Abstract
Female zebra finches display a preference for songs of males raised with tutors compared to those from males without tutors. To determine howthis behavioral preference may bemediated by auditory perception sites, the social behavior network, and the dopamine reward system, and whether responses of these regions are affected by estradiol, females were treated with hormone or blank implants.An auditory choice test was conducted followed by exposure to tutored or untutored song or silence to examine induction of the immediate early gene, ZENK. Birds spent significantly more time near tutored than untutored song, regardless of estrogen treatment, and estradiol significantly decreased the density of ZENK immunore-active neurons within the ventromedial hypothalamus. These results suggest that selective neural and behavioral responses can be induced by both high quality vocalizations and estradiol, although they are not necessarily correlated.
Keywords: Immediate early gene, Estrogen, Song preference, Auditory perception, Songbird
1. Introduction
Song is an important signal for reproductive interactions between zebra finches [95]; females use male songs to make mate choice decisions. Females are less likely to pair with males that have been surgically altered to produce low quality song or have a lower song output [90]. They also show preferences for particular song types, often indicated by increased time spent near a vocalization [39,60,62], and/or with copulatory solicitation displays [5,81]. In the zebra finch, females prefer the songs of males that were raised with a male tutor and produce a normal song, compared to those from males reared without tutors [39], which are similar in pattern and duration to tutored song but contain fewer notes and more variation in frequency, primarily due to reduced infusion of call notes with stable frequencies into the song bout [73].
This preference may be the result of a selective response to songs of higher quality in a number of neural systems. For example, immediate early genes are induced within auditory perception regions including the caudomedial nidopallium (NCM) and the caudomedial mesopallium (CMM) after exposure to song [52,53]. In juveniles, increased immediate early gene expression is observed in these regions following exposure to conspecific versus heterospecific song [3,4] and tutored versus untutored song [91]. Adult females also display an increased density of immediate early gene immunoreactive nuclei with conspecific song versus heterospecific song [2], but responses to varying qualities of zebra finch song have not been examined in adult females. In other songbird species, however, immediate early gene expression is induced in auditory perception regions by high quality songs that elicit a behavioral preference in females [24,44,50,86].
Brain regions outside the auditory perception system, especially those important for social behavior, are likely also affected by male song. Newman [61] describes a network of interconnected nuclei (including the medial amygdala, bed nucleus of the stria terminalis, lateral septum, midbrain, ventromedial hypothalamus, anterior hypothalamus, andmedial preoptic area) that is responsive to social stimuli in a number of species (also reviewed by [26] and discussed in [49]). Under the influence of gonadal steroids, these areas mediate multiple social behaviors, including reproduction and aggression [61]. The response of these regions to male song stimuli has not been investigated in female zebra finches, but some areas, including the ventromedial hypothalamus and nucleus taeniae (which shares homology with a portion of the mammalian amygdala [12]), have been implicated in the control of social behaviors in ring doves [12,25] and starlings [12]. In addition, in other avian species some regions within this network are responsive to auditory stimuli [13,14], including song [49].
The mesolimbic dopamine reward system may also play a role in the response of female zebra finches to male song. It is conserved amongmammals and birds [21], and is activated following exposure to rewarding stimuli. For example, in female rodents, dopamine is released in this network following reproductive interactions with males [54,68] and exposure tomale odors [57]. In birds, when male starlings are producing sexually motivated song, increased immediate early gene expression is observed within the ventral tegmental area [30,79]. One study in female starlings, however, indicates that the dopaminergic activity may have an inhibitory effect, which is related to breeding condition [78]. Breeding females display decreased phosphorylated tyrosine hydroxylase immunoreactivity in the ventromedial hypothalamus and lateral septum when they are exposed to song compared to females exposed to no song. In contrast, non-breeding females displayed the opposite pattern, with an increased immunoreactivity in these areas in response to song.
It seems likely that estradiol acts in some or all of these neural systems to facilitate a reproductive response. All brain areas in the social behavior network express estrogen receptors in zebra finches [22,23,34]. They are also found in parts of the ventral tegmental area [47] and in auditory perception regions [22,23,34]. In addition, high estradiol levels correlate with periods of reproductive behavior such as nest building, egg laying, and incubation in female birds [37,87]. In many song response studies, females are treated with estradiol to facilitate reproductive behaviors [5,17,50,80,81], but studies in canaries have indicated that this manipulation is unnecessary for the induction of copulatory solicitation displays in response to song [42,58]. Until now, the effect of estradiol on neural responses to song has only been investigated in seasonal breeding birds; it increases immediate early gene expression in the auditory perception regions in white-crowned sparrows [48]. The influence of estradiol in neural systems in an opportunistic breeder such as the zebra finch is unknown. While hormones have not been measured across the reproductive cycle in zebra finches, presumably fluctuations in estrogen are similar to those in other avian species, which raises the question of how they alter the neural and behavioral responses of these females.
In sum, solid data from a variety of sources provide intriguing information on the neruroendocrine systems involved in perception of and responses to reproductively relevant auditory cues in female songbirds. A complete picture is still lacking, however. In an attempt to begin to synthesize and expand information about critical factors, we used a behavioral assay and immunohistochemistry for the immediate early gene ZENK in the same birds to simultaneously investigate potential roles of the auditory perception, social behavior and reward systems in the preference for tutored versus untutored song, as well as how the neural and behavioral responses may be mediated by estradiol.
2. Materials and methods
2.1. Animals
Adult female zebra finches were taken from the breeding colony at Michigan State University. The birds were kept on a 12:12 light:dark cycle and provided seed and water ad libitum, with hard-boiled eggs mixed with bread and either spinach or orange given once a week. Females used in the study were raised in mixed sex breeding aviaries for at least 100 days, to ensure that they had reached sexual maturity and were exposed to normal song during development. After this period, they were removed and housed in a single-sex aviary, which allowed auditory, but not visual or tactile contact with males, for at least 2 weeks prior to testing. All procedures were approved by the Michigan State University Animal Use and Care Committee and adhered to the guidelines of the National Institutes of Health.
2.2. Hormone treatment
Estradiol implants were produced by packing 2 mm of 17β-estradiol into 5 mm of Silastic tubing (i.d. 0.76, o.d. 1.65 mm) and sealing each end with silicone. Blank capsules were left unpacked. Females were anesthetized with isoflurane and either an estradiol or a blank capsule was inserted subcutaneously above the breast muscle. The incision was sealed with collodion adhesive, and the female was placed in an individual cage for 5 days to recover.
2.3. Song stimuli
Recordings of tutored (males raised with other males present) and untutored (males isolated from adult males from post-hatch day 18 after hatching) zebra finch songs were received from Dr. Adkins-Regan at Cornell University [39]. Two types of sound files were produced. In one, both types of songs were used to produce 10 different 20-min sound clips for behavioral testing. Each clip was generated by combining one song of each type from the pool of six untutored and tutored males into a stereo file. Although there were large differences between the tutored and untutored songs, pairs were made of one tutored and one untutored song with similar phrase lengths and relatively high similarity scores (which indicate note type, spectral characteristics, duration, order, and time between notes [88]), determined by comparing phrases from each males song with the freeware Sound Analysis Pro (http://ofer.sci.ccny.cuny.edu/htm/body sound analysis.html). Each song was normalized for amplitude in Adobe Audition (Adobe Systems, Inc., San Jose, CA), and following the introductory notes, it was repeated for a total of 20 min. A file was then synthesized to simultaneously play a tutored song from one side of the testing chamber (see below) and an untutored one from the other side, counterbalanced across tests.
