1a1 was present in odontoblasts, although the level of the expression appeared lower than that of wild type (Fig. 3D). The expression of Dspp, which was not detectable at E18.5 in the mutant, finally appeared in odontoblasts at this stage (Fig. 3F). In fact, we observed Col1a1 and Dspp expression in Tgfbr2fl/fl;Wnt1-Cre samples as early as 5 days after transplantation (E14.5 + 5), a time that should essentially represent a newborn stage (Fig. 3I,J). The level and pattern of Amelogenin expression in the mutant was indistinguishable from that of wild type (Fig. 3G,H). These data are consistent with a requirement for TGF-β signaling in the secretion of the extracellular matrix and the formation of dentin, but not in the terminal differentiation of odontoblasts in vivo.
Fig. 3. Expression of odontoblastic and ameloblastic differentiation markers in Tgfbr2fl/fl;Wnt1-Cre tooth germ cultured under a kidney capsule.
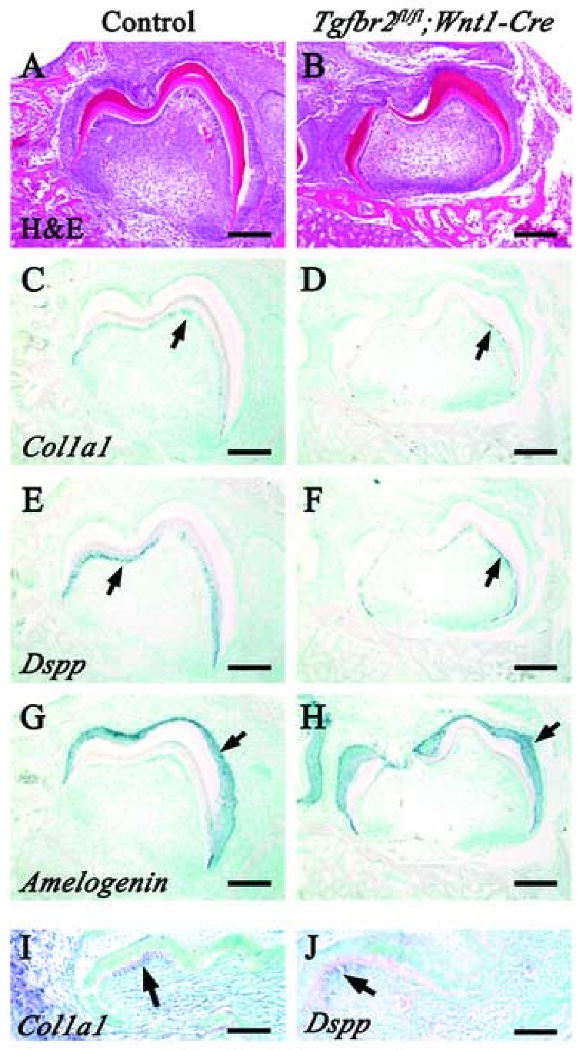
H&E staining and in situ hybridization of Dspp, Col1a1 and Amelogenin in wild type (A,C,E,G) and Tgfbr2fl/fl;Wnt1-Cre (B,D,F,H) samples after 14 days cultivation under a kidney capsule. (C,D) Expression of Col1a1 (arrows) in Tgfbr2fl/fl;Wnt1-Cre transplants is detectable in a similar pattern to wild type, although the level of the expression is reduced. (E,F) Expression of Dspp (arrows) is detectable in wild type and Tgfbr2fl/fl;Wnt1-Cre odontoblasts, but the level of expression is reduced in the Tgfbr2fl/fl;Wnt1-Cre sample. (G,H) The level and pattern of Amelogenin expression (arrows) in the Tgfbr2fl/fl;Wnt1-Cre sample are indistinguishable from wild type. (I,J) In situ hybridization of Col1a1 (I) and Dspp (J) in wild type samples after 5 days cultivation under a kidney capsule. Both Col1a1 and Dspp are already expressed in odontoblasts at the tip of the cusp at this stage (arrow). Scale bars: 100 μm in A-H; 50 μm in I and J.
