Abstract
BACKGROUND
The spinal cord is an important site where volatile anesthetics decrease sensation and produce immobility. Beyond this knowledge, our understanding of a site of anesthetic action is limited. Previous evidence suggests that dorsal horn neurons with ascending projections may be more susceptible to depression by general anesthetics than local spinal interneurons. In this study we evaluated the effects of volatile and injectable general anesthetics on lumbar dorsal horn neurons with and without ascending projections.
METHODS
Thirty-seven adult male rats underwent laminectomies at C1, for placement of a stimulating electrode, and T13/L1, for extracellular recording from the spinal cord dorsal horn. Neuronal responses to heat were evaluated under two doses of halothane, isoflurane, or propofol anesthesia.
RESULTS
Under both halothane and isoflurane anesthesia, increasing the dose from 0.8 to 1.2 minimum alveolar concentration (MAC) had no significant effect on heat-evoked responses in neurons that had ascending projections identified via antidromic stimulation (AD) or those without ascending projections (nAD). Heat responses in AD neurons 1 min after IV administration of 3 and 5 mg/kg of propofol were reduced to 60% ± 18% (mean ± se) and 39% ± 14% of control respectively. Similarly, in nAD neurons responses were reduced to 56% ± 14% and 50% ± 10% of control by 3 and 5 mg/kg propofol respectively.
CONCLUSIONS
Our findings suggest, at peri-MAC concentrations, these general anesthetics do not preferentially depress lumbar dorsal horn neurons with ascending projections compared to those with no identifiable ascending projections.
Current evidence suggests that volatile anesthetics act primarily within the spinal cord to produce some of their desired clinical effects, including decreased sensation and immobility.1,2 However, there are little data regarding how anesthetics affect classes of spinal neurons differentiated by their axonal projection patterns. Soja et al.3 found that spinoreticular and dorsal spinocerebellar tract neurons were both depressed by thiopental. Interestingly, these neurons remained depressed after the animals had behaviorally recovered from anesthesia, a period when the animals would be expected to display vigorous withdrawal reflexes in response to noxious stimulation. This suggests that spinal neurons with ascending projections may be more sensitive to depression by thiopental than spinal neurons with no identifiable ascending projections, e.g., neurons participating in segmental withdrawal reflex pathways. However, another study reported no difference in the dose-depressant effects of sodium pentobarbital on nociceptive responses of lumbar dorsal horn neurons based on whether or not they were antidromically activated from the mid-thoracic spinal cord.4
Neurons with different functional characteristics (ascending sensory neurons, interneurons, or motor neurons) have been proposed to differ in their ion channel and receptor distributions. For example, spinal neurons with ascending projections may express greater numbers of γ-aminobutyric acid (GABAA) receptors and fewer N-methyl-d-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors than neurons without ascending projections.5,6 Thus, anesthetics that differ in their receptor modulating profiles would be expected to have a differential effect on these neuronal classes. Inhaled anesthetics have effects on a variety of receptors7 whereas the primary mechanism of propofol anesthesia is thought to involve enhancement of GABAA receptors.8 The inhaled anesthetics, isoflurane and halothane, both with mixed receptor effects, have demonstrated different effects on dorsal horn neuronal responses to noxious stimulation.9
The aim of the present study was to determine if isoflurane, halothane, and propofol in the concentration range that ablates movement, would have differential effects on evoked responses of dorsal horn neurons with ascending projections versus neurons without identifiable ascending projections. We hypothesized that spinal neurons with ascending projections would be more sensitive to the depressant effects of increased anesthetic dose.
METHODS
Animals
Thirty-seven adult male Sprague-Dawley rats weighing 540 ± 6 g (mean ± se) were used in this experiment which had been approved by the Animal Care and Use Committee of the University of California, Davis. Rats received food and water ad libitum and were housed in a temperature-controlled room (set to 21°C) with 12-h light–dark cycle.