The other type of sound file was used for exposure prior to immediate early gene analysis. In this case, twelve 30-min sound clips (six tutored and six untutored) were created in Adobe Audition. For each file, 30-s song clips from three different randomly chosen males within the same group (tutored or untutored) were played sequentially with 30 s of silence separating them. This compilation was repeated for a total of 30 min.
2.4. Choice test
Five days after implant surgery, females were taken to a room containing a wood and plexiglass chamber of (215 cm L × 60 cm W × 60 cm H; [1] modeled after [56]). It consisted of three zones: the center (with one perch), and the left and right (with three perches each). The bird was placed into the cage through a center door and allowed to freely move between the zones. After 70 min for acclimation, a randomly chosen song file was broadcast from speakers, one located at each end of the chamber, for 20 min at approximately 60 dB. Behaviors were videotaped and the time spent in each zone was quantified. In addition, two other behavioral measures (jumps between perches and calls) were taken. To ensure that the females adequately received the song stimulus, animals that were completely unresponsive during the song presentation (i.e., did notmove or call)were not used (20 individuals across the groups were removed; leaving sample sizes of 20 estradiol-treated and 20 control birds).
Following song presentation, each female was captured and blood was collected by wing vein puncture, centrifuged at 10,000 rpm for 10 min at 4 °C and plasma stored at −80 °C until radioimmunoassay to quantify estradiol concentration. Females were returned to their individual cages and taken back to the colony room.
Behavioral data were compared using a mixed-model ANOVA (treatment between animals and time spent within each zone within animals). The proportion of perch jumps and flights in the tutored zone were separately analyzed between treatment groups using Mann–Whitney U-tests, because some individuals did not display these behaviors and the data were not normally distributed. Statview (SAS Institute; Carey, NC) was used for all statistical analyses.
2.5. Immediate early gene analysis
Two days after the choice test, the 40 females were individually taken to a novel room for stimulus exposure to examine ZENK induction (as in [2–4]). They remained in their individual cages during the test, which were placed within a sound-isolated box. After a 30-min acclimation period (as in [2]), a randomly chosen 30-min file of tutored or untutored song or silence was broadcast from a speaker three inches from the cage at 60 dB. Each group contained six to eight individuals. In a few cases, a brain region could not be quantified due to histological artifact; final sample sizes are indicated in the figures and table. To reduce habituation [15], no female was exposed to song from the same male in the choice test and stimulus exposure for ZENK induction.
The song exposure was videotaped, and calls and receptive behaviors were later quantified. Following song presentation, the female remained in silence for 1 h before being overdosed with 0.12 cc of equithesin and perfusion with 0.1 M phosphate buffered saline (PBS) and 4% paraformaldehyde. Brains were dissected from the skull and postfixed for 15 min in 4% paraformaldehyde. They were embedded in gelatin, fixed for another hour in 4% paraformaldehyde and placed in 30% sucrose in 0.1 M PBS at −20 °C until sectioning. The embedded tissue was sectioned frozen into four series at 30µm in the sagittal plane and stored in cryoprotectant at −20 °C until immunohistochemical processing.
Immunohistochemistry was performed in a method adapted from Bailey et al. [2] and Bailey and Wade [3,4]. Cryoprotected tissue was rinsed 6× 5 min in PBS, reacted for 15 min in 0.5% hydrogen peroxide (H202) in PBS and blocked for 1 h in 5% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS with 0.3% Triton-X-100 (PBST). The tissue was then incubated in primary antibody (Santa Cruz Biotech; catalog #sc-189, 0.1µg/ml) in PBST for 2 days at 4 °C. It was rinsed 3× 5 min in PBS and incubated for 1 h in Biotin-SP conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories; 1:500 dilution) in PBST. After a 3× 5 min PBS rinse, tissue was exposed to an ABC reagent (Elite kit, Vector Laboratories, Burlingame, CA) for 1 h. It was then rinsed 3× 5 min in PBS and incubated in diaminobenzadine (Sigma, St. Louis, MO; 0.5 mg/ml) plus 0.0075% H202 for 7 min. After PBS rinses, sections were mounted, dehydrated, cleared in xylene and coverslipped with DPX.
ZENK immunoreactivity in the auditory regions (NCM and CMM), social behavior areas (preoptic area [POA], ventromedial hypothalamus [VMH], bed nucleus of the stria terminalis [BST], nucleus taeniae [TnA], ventrolateral subdivision of the caudal lateral septum [LSc. vl], and midbrain central gray [CGt]), and reward system (nucleus accumbens [Ac] and the ventral tegmental area [VTA]) was assessed using an Olympus BX60 microscope (Fig. 1 and Fig. 2). The placement and size of the sampling regions, which is detailed in the figure captions, was determined after an initial scan of the tissue to locate the extent of labeling. Immunoreactive cells were hand-counted by an observer blind to treatment and auditory stimulus within the sampling regions. The NCM and CMM were analyzed within the same medial sections, which were identified by the connection of the nidopallium to the rest of the telencephalon and the presence of the septopallio-mesencephalic tract (TSM). For the nucleus accumbens and ventral tegmental area, alternate sections in some animals were subjected to tyrosine hydroxylase immunohistochemistry to determine the correct placement of the sampling region (Fig. 2). The methods were the same as those described for ZENK immunohistochemistry, but using tyrosine hydroxylase primary antibody (Immunostar; catalog # 22941, 1:10,000 dilution) and donkey anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). As the precise border of the Ac is not completely distinguished, the box was placed in the ventromedial portion of the medial striatum in the most medial sections, as discussed in Reiner et al. [74].
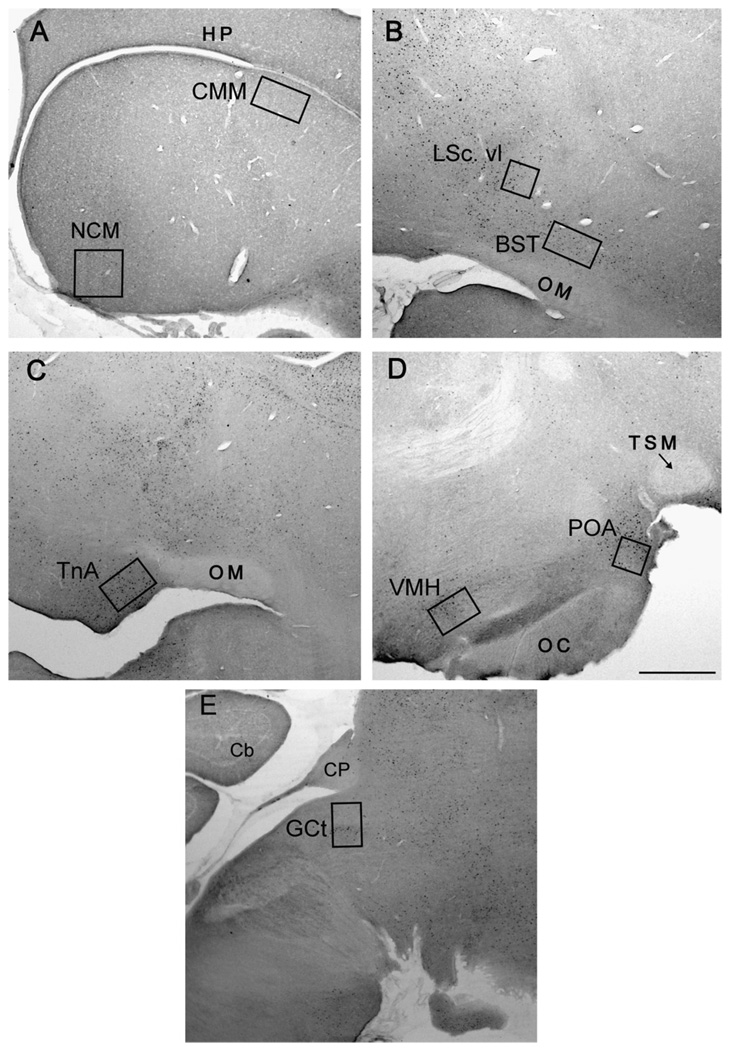
Fig. 1.