TGF-β signaling is required for cytological differentiation of odontoblasts
To determine if there were any dentinal tubule structure defects in Tgfbr2fl/fl;Wnt1-Cre samples, we examined the ultrastructure of mineralized dentin using scanning electron microscopy (SEM). Dentinal tubules were clearly visible in wild type (Fig. 4A). In contrast, we observed no dentinal tubules in the Tgfbr2fl/fl;Wnt1-Cre sample, although the structure of the enamel was normal (Fig. 4B).
Fig. 4. Loss of dentinal tubules in Tgfbr2fl/fl;Wnt1-Cre tooth germ.
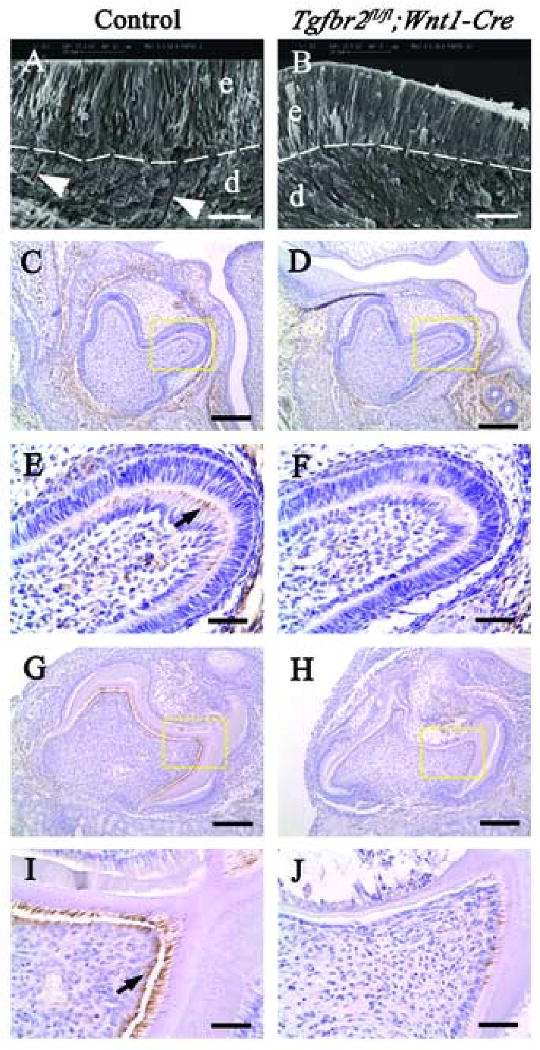
(A,B) SEM analysis of wild type and Tgfbr2fl/fl;Wnt1-Cre teeth after 28 days cultivation under a kidney capsule. Dentinal tubules are clearly visible in the wild type dentin layer (arrowheads in A), but not in the Tgfbr2fl/fl;Wnt1-Cre dentin (B). The structure of the Tgfbr2fl/fl;Wnt1-Cre enamel is indistinguishable from wild type. Broken line; dentin-enamel junction, d; dentin, e; enamel. (C-J) Nestin immunohistochemistry in wild type and Tgfbr2fl/fl;Wnt1-Cre tooth germ at E18.5 (C-F) and after 14 days cultivation under kidney capsules (G-J). E, F, I, and J are enlarged views of the yellow dashed boxes in C, D, G, and H, respectively. Nestin expression is detectable in wild type odontoblasts at the tip of the cusp (E, arrow), but not in Tgfbr2fl/fl;Wnt1-Cre odontoblasts (F). After cultivation under the kidney capsule, nestin is highly expressed in odontoblasts and in the dentin matrix area in wild type transplants (G,I). In Tgfbr2fl/fl;Wnt1-Cre transplants, nestin was barely detectable in odontoblasts (H,J). Scale bars: 20 μm in A, B; 100 μm in C, D, G and H; 25 μm in E, F, I and H.