Surgical Preparation
Rats were anesthetized with isoflurane (5%) or halothane (4%) in oxygen (1 L/min) delivered into a chamber until the righting reflex was lost. Rats were then removed from the chamber and anesthetic delivered via facemask at concentrations sufficient to prevent movement in response to toe pinch. A rectal temperature probe was placed and the rat positioned over a heating pad to maintain core body temperature at 38 ± 1°C. A 12- or 14-gauge tracheostomy tube was placed and mechanical ventilation commenced (CIV101, Columbus Instruments, Columbus, OH). Respiratory gas was sampled via a cannula placed into the anesthetic circuit at the connection of the tracheostomy tube. A calibrated gas analyzer (Rascal II, Datex-Ohmeda, Helsinki, Finland) was used to monitor anesthetic concentrations and partial pressure of carbon dioxide (Pco2). Mechanical ventilation was adjusted to maintain an expired Pco2 of 25–35 mm Hg. The carotid artery was cannulated with PE50 tubing to enable blood pressure measurement (PB240, Puritan Bennet, Pleasanton, CA). Unilateral carotid ligation in the rat has been shown not to result in cerebral ischemia.10 The jugular vein was cannulated with PE50 tubing for delivery of drugs and fluids. IV lactated Ringer’s solution or an artificial colloid solution (Hextend, BioTime, Berkley, CA) was delivered as required for maintenance of mean arterial blood pressure (MAP) greater than 60 mm Hg.
A midline incision was made over the back, the paraspinous musculature was dissected free from the spinous processes of T12 to L2, and dorsal laminectomies performed at T13 and L1 to expose the lumbar spinal cord. Connective tissue and muscle were dissected from the dorsolateral surfaces of T12 and L2 to enable placement of vertebral clamps. A second midline incision was made over the cervical spine and a dorsal laminectomy performed to expose the C1 cervical spinal cord segment.
For both the isoflurane and halothane groups the animals were anesthetized with the respective drug and prepared as described above. After surgical preparation the minimum alveolar concentration (MAC) of the inhaled anesthetic was determined in each animal. Briefly, after equilibration (15 min for isoflurane or 20 min for halothane), a 12-in. hemostat was applied across the tail to the first ratchet lock and oscillated at approximately 1 Hz for 60 s. If the animal moved, which consisted of lifting of the head or gross limb movement, the anesthetic concentration was increased by 10%–20%. If the animal did not move, the anesthetic concentration was decreased by 10%–20%. After allowing equilibration the animal was retested. This was continued until the two concentrations that just allowed, and just prevented, movement were determined. The arithmetic mean of these two concentrations was deemed MAC for that anesthetic in that animal.
The rat was secured into a stereotaxic frame (D. Kopf Instruments, Tujunga, CA) using the vertebral clamps and ear bars. The dura was removed from the exposed segments of the cervical and lumbar cord. A thin layer of warm transparent agar was poured over the lumbar spinal cord. During neuronal recording pancuronium (0.5 mg/kg/h) was administered.
Neuron Classification and Recording Techniques
A 10-Mω tungsten microelectrode (FHC, Bowdoinham, ME) was advanced into the spinal cord dorsal horn in 5 µm increments using a hydraulic drive (D. Kopf Instruments). Action potentials were amplified (P511, Grass, Braintree, MA), band-pass filtered between 300 and 3000 Hz, displayed and recorded with a PowerLab interface and Chart5 software (ADInstruments, Colorado Springs, CO).
Innocuous mechanical stimulation and intermittent noxious pinch of the hindpaw ipsilateral to the recording electrode were used to search for single units. Units responding to both nonnoxious (light touch) and noxious (pinch) stimuli were classified as wide-dynamic-range (WDR) neurons. Those units responding only to noxious stimuli (pinch) were classified as nociceptive-specific (NS) neurons. Receptive fields for the single units were mapped using touch for WDR and pinch for NS neurons. The depth of the recorded neuron from the surface of the spinal cord was based on depth readings on the hydraulic microdrive. After a single unit had been isolated, responses to noxious thermal stimulation were recorded. The receptive field on the hindpaw was centered over a Peltier thermode11 and heated to 54°C for 10 s from an adapting temperature of 35°C with a rate of increase 10°C/s.