Placement of sampling regions within auditory perception and social behavior areas in sagittal sections. Panel A contains boxes for the caudomedial nidopallium (NCM; 310 µm × 320 µm) and caudomedial mesopallium (CMM; 190 µm × 345 µm), panel B for the bed nucleus of the stria terminalis (BST; 190 µm × 345 µm) and ventrolateral subdivision of the caudal lateral septum (LSc. vl; 200 µm × 200 µm), panel C for the nucleus taeniae (TnA; 200 µm × 320 µm), panel D for the ventromedial hypothalamus (VMH; 190 µm × 290 µm) and preoptic area (POA; 200 µm × 200 µm), and panel E demonstrates the midbrain central gray (GCt; 190 µm × 290 µm). The hippocampus (HP), tractus occipito-mesencephalicus (OM), tractus septopallio-mesencephalicus (TSM), optic chiasm (OC), posterior commisure (CP), and cerebellum (Cb) were used as landmarks for location of sections and placement of boxes. The more rostral portion of each photo is towards the right edge. Scale bar (in panel D) = 500 µm.
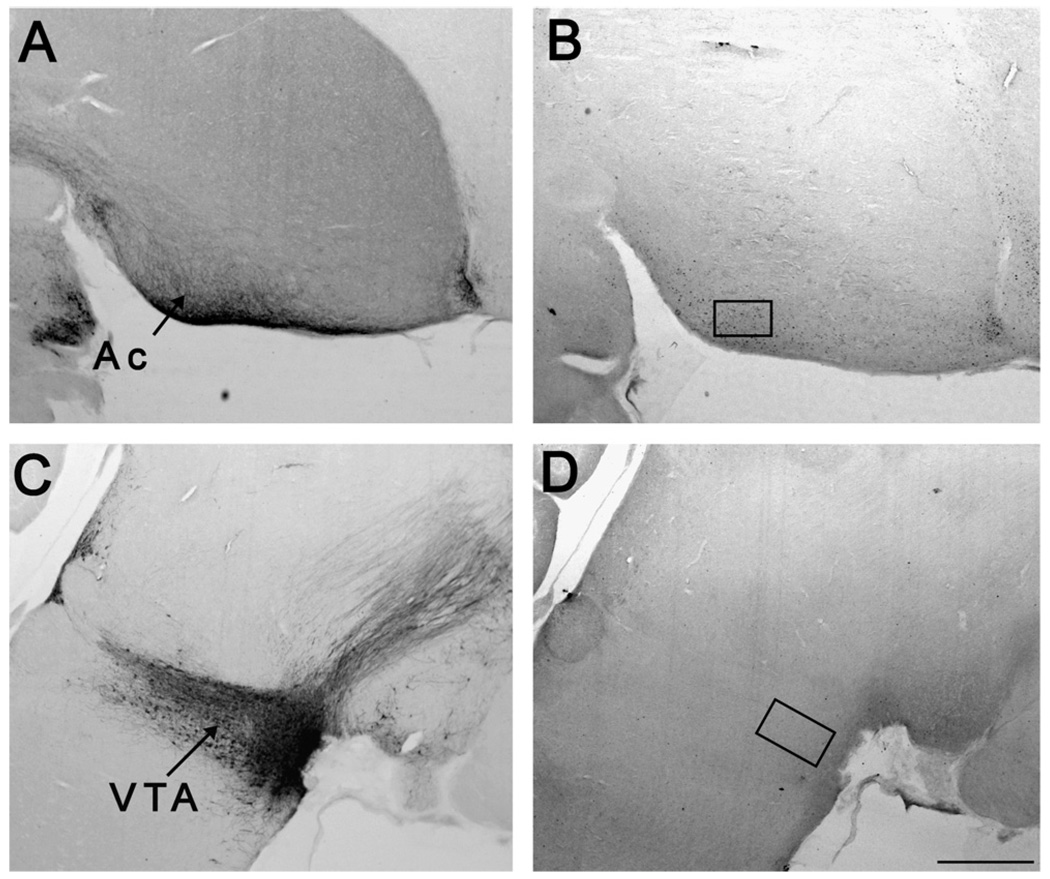
Fig. 2.
Placement of sampling regions within the reward system. Panels A and C contain sections subjected to tyrosine hydroxylase immunohistochemistry sliced in the sagittal plane. Panels B and D contain ZENK labeling. Panel B displays the location of the box for the nucleus accumbens (Ac; 190 µm × 305 µm), and panel D displays the ventral tegmental area (VTA; 210 µm × 368 µm). The more rostral portion of each photo is towards the right edge. Scale bar = 300 µm.
The density of ZENK immunoreactive nuclei was determined by dividing the average number of immunoreactive cells for each animal (at least three sections per animal were analyzed for each brain region) by the area of the sampling box. Effects of treatment and song exposure type on ZENK density were analyzed using a two-way ANOVA followed by post hoc Tukey–Kramer tests where appropriate.
2.6. Radioimmunoassay
A single radioimmunoassay was conducted in a manner adapted from Lovern and Wade [45]. Parallelism was first demonstrated with recently collected zebra finch plasma (data not shown). For the full assay, a mean of 31.4 µl of plasma from each animalwas incubated with radioactive tracer [3H] estradiol (70 Ci/mmol; PerkinElmer, Boston, MA) at 4 °C overnight. Steroids were then extracted twice with diethyl ether, and samples were dried under nitrogen. They were resuspended in PBS and stored overnight at 4 °C. A competitive binding assay was completed in duplicate samples and a serially diluted standard curve (0.98–250 pg estradiol) in triplicate, by adding an estradiol antibody (NEG307H; Biogenesis, Kingston, NH) with [3H] estradiol and incubating overnight at 4 °C. Water blanks were added as controls (n = 4) and six aliquots of a known concentration of estradiol were used to determine intra-assay precision. The next day, dextran-coated charcoal was added and centrifuged at 2200 rpm for 10 min at 4 °C in order to remove unbound tracer. The remaining sample was combined with scintillation fluid (UltimaGold; Perkin and Elmer, Boston, MA) and analyzed with a scintillation counter (Beckman 6500, Fullerton, CA). Estradiol levels were calculated by standardizing samples for individual recovery and the volume assayed and compared to the standard curve. The intra-assay coefficient of variance was 6%. A Mann–Whitney U-test was used to compare the estradiol and blank treated groups.