Previous studies have shown that nestin, a member of the intermediate filament family, is expressed in fully differentiated odontoblasts, especially in odontoblast processes (About et al., 2000; Terling et al., 1995). We investigated odontoblast process development in the Tgfbr2fl/fl;Wnt1-Cre sample by analyzing nestin expression. Nestin expression began at the tip of the cusps in wild type at E18.5 (Fig. 4C,E), but failed to express in the Tgfbr2fl/fl;Wnt1-Cre sample (Fig. 5D,F). After cultivation under the kidney capsule, we observed strong nestin expression in odontoblasts and in the dentin matrix area, the region containing odontoblast processes, in the wild type sample (Fig. 4G,I). In Tgfbr2fl/fl;Wnt1-Cre transplants, we found that nestin was barely detectable in odontoblasts (Fig. 4H,J). Thus, TGF-β signaling is required for the formation of odontoblast processes.
Fig. 5. TGF-β induces nestin expression in odontoblasts.

Nestin and Dspp expression in wild type and Tgfbr2fl/fl;Wnt1-Cre E18.5 tooth germ implanted with BSA or TGF-β2 beads. Nestin expression is analyzed by immunohistochemistry (A-F) and Dspp expression is analyzed by in situ hybridization (G,H). Enlarged areas of A, C, E and G are shown in B, D, F and H, respectively. (A,B) Nestin expression is detectable in odontoblasts adjacent to the dentin-enamel junction in wild type tooth germ (arrow), but BSA beads do not induce nestin expression in dental papilla cells. (C,D) There is no expression of nestin in odontoblasts adjacent to the dentin-enamel junction in Tgfbr2fl/fl;Wnt1-Cre mutant tooth germ, and nestin is not induced in dental papilla cells around TGF-β2 beads. (E,F) In wild type tooth germ, nestin expression is again visible in odontoblasts adjacent to the dentin-enamel junction (arrow in E) and is induced in dental papilla cells around TGF-β2 beads (arrowhead). Cells expressing nestin around beads appear differentiated cytologically with an enlargement of the cytoplasm and the production of processes (arrow in F). (G,H) Dspp mRNA expression in wild type tooth germ from an adjacent section to E and F shows the same pattern as nestin. Arrow indicates the Dspp expression in odontoblasts adjacent to the dentin-enamel junction, and the arrowhead highlights that in dental papilla cells around the TGF-β2 bead. (I) Double immunofluorescence for nestin and NF-M in E18.5 tooth germ implanted with TGF-β2 bead. Nestin is expressed spontaneously in odontoblasts adjacent to epithelial cells (arrow), and its expression is induced in dental papilla cells by TGF-β2 (arrowhead). NF-M expression is not observed in any area of tooth germ. (J) Antibodies against NF-M can react with neurons in trigeminal ganglia. E, dental epithelium; P, dental papilla. Scale bars: 100 μm in A, C, E, G, I and J; 20 μm in B, D, F, and H.
Next, we treated the lower 1st molar explant with either TGF-β2 or BSA control beads and evaluated nestin expression. Wild type tooth explants treated with BSA for 5 days showed no expression of nestin around the beads, whereas odontoblasts adjacent to the dentin-enamel junction did express it (Fig. 5A,B). When we treated Tgfbr2fl/fl;Wnt1-Cre mutant tooth explants with TGF-β2 beads, there was no expression of nestin (Fig. 5C,D). In contrast, TGF-β2 beads did induce the expression of nestin in wild type dental papilla cells, and those cells were cytologically differentiated with an enlargement of the cytoplasm (Fig. 5E,F). However, nestin is expressed in neuronal cells as well as odontoblasts. We performed in situ hybridization using a Dspp probe in order to confirm whether nestin positive cells around the TGF-β2 beads were differentiated odontoblasts. In fact, we observed Dspp positive cells around the TGF-β2 beads and around the odontoblasts adjacent to the dentin-enamel junction (Fig. 5G,H). Furthermore, we performed double immunofluorescence using anti-Neurofilament M (NF-M) and anti-nestin antibody. Nestin expression was induced in cells around the TGF-β2 beads. However, we were unable to detect any NF-M positive cells in the dental pulp cell population (Fig. 5I). These results suggest that TGF-β signaling is required for odontoblast cytological differentiation via the regulation of nestin expression.