3. Results
3.1. Behavior
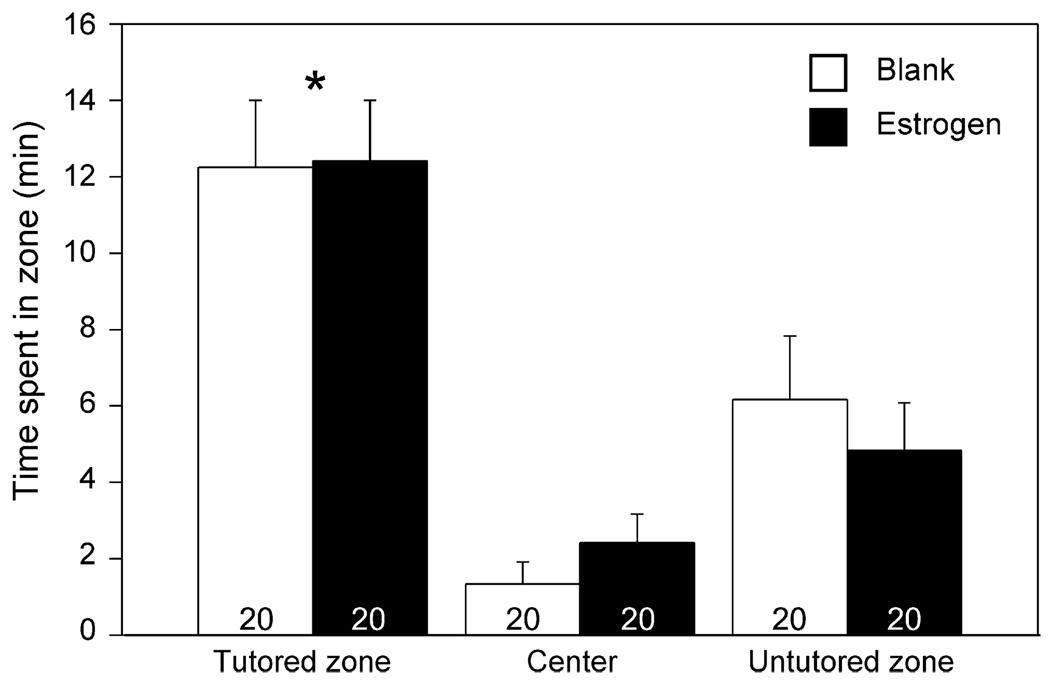
Females spent significantly more time in the zone near the tutored song than that neighboring the untutored song or in the center (F = 19.238, p < 0.01; Tukey–Kramer, both p < 0.05). However, estrogen did not affect this behavior (F = 0.561, p = 0.56), perch jumps (Mann–Whitney U, p = 0.371) or calls (Mann–Whitney U, p=0.112; Fig. 3).
Fig. 3.
Total time spent within the tutored, center and untutored zones in the behavioral test. Sample sizes are indicated on the graph. *Tutored significantly greater than untutored and center zones.
During the ZENK induction, only one female displayed a tail quiver (associated with receptivity [89]), so these data were not analyzed statistically. No significant main effects of hormone, song type or interaction between them were detected in calling (data not shown).
3.2. ZENK expression
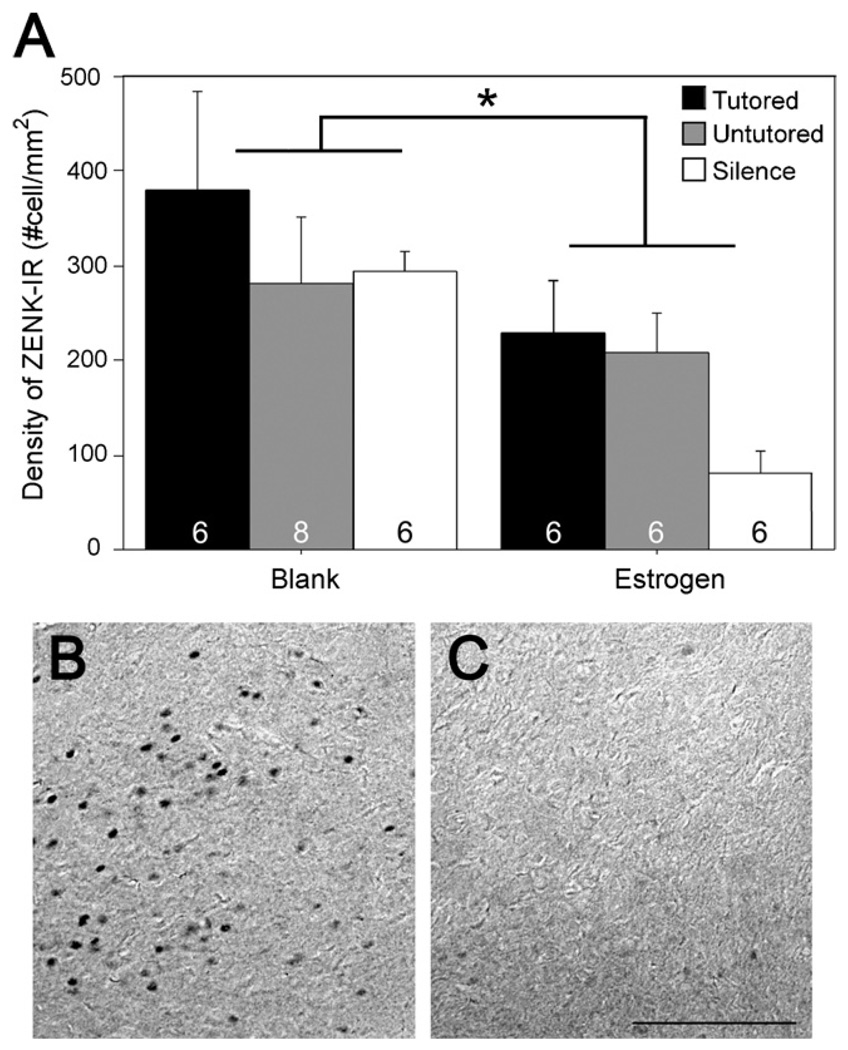
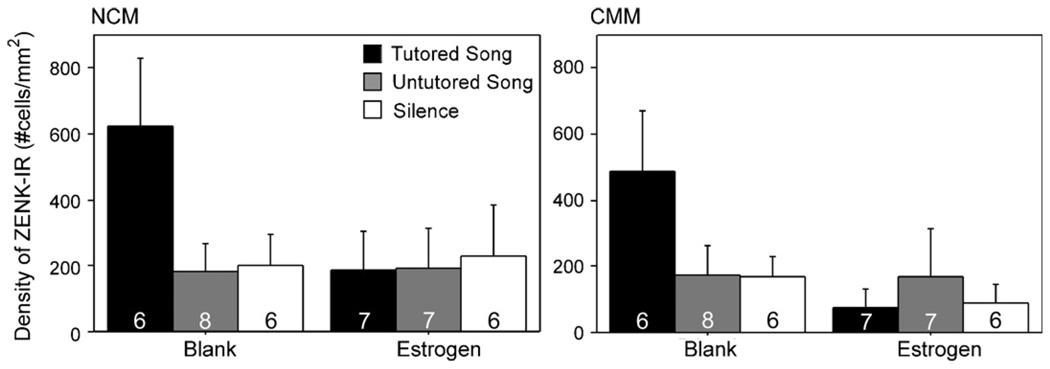
The clearest effect of estradiol was detected in the VMH. Amain effect of treatment was observed, such that estradiol reduced the density of ZENK immunoreactive (IR) nuclei (F = 8.44, p = 0.007; Fig. 4). No significant main effects of hormone or song type or interactions between them were observed in any of the other seven brain regions with this type of analysis (Table 1). However, the pattern observed in the auditory regions was striking. While the effect of stimulus exposure was not statistically significant (NCM: F = 1.72, p = 0.194; CMM: F = 0.98, p = 0.385), the mean density of ZENK-IR nuclei in the NCM and CMM of the blank-treated birds that heard tutored song was three times greater than those exposed to untutored song or silence (Fig. 5). In fact, this increase was observed only in the one group, and a trend for estradiol treatment to reduce it existed in the CMM (F = 3.61, p = 0.066). A similar, albeit weaker, pattern was observed in the NCM (F = 1.46, p = 0.236).
Fig. 4.
ZENK-IR nuclei in the ventromedial hypothalamus of female zebra finches. Panel A displays the quantification of densities of ZENK-IR for each treatment group and song exposure type. Sample sizes are indicated on the graph. A main effect of treatmentwas detected (*); estradiol decreased the expression of ZENK. Panels B and C depict ZENK in the ventromedial hypothalamus of birds exposed to tutored song. Panel B = blank treated female, Panel C = estrogen treated female. Scale bar = 100 µm.
Table 1.
Mean densities of ZENK-IR in various brain regions presented as number of IR nuclei/mm2 (mean ± standard error). Due to histological artifact, only seven animals were analyzed in the Ac in the blank, untutored group. No main effects or interactions were detected, all F < 3.02, p > 0.06.
| Blank-treated | Estradiol-treated | |||||
|---|---|---|---|---|---|---|
| Tutored (n = 6) | Untutored (n = 7 or 8) | Silence (n = 6) | Tutored (n = 6) | Untutored (n = 6) | Silence (n = 6) | |
| POA | 414.5 ± 117 | 500.2 ± 126 | 591.0 ± 99 | 467.0 ± 168 | 279.7 ± 81 | 389.7 ± 89 |
| BST | 1056.0 ± 255 | 945.3 ± 226 | 1106.5 ± 254 | 494.8 ± 187 | 935.6 ± 338 | 694.4 ± 352 |
| TnA | 125.0 ± 73 | 67.3 ± 26 | 30.5 ± 11 | 52.8 ± 23 | 91.9 ± 46 | 216.4 ± 215 |
| LSc. vl | 591.0 ± 191 | 376.6 ± 146 | 434.2 ± 164 | 120.8 ± 58 | 323.6 ± 207 | 256.9 ± 254 |
| GCt | 61.3 ± 32 | 54.8 ± 26 | 132.5 ± 54 | 23.4 ± 11 | 83.7 ± 45 | 39.1 ± 19 |
| Ac | 552.8 ± 252 | 837.2 ± 252 | 1286.7 ± 224 | 888.4 ± 280 | 1049.6 ± 223 | 558.8 ± 135 |
| VTA | 61.8 ± 20 | 52.2 ± 17 | 74.2 ± 30 | 54.0 ± 40 | 67.3 ± 25 | 13.3 ± 7 |
Abbreviations: preoptic area (POA), bed nucleus of the stria terminalis (BST), nucleus taeniae (TnA), ventrolateral subdivision of the caudal lateral septum (LSc. vl), midbrain central gray (GCt), nucleus accumbens (Ac), ventral tegmental area (VTA).
Fig. 5.
Densities of ZENK expression within the caudomedial nidopallium (NCM) and caudomedial mesopallium (CMM) in female zebra finches. Sample sizes are indicated on the graphs.
3.3. Plasma estradiol
Estrogen treatment reliably increased circulating estradiol levels (Mann–Whitney U = 73, p = 0.002). Estradiol-treated birds had a mean of 5.7 ng/ml ± 1.3 S.E., while the value for control birds was 2.2 ng/ml ± 0.46 S.E. However, a number of the samples from blank-treated females fell below the range of the sensitivity of the assay, so to be conservative, the minimum detectable value (15.63 pg/sample; equivalent to an average of 1.84 ng/ml, depending on sample volume and recovery) was assigned.
4. Discussion
4.1. Behavioral response
Female zebra finches spent more time near the tutored song than untutored song or silence. Lauay et al. [39] also detected a similar propensity for increased time spent near tutored over untutored song in female zebra finches, although they did not examine the effects of estrogen or the neural response to these two types of song.
The tendency to spend increased time nearer this type of stimulus may be related to reproduction. Females prefer songs with characteristics reflecting increased male energy input (i.e., long songs, increased song rate, and difficult syllables [5,60,62]). In addition, some of these characteristics correlate with high paternal effort (nestling feeding [9,18]) and offspring survival [29]. Low song quality may also indicate developmental stress, and as a result, lower the fitness of a potential mate [63]. It therefore seems reasonable to hypothesize that females spend more time near tutored song because it indicates a higher quality mate than untutored song. It is possible, however, that the females simply find the untutored song aversive.
The widespread nature of song preference behavior might suggest that its function is broader than reproduction and mate choice. For example, male birds from a variety of species prefer song from conspecifics [8,10] and their own fathers [16,75,76]. In addition, juvenile zebra finches prefer song from their tutor [31,32] prior to the period when they become reproductively active. The lack of an effect of estradiol on the behavioral response is also consistent with the idea that the tendency to spend time with a specific song type is dissociated from mate choice and reproduction in the female zebra finch. Alternatively, it is possible that the hormone is required to display this behavioral preference, and the concentration of circulating estradiol in control females was above the threshold (similar to untreated canaries [42,58]).
4.2. Neural response
4.2.1. Auditory regions
Statistically significant differences in ZENK expression due to auditory stimulus were not detected in the NCM or CMM. However, in control but not estradiol-treated animals, the average density of these cells was approximately three times greater in birds exposed to tutored song than the other two stimuli. The effect of estradiol on ZENK expression in these areas also did not reach statistical significance, and no interaction was detected between the hormone and auditory stimulus type. One therefore needs to be very careful in suggesting conclusions about potential effects of the hormone. However, a number of factors suggest that potential biological relevance might be considered. For example, specific large mean differences in ZENK expression were seen in just the auditory areas of control birds exposed to high quality song compared to both low-quality song and silence (see Fig. 5). This pattern parallels previous work in zebra finches in which immediate early gene expression in auditory regions was increased following exposure to stimuli of greater relevance [2–4,53,91]. If a mixed-model 3-way ANOVA is used to consider patterns across brain regions, a song × treatment × region interaction is detected. This interaction partly stems from the fact that NCM and CMM are the only regions to show this dramatic difference between tutored and untutored song and silence and that this effect is only observed in blank treated individuals. Thus, it is likely that estrogen inhibits this selective ZENK response in these regions. However, the lack of a significant main effect of song type in the auditory regions in the present study may be the result of the reduced requirement to detect variations in song in adulthood in this species. The other species in which this phenomenon has been investigated are primarily seasonal breeders, in which the females also sing [7,65,92] and/or male song is variable in adulthood [43,77,85]. Zebra finches, in contrast, are aseasonal, the females do not sing, and male zebra song is stable in adulthood [89]. A difference in ZENK response to tutored and untutored song was observed in juvenile female zebra finches [85]. However, this plasticity may be lost once females have formed a template and learned the basic characteristics of species-appropriate song.