Discussion
Odontoblasts represent one of the primary fates of CNC cells entering the first branchial arch. TGF-β is known to mediate epithelial-mesenchymal interactions, and TGF-β ligands are conspicuously expressed in the dental epithelium and the CNC-derived mesenchyme throughout different stages of tooth development. We have shown here that there is a cell-autonomous requirement for TGF-β signaling during terminal differentiation of the CNC-derived odontoblasts and dentin matrix formation.
TGF-β is secreted by differentiated odontoblasts and regulates the biological activity of these cells through an autocrine mode of action (Begue-Kirn et al., 1994; Begue-Kirn et al., 1992). Studies using mice with Tgfb1 null mutation or overexpression of Tgfb1 support an essential role for TGF-β1 signaling in dentin mineralization (D'Souza et al., 1998; Thyagarajan et al., 2001). Although there was a delay in odontoblast differentiation in the Tgfbr2 mutant, all CNC-derived odontoblast cells expressed Dspp, ColIa1 and formed dentin matrix following kidney capsule transplantation. However, exogenous TGF-β2 induced ectopic Dspp expression in wild type dental mesenchymal cells. Therefore, we conclude that TGF-β signaling is not required for the functional differentiation of odontoblasts during tooth morphogenesis in vivo, even though exogenous TGF-β can induce odontoblast differentiation. The delay in amelogenin expression in ameloblasts in Tgfbr2fl/fl;Wnt1-Cre mice suggests that normal odontoblast differentiation is required for ameloblast differentiation. We did not observe any ameloblast differentiation defect in K14-Cre;Tgfbr2 mutant mice, in which TGF-β signaling was completely blocked in the dental epithelium throughout tooth development (our unpublished data), implying that TGF-β signaling is dispensable during ameloblast differentiation.
Dentin and enamel are formed and calcified in Tgfbr2fl/fl;Wnt1-Cre mice, although the dentin thickness was dramatically reduced when compared to that of wild type. Specifically, dentinal tubules and dentin matrix were malformed in the Tgfbr2fl/fl;Wnt1-Cre sample. Type I collagen is the major organic component of the dentin matrix, and type I procollagen is composed of two polypeptide chains, α1(I) and α2(I). The synthesis of these two polypeptide chains is under the control of two separate genes, COL1A1 and COL1A2 (Cutroneo, 2003; Verrecchia and Mauviel, 2004). There is a potential TGF-β activation region located upstream of both COL1A1 and COL1A2 genes (Ritzenthaler et al., 1991). However, we have been unable to detect an induction of Col1a1 mRNA by TGF-β2 stimulation in dental pulp cell culture within 1 hour (data not shown). Therefore, the question remains open whether TGF-β induces Col1a1 directly or as a secondary consequence of activation of other signaling targets.
Nestin is expressed predominantly in the developing nervous system and muscles, and it is replaced by neurofilament and glia fibrillary acid protein (GFAP) in nervous tissue and by desmin in muscle (Kachinsky et al., 1994; Sejersen and Lendahl, 1993). Previous studies have shown that nestin is expressed in developing and adult human teeth under normal and pathological conditions such as carious lesions and dental wound healing. In particular, nestin expression is evident in odontoblast processes up to the dentin-enamel junction where dentin matrix is produced. It has also been reported that nestin expression becomes progressively restricted to differentiating odontoblasts and is absent from older permanent teeth (About et al., 2000). These studies suggest that nestin expression is involved in the cytological differentiation of odontoblasts, especially in generating the odontoblast process.