From a more technical perspective, we cannot completely eliminate the possibility that the modest differences in exposure protocols during the behavior test (in which they were exposed to both song types simultaneously in a large choice cage) and ZENK induction (in which they were only exposed to one auditory stimulus in a sound isolated box) affected the animals somewhat differently, such that they showed a behavioral preference for tutored song, but not increased ZENK expression. However, in the ZENK induction test they remained within their home cage, which fit inside the sound-isolated box with the interest of reducing stress from a novel environment.
Regardless of the results in blank treated animals, it is clear that when our females were treated with estradiol, the response to all auditory stimuli was nearly identical. This result differs from the findings of Maney et al. [48,49], in which estrogen treatment resulted in a greater immediate early gene response to conspecific song compared to tones. In the present study, however, we examined the difference in ZENK expression to variations in quality of song, whereas Maney et al. [48,49] compared normal song to a tone stimulus. It is unknown how estradiol may mediate neural responses in auditory perception sites, but it is plausible that it acts on different signaling mechanisms. That is, separate pathways might (1) provide information that an auditory signal is an approximation of song, and (2) assess particular features (i.e., relative quality) of the song. If so, it is also possible that estradiol facilitates a neural response in one case and inhibits it in the other. Some estrogen receptors are located along the caudal edge of the NCM, as well as within the CMM [22,23,34], so regulation may occur directly at these sites or through connections with other brain regions. LeBlanc et al. [41] observed an increase in density of tyrosine hydroxylase immunoreactivity in the NCM following estrogen treatment, and predicted that estradiol plays a neuromodulatory role on responses within the auditory perception regions. It will be important to further investigate all of these ideas in future experiments.
4.2.2. Social behavior network
Although estradiol did not alter behavior in the present study, it did affect the neural response specifically in the VMH. This region contains a high concentration of estrogen receptors in a wide range of vertebrates, including rodents [e.g., 20,36,40,66,82,83,94] and birds [6,28,55], including zebra finches [22,23,34]. In addition, the VMH plays a crucial role in the control of reproduction in female rodents [67] and also appears to be important in birds [25]. However, rather than an increase in immediate early gene expression as has been observed in rodents following exposure to reproductive stimuli [69], we detected less ZENK-IR in the VMH with hormone administration. Treatment with estradiol alone can result in increases in immediate early gene expression [11,33], but similar to the present data, decreases have also been documented. For example, Tetel et al. [89] and Pfaus et al. [70] observed decreases in the induction of FOS-IR following small amounts of vaginocervical stimulation in the VMH when female rats were treated with estradiol. In addition, a decrease in immediate early gene expression in the preoptic area and amygdala occurs following testosterone administration in male anole lizards [59].
At least two potential explanations for this estradiol-induced decrease in immediate early gene expression exist. First, although these genes are often used to indicate regions that are “activated” by a certain stimulus, expression may also indicate stimulation of inhibitory neurons such as those expressing GABA. In male gerbils, a large proportion of cells expressing immediate early genes following mating are GABAergic [84]. Estradiol treatment can result in a decrease in bound GABAA receptor [64] and responsiveness of GABAB receptors [38] possibly resulting in disinhibition. Conversely, estrogen can cause an increase in GABA levels in the VMH of female rats [46]. It is possible that estradiol reduces the activity of GABA neurons in the VMH in the present study, which is indicated by a decrease in ZENK expression. In zebra finches, song exposure results in GABA neurons expressing ZENK within the auditory perception regions [71,72]. As GABA neurons are present in female birds [19,27], and can be regulated by social stimuli, as in rodents, it is possible that they may also be affected by estradiol in zebra finches. Second, it is possible that the decrease in ZENK expression observed in this study may be partially due to a decrease in activation of dopaminergic neurons in the VMH as described in Riters et al. [78]. In that study, decreased levels of phosphorylated tyrosine hydroxylase were observed following exposure to song in only breeding season female starlings. The authors hypothesize that these neurons may serve an inhibitory function within the VMH during reproductive behavior [78].
Maney et al. [49] conducted a study similar to ours in white-crowned sparrows; the response to song and how it is modulated by estradiol was examined in the social behavior network. In that study, increased ZENK responses were observed within the TnA, VMH, BST, and LSc. vl, and estrogen enhanced this response in all the examined social behavior network regions, except the medial VMH and the anterior medial hypothalamus. We did not observe changes in the responses of these regions to song stimuli, and we did not observe modulation of the response to song in these regions by estrogen. There are multiple potential explanations for these discrepancies. For example, housing conditions differed. Our animals were maintained in a colony room with hundreds of zebra finches, whereas the white-crowned females were housed with several other females in sound-isolated booths for the extent of the study in Maney et al. [49]. Perhaps more important, however, are biological differences between zebra finches and white-crowned sparrows that may lead to differences in their responses within this network to song. Female zebra finches do not sing and adult males produce a simple and stable song in adulthood [93]. White-crowned sparrows, in contrast, have several song dialects [51] and females can also produce song [35]. It is possible that if female white-crowned sparrows produce song when hearing the song stimulus, it could affect the response of regions in the social behavior network. Some of these regions are activated in males when they sing [i.e. 30]. In addition, differences in neural responses may reflect song complexity. For example, because male zebra finch song is so simple and stable, and females do not sing, female zebra finches may not need to utilize these regions to interpret the meaning of the song stimulus, whereas birds such as white-crowned sparrows that have more variable song that can be used in more contexts might require additional regions for processing. Similarly, female zebra finches may require additional stimulation to activate these regions. Song might be interpreted by only the auditory perception regions, and other areas of the brain may only be activated when other stimuli are present, such as courtship displays (visual input) or physical bonding (tactile input). Differing effects of estrogen between the species might relate to varying levels of reliance on the hormone for reproduction in seasonal (white-crowned sparrows) and opportunistic (zebra finches) breeders. Determining which, if any, of these possibilities led to the differences in ZENK response between the present study and that of Maney et al. [49] on white-crowned sparrows could lead to insights on the specific roles of these brain regions.
4.3. Dissociation between behavior and immediate early gene expression
Dissociations between behavior and ZENK expression were observed on two levels. First, although the females displayed a strong propensity to spend time near the tutored song, no differences in ZENK expression between auditory stimuli were observed in the social behavior network or reward system, and more work must be done on the auditory perception regions before solid conclusions can be drawn. Second, the reduction of ZENK expression by estrogen in the VMH with the lack of an effect of the hormone on behavior in the choice test suggests that an increased ZENK response within the VMH is not necessary for the display of differential behavioral responses to high quality song.
These data suggest a number of possibilities. For example, another immediate early gene may be involved in the regulation of the behavior. Consistent with that idea, some differences between the activities of FOS and ZENK have been detected in juvenile zebra finches [3], although similar patterns of induction of the two immediate early genes have also been observed following song presentation to adult female songbirds [86,93]. Additionally, several other immediate early genes (such as c-jun) exist that may also be important to the changes that occur during the display of this behavior. It is also possible that some other neural system (in addition to the three networks examined here) is vital to mediation of this behavior.
4.4. Conclusion and future directions
In summary, estrogen treatment does not affect the tendency to spend time near high quality, tutored song as measured in the present study, but it does suppress ZENK induction in the VMH. The present data suggest that the maintenance of this behavior does not require higher concentrations of estradiol and may not be associated with the ZENK expression detected within the examined brain regions. In addition, the fact that estrogen’s inhibition of the neural response is relatively specific, and does not occur in most of the brain regions investigated, suggests that it serves some function, although the nature of it is not clear at this point. Determining the phenotype of the affected cells will help to elucidate their function(s).
Acknowledgements
This research was funded by the National Institutes of Health (R01-MH55488 and K02-MH65907). We thank Elizabeth Adkins-Regan for providing us with the tutored and untutored song files, Dave Bailey for assistance with the behavioral testing procedure, Yu Ping Tang for advice with immunohistochemistry, Stephany Latham for technical assistance, and Katie Licht for coding the song preference tests.
References
- 1.Bailey DJ. Department of Psychology. East Lansing: Michigan State University; 2006. Neurobiology of song learning and perception in the zebra finch (Taeniopygia guttata), with a focus on the role of the hippocampus; p. 190. [Google Scholar]
- 2.Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Mol Brain Res. 2003;116:147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DJ, Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res. 2005;162(1):108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Ballentine B, Hyman J, Nowicki S. Vocal performance influences female response to male bird song: an experimental test. Behav Ecol. 2004;15(1):163–168. [Google Scholar]
- 6.Balthazart J, Gahr M, Surlemont C. Distribution of estrogen receptors in the brain of the Japanese quail: an immunocytochemical study. Brain Res. 1989;501(2):205–214. doi: 10.1016/0006-8993(89)90638-0. [DOI] [PubMed] [Google Scholar]
- 7.Baptista LF, Petrinovich L. Song development in the white-crowned sparrow—social-factors and sex-differences. Anim Behav. 1986;34(5):1359–1371. [Google Scholar]
- 8.Braaten RF, Reynolds K. Auditory preference for conspecific song in isolation-reared zebra finches. Anim Behav. 1999;58(1):105–111. doi: 10.1006/anbe.1999.1134. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan KL, Catchpole CK. Song as an indicator of male parental effort in the sedge warbler. Proc Biol Sci. 2000;267(1441):321–326. doi: 10.1098/rspb.2000.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calhoun S, Hulse SH, Braaten RF, Page SC, Nelson RJ. Responsiveness to conspecific and alien song by canaries (Serinus canaria) and European starlings (Sturnus vulgaris) as a function of photoperiod. J Comp Psychol. 1993;107(3):235–241. [Google Scholar]
- 11.Cattaneo E, Maggi A. c-fos induction by estrogen in specific rat brain areas. Eur J Pharmacol. 1990;188(2–3):153–159. doi: 10.1016/0922-4106(90)90050-8. [DOI] [PubMed] [Google Scholar]
- 12.Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53(5–6):243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- 13.Cheng MF, Peng JP. Reciprocal talk between the auditory thalamus and the hypothalamus: an antidromic study. Neuroreport. 1997;8(3):653–658. doi: 10.1097/00001756-199702100-00015. [DOI] [PubMed] [Google Scholar]
- 14.Cheng MF, Peng JP, Johnson P. Hypothalamic neurons preferentially respond to female nest coo stimulation: demonstration of direct acoustic stimulation of luteinizing hormone release. J Neurosci. 1998;18(14):5477–5489. doi: 10.1523/JNEUROSCI.18-14-05477.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew SJ, Mello CV, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci. 1995;92(8):3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton N. Song discrimination learning in zebra finches. Anim Behav. 1988;36:1016–1024. [Google Scholar]
- 17.Clayton N, Prove E. Song discrimination in female zebra finches and Bengalese finches. Anim Behav. 1989;38:352–362. [Google Scholar]
- 18.Dolby AS, Clarkson CE, Haas ET, Miller JK, Havens LE, Cox BK. Do song-phrase production rate and song versatility honestly communicatemale parental quality in the Gray Catbird? J Field Ornithol. 2005;76(3):287–292. [Google Scholar]
- 19.Domenici L, Waldvogel HJ, Matute C, Streit P. Distribution of GABA-like immunoreactivity in the pigeon brain. Neuroscience. 1988;25(3):931–950. doi: 10.1016/0306-4522(88)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptorimmunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol. 1991;305(4):591–612. doi: 10.1002/cne.903050406. [DOI] [PubMed] [Google Scholar]
- 21.Durstewitz D, Kroner S, Gunturkun O. The dopaminergic innervation of the avian telencephalon. Prog Neurobiol. 1999;59(2):161–195. doi: 10.1016/s0301-0082(98)00100-2. [DOI] [PubMed] [Google Scholar]
- 22.Gahr M, Guttinger HR, Kroodsma DE. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. J Comp Neurol. 1993;327(1):112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- 23.Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44(4):509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 24.Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Gibson MJ, Cheng MF. Neural mediation of estrogen-dependent courtship behavior in female ring doves. J Comp Physiol Psychol. 1979;93:855–867. [Google Scholar]
- 26.Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc Biol Sci. 2005;272(1560):227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granda RH, Crossland WJ. GABA-like immunoreactivity of neurons in the chicken diencephalon and mesencephalon. J Comp Neurol. 1989;287(4):455–469. doi: 10.1002/cne.902870405. [DOI] [PubMed] [Google Scholar]
- 28.Halldin K, Axelsson J, Holmgren C, Brunstrom B. Localization of estrogen receptor-alpha and -betamRNA in brain areas controlling sexual behavior in Japanese quail. J Neurobiol. 2006;66(2):148–154. doi: 10.1002/neu.20199. [DOI] [PubMed] [Google Scholar]
- 29.Hasselquist D, Bensch S, von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. [Google Scholar]
- 30.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50(5):726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houx BB, ten Cate C. Do stimulus–stimulus contingencies affect song learning in zebra finches (Taeniopygia guttata)? J Comp Psychol. 1999;113(3):235–242. [Google Scholar]
- 32.Houx BB, ten Cate C. Song learning from playback in zebra finches: is there an effect of operant contingency? Anim Behav. 1999;57(4):837–845. doi: 10.1006/anbe.1998.1046. [DOI] [PubMed] [Google Scholar]
- 33.Insel TR. Regional induction of c-fos-like protein in rat brain after estradiol administration. Endocrinology. 1990;126(4):1849–1853. doi: 10.1210/endo-126-4-1849. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Steroid Biochem Mol Biol. 1996;59(2):135–145. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- 35.Kern MD, King JR. Testosterone-induced singing in female white-crowned sparrows. Condor. 1972;74(2):204–209. [Google Scholar]
- 36.Koch M, Ehret G. Immunocytochemical localization and quantitation of estrogen-binding cells in the male and female (virgin, pregnant, lactating) mouse brain. Brain Res. 1989;489(1):101–112. doi: 10.1016/0006-8993(89)90012-7. [DOI] [PubMed] [Google Scholar]
- 37.Korenbrot CC, Schomberg DW, Erickson CJ. Radioimmunoassay of plasma estradiol during the breeding cycle of ring doves (Streptopelia risoria) Endocrinology. 1974;94:1126–1132. doi: 10.1210/endo-94-4-1126. [DOI] [PubMed] [Google Scholar]
- 38.Lagrange AH, Wagner EJ, Ronnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64(2):114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- 39.Lauay C, Gerlach NM, Adkins-Regan E, DeVoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim Behav. 2004;68:1249–1255. [Google Scholar]
- 40.Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129(6):3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstemcatecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 42.Leboucher D, Kreutzer M, Dittami J. Copulation-solicitation displays in female canaries (Serinus canaria): are oestradiol implants necessary? Ethology. 1994;97:190–197. [Google Scholar]