We have shown in this study that nestin is expressed in odontoblasts at the tip of the cusp region in wild type samples at E18.5, but it is missing in the Tgfbr2fl/fl;Wnt1-Cre mutant. Following transplantation under the kidney capsule, nestin expression is clearly visible in wild type odontoblasts and their processes within the dentin matrix, whereas it is rarely observed in Tgfbr2fl/fl;Wnt1-Cre teeth. These results are consistent with the morphological phenotype of our mutant tooth, which has no dentinal tubules in the dentin. The dentin in our mutant consisted of a very thin monolayer, and it is well mineralized, suggesting that the defect in dentin formation in this model is downstream of the defect of odontoblast process formation. Newly differentiated odontoblasts are relatively closely packed and characterized by numerous cytoplasmic processes oriented toward the basement membrane of the inner enamel epithelium. As the odontoblast continued to mature, the many small cytoplasmic processes are replaced by a single major cytoplasmic process, the odontoblast process, which becomes the major secretory pole of the cell. (Sasaki and Garant, 1996). In the Tgfbr2 mutant, odontoblast process formation is perturbed, resulting in reduced dentin formation.
There is limited information regarding the regulation of nestin expression during tooth development. Application of exogenous BMP-4 in dental pulp tissue induces the expression of nestin in organ culture (About et al., 2000). In this study, we found no difference in the distribution and expression level of Bmp4 in the Tgfbr2fl/fl;Wnt1-Cre mutant as compared to wild type (data not shown). Our study provides the novel finding that exogenous TGF-β2 can induce ectopic nestin expression in wild type dental mesenchymal cells, but not in Tgfbr2fl/fl;Wnt1-Cre cells. We have also cultured dental pulp cells with TGF-β2 stimulation, however we have been unable to detect an induction of Nestin mRNA (data not shown). So, it remains unclear whether induction of nestin expression by TGF-β2 is direct or indirect.
In conclusion, the present study demonstrates that TGF-β signaling is critical for the functional differentiation of the CNC-derived odontoblasts in vivo. Compromised type I collagen and odontoblast process formation result in reduced dentin matrix formation, dentin thickness and smaller tooth size in the Tgfbr2fl/fl;Wnt1-Cre mutant. This animal model will provide additional useful information regarding the mechanism of TGF-β signaling in regulating dentin matrix formation.
Acknowledgments
We thank Dr. Julie Mayo for critical reading of the manuscript and Dr. Harold Moses for the Tgfbr2fl/fl mice. We also wish to thank Dr. Hidemitsu Harada for critical advice and suggestions. This study was supported by grants from the National Institute of Dental and Craniofacial Research, NIH (DE012711 and DE014078) to Yang Chai.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol. 2000;157:287–95. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begue-Kirn C, Smith AJ, Loriot M, Kupferle C, Ruch JV, Lesot H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol. 1994;38:405–20. [PubMed] [Google Scholar]
- Begue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, Lesot H. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992;36:491–503. [PubMed] [Google Scholar]
- Cam Y, Neumann MR, Oliver L, Raulais D, Janet T, Ruch JV. Immunolocalization of acidic and basic fibroblast growth factors during mouse odontogenesis. Int J Dev Biol. 1992;36:381–9. [PubMed] [Google Scholar]
- Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42:219–23. doi: 10.1016/S0003-9969(96)00115-X. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- Cutroneo KR. How is Type I procollagen synthesis regulated at the gene level during tissue fibrosis. J Cell Biochem. 2003;90:1–5. doi: 10.1002/jcb.10599. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- D'Souza RN, Cavender AC, Sood R, Tarnuzzer R, Dickinson D, Roberts A, Letterio J. Dental abnormalities in mice lacking a functional transforming growth factor-beta1 (TGF-beta1) gene indicate a role for TGF-beta1 in biomineralization. Int J Oral Biol. 1998;23:119–31. [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–24. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990;5:717–23. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- Graham A, Lumsden A. The role of segmentation in the development of the branchial region of higher vertebrate embryos. Birth Defects Orig Artic Ser. 1993;29:103–12. [PubMed] [Google Scholar]
- Heikinheimo K. Stage-specific expression of decapentaplegic-Vg-related genes 2, 4, and 6 (bone morphogenetic proteins 2, 4, and 6) during human tooth morphogenesis. J Dent Res. 1994;73:590–7. doi: 10.1177/00220345940730030401. [DOI] [PubMed] [Google Scholar]
- Heikinheimo K, Happonen RP, Miettinen PJ, Ritvos O. Transforming growth factor beta 2 in epithelial differentiation of developing teeth and odontogenic tumors. J Clin Invest. 1993;91:1019–27. doi: 10.1172/JCI116258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helder MN, Karg H, Bervoets TJ, Vukicevic S, Burger EH, D'Souza RN, Woltgens JH, Karsenty G, Bronckers AL. Bone morphogenetic protein-7 (osteogenic protein-1, OP-1) and tooth development. J Dent Res. 1998;77:545–54. doi: 10.1177/00220345980770040701. [DOI] [PubMed] [Google Scholar]
- Imai H, Osumi-Yamashita N, Ninomiya Y, Eto K. Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev Biol. 1996;176:151–65. doi: 10.1006/dbio.1996.9985. [DOI] [PubMed] [Google Scholar]
- Inage T, Toda Y. Gene expression of TGF-beta 1 and elaboration of extracellular matrix using in situ hybridization and EM radioautography during dentinogenesis. Anat Rec. 1996;245:250–66. doi: 10.1002/(SICI)1097-0185(199606)245:2<250::AID-AR11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bringas P, Jr, Mogharei A, Zhao J, Deng C, Chai Y. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel's cartilage development. Dev Dyn. 2002;224:69–78. doi: 10.1002/dvdy.10088. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–80. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Joseph BK, Savage NW, Young WG, Gupta GS, Breier BH, Waters MJ. Expression and regulation of insulin-like growth factor-I in the rat incisor. Growth Factors. 1993;8:267–75. doi: 10.3109/08977199308991572. [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;165:216–28. doi: 10.1006/dbio.1994.1248. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, Couly GF. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev Biol. 1993;159:24–49. doi: 10.1006/dbio.1993.1219. [DOI] [PubMed] [Google Scholar]
- Noden DM. Cell movements and control of patterned tissue assembly during craniofacial development. J Craniofac Genet Dev Biol. 1991;11:192–213. [PubMed] [Google Scholar]
- Ritzenthaler JD, Goldstein RH, Fine A, Lichtler A, Rowe DW, Smith BD. Transforming-growth-factor-beta activation elements in the distal promoter regions of the rat alpha 1 type I collagen gene. Biochem J. 1991;280(Pt 1):157–62. doi: 10.1042/bj2800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch JV. Patterned distribution of differentiating dental cells: facts and hypotheses. J Biol Buccale. 1990;18:91–8. [PubMed] [Google Scholar]
- Ruch JV, Lesot H, Begue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–9. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Russo LG, Maharajan P, Maharajan V. Basic fibroblast growth factor (FGF-2) in mouse tooth morphogenesis. Growth Factors. 1998;15:125–33. doi: 10.3109/08977199809117188. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Garant PR. Structure and organization of odontoblasts. Anat Rec. 1996;245:235–49. doi: 10.1002/(SICI)1097-0185(199606)245:2<235::AID-AR10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106(Pt 4):1291–300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–56. [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A. The role of growth factors in determination and differentiation of the odontoblastic cell lineage. Proc Finn Dent Soc. 1992;88 1:357–68. [PubMed] [Google Scholar]
- Thyagarajan T, Sreenath T, Cho A, Wright JT, Kulkarni AB. Reduced expression of dentin sialophosphoprotein is associated with dysplastic dentin in mice overexpressing transforming growth factor-beta 1 in teeth. J Biol Chem. 2001;276:11016–20. doi: 10.1074/jbc.M010502200. [DOI] [PubMed] [Google Scholar]
- Trainor P, Krumlauf R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol. 2000;2:96–102. doi: 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Vainio S, Thesleff I. Associations between transforming growth factor beta 1 RNA expression and epithelial-mesenchymal interactions during tooth morphogenesis. Development. 1991;113:985–94. doi: 10.1242/dev.113.3.985. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873–80. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]