- 43.Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav. 2001;40(2):160–168. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- 44.Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64(3):275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- 45.Lovern MB, Wade J. Sex steroids in green anoles (Anolis carolinensis): uncoupled maternal plasma and yolking follicle concentrations, potential embryonic steroidogenesis, and evolutionary implications. Gen Comp Endocrinol. 2003;134(2):109–115. doi: 10.1016/s0016-6480(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 46.Luine VN, Grattan DR, Selmanoff M. Gonadal hormones alter hypothalamic GABA and glutamate levels. Brain Res. 1997;747(1):165–168. doi: 10.1016/s0006-8993(96)01255-3. [DOI] [PubMed] [Google Scholar]
- 47.Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in cate-cholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311(3):189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- 48.Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23(6):1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- 49.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- 50.Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- 51.Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- 52.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14(11 Pt 1):6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109(2):354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 55.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsong birds. J Comp Neurol. 1999;407(1):115–129. [PubMed] [Google Scholar]
- 56.Miller DB. The acoustic basis of mate recognition by female zebra finches (Taeniopygia guttata) Anim Behav. 1979;27(2):376–380. [Google Scholar]
- 57.Mitchell JB, Gratton A. Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci. 1994;5(4):317–329. doi: 10.1515/revneuro.1994.5.4.317. [DOI] [PubMed] [Google Scholar]
- 58.Nagle L, Kreutzer ML, Vallet E. Obtaining copulation solicitation displays in female canaries without estradiol implants. Experientia. 1993;49:1022–1023. [Google Scholar]
- 59.Neal JK, Wade J. Effects of season, testosterone and female exposure on c-fos expression in the preoptic area and amygdala of male green anoles. Brain Res. 2007;1166:124–131. doi: 10.1016/j.brainres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neubauer RL. Super-normal length song preferences of female zebra finches (Taeniopygia guttata) and a theory of the evolution of bird song. Evol Ecol. 1999;13:365–380. [Google Scholar]
- 61.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 62.Nolan PM, Hill GE. Female choice for song characteristics in the house finch. Anim Behav. 2004;67:403–410. [Google Scholar]
- 63.Nowicki S, Searcy WA, Peters S. Brain development, song learning and mate choice in birds: a reviewand experimental test of the “nutritional stress hypothesis”. J Comp Physiol A. 2002;188:1003–1014. doi: 10.1007/s00359-002-0361-3. [DOI] [PubMed] [Google Scholar]
- 64.O’Connor LH, Nock B, McEwen BS. Regional specificity of gamma-aminobutyric acid receptor regulation by estradiol. Neuroendocrinology. 1988;47(6):473–481. doi: 10.1159/000124958. [DOI] [PubMed] [Google Scholar]
- 65.Pavlova D, Pinxten R, Eens M. Female song in European starlings: sex differences, complexity, and composition. Condor. 2005;107:559–569. [Google Scholar]
- 66.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 67.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press, Ltd; 1994. pp. 107–220. [Google Scholar]
- 68.Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693(1–2):21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 69.Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44(4):397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 70.Pfaus JG, Marcangione C, Smith WJ, Manitt C, Abillamaa H. Differential induction of Fos in the female rat brain following different amounts of vaginocervical stimulation: modulation by steroid hormones. Brain Res. 1996;741(1–2):314–330. doi: 10.1016/s0006-8993(96)00985-7. [DOI] [PubMed] [Google Scholar]
- 71.Pinaud R, Mello CV. GABA immunoreactivity in auditory and song control brain areas of zebra finches. J Chem Neuroanat. 2007;34(1–2):1–21. doi: 10.1016/j.jchemneu.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinaud R, Velho TA, Jeong JK, Tremere LA, Leao RM, von Gersdorff H, et al. GABAergic neurons participate in the brain’s response to birdsong auditory stimulation. Eur J Neurosci. 2004;20(5):1318–1330. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- 73.Price P. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol. 1979;93:260–277. [Google Scholar]
- 74.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473(3):377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riebel K, Smallegange IM. Does zebra finch (Taeniopygia guttata) preference for the (Familiar) father’s song generalize to the songs of unfamiliar brothers. J Comp Psychol. 2003;117(1):61–66. doi: 10.1037/0735-7036.117.1.61. [DOI] [PubMed] [Google Scholar]
- 76.Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc R Soc Lond B. 2002;269:729–733. doi: 10.1098/rspb.2001.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and themedial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 78.Riters LV, Olesen KM, Auger CJ. Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen Comp Endocrinol. 2007;154(1–3):137–149. doi: 10.1016/j.ygcen.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 79.Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155(2):307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Searcy WA, Capp MS. Estradiol dosage and the solicitation display assay in red-winged blackbirds. Condor. 1997;99:826–828. [Google Scholar]
- 81.Searcy WA, Marler P. A test for responsiveness to song structure and programming in female sparrows. Science. 1981;213(4510):926–928. doi: 10.1126/science.213.4510.926. [DOI] [PubMed] [Google Scholar]
- 82.Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138(12):5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- 83.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 84.Simmons DA, Yahr P. GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils. J Comp Neurol. 2003;459(3):290–300. doi: 10.1002/cne.10605. [DOI] [PubMed] [Google Scholar]
- 85.Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. J Neurobiol. 1995;28(1):114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- 86.Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- 87.Sockman KW, Schwabl H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen Comp Endocrinol. 1999;114:257–268. doi: 10.1006/gcen.1999.7252. [DOI] [PubMed] [Google Scholar]
- 88.Tchernichovski O, Schwabl H, Nottebohm F. Context determines the sex appeal of male zebra finch song. Anim Behav. 1998;55:1003–1010. doi: 10.1006/anbe.1997.0673. [DOI] [PubMed] [Google Scholar]
- 89.Tetel MJ, Getzinger MJ, Blaustein JD. Estradiol and progesterone influence the response of ventromedial hypothalamic neurons to tactile stimuli associated with female reproduction. Brain Res. 1994;646(2):267–272. doi: 10.1016/0006-8993(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 90.Tomaszycki ML, Adkins-Regan E. Experimental alteration of male song quality and output affects female mate choice and pair bond formation in zebra finches. Anim Behav. 2005;70(4):785–794. [Google Scholar]
- 91.Tomaszycki ML, Sluzas EM, Sundberg KA, Newman SW, DeVoogd TJ. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: effects of rearing environment. J Neurobiol. 2006;66(11):1175–1182. doi: 10.1002/neu.20275. [DOI] [PubMed] [Google Scholar]
- 92.Vallet E, Kreutzer M, Gahr M. Testosterone induces sexual release quality in the song of female canaries. Ethology. 1996;102:617–628. [Google Scholar]
- 93.Velho TA, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22(7):1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- 94.Vito CC, DeBold JF, Fox TO. Androgen and estrogen receptors in adult hamster brain. Brain Res. 1983;264(1):132–137. doi: 10.1016/0006-8993(83)91130-7. [DOI] [PubMed] [Google Scholar]
- 95.Zann RA. The zebra finch: a synthesis of field and laboratory studies. In: Perrins CM, editor. Oxford Ornithology Series. New York: Oxford University Press; 1996. [Google Scholar]