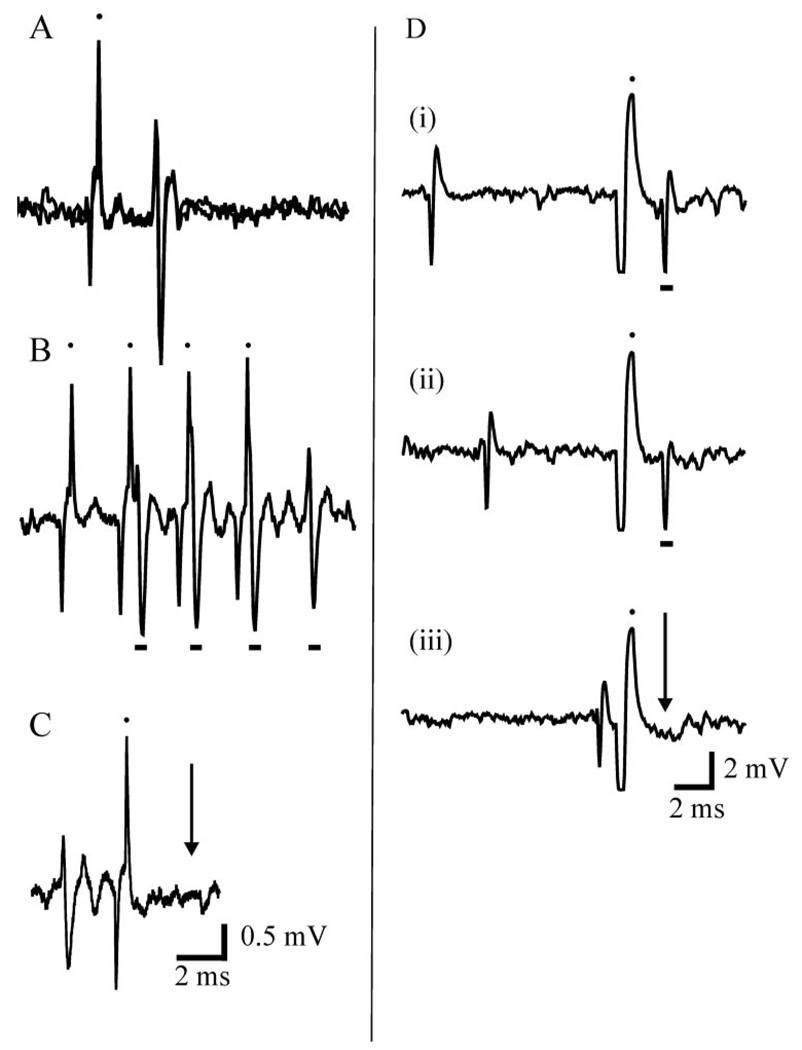
A tungsten concentric bipolar stimulating electrode (Frederick Haer, Bowdoinham, ME) was placed in the cervical cord at the C1 segment approximately 1 mm lateral to midline. Both the position and depth (1.6 ± 0.04 mm from cord surface) of the electrode were adjusted to maximize field potentials recorded from the electrode in response to manual stimulation of the contralateral hindpaw (i.e., evoked activity in the anterolateral tract). The cervical electrode was then switched from recording to stimulation. To identify lumbar dorsal horn neurons with ascending projections, 1 mA 0.5 ms pulses were delivered through the cervical electrode at a frequency of 5 Hz while recording from the contralateral lumbar cord. A unit was determined to be antidromically activated if it met all three of the following criteria: there was a consistent latency from stimulation to spike (Fig. 1A), spikes were able to follow stimulation frequencies of 300 Hz or more (Fig. 1B), and spontaneously occurring or orthodromically elicited spikes were able to block the antidromic spike (i.e., collision, Fig. 1C).
Figure 1.
Necessary criterion for antidromic activation. A: Overlay of two tracings from the same neuron demonstrating the constant latency of the spike produced by the antidromic stimulus (black dots denote stimulus artifacts). B: The antidromically elicited spike (denoted by the black bar) is able to follow high frequency stimulation (>300 Hz) C: The unit demonstrates collision, such that an orthodromically elicited spike occurring within a critical period is able to block the antidromic response. The arrow denotes the time when the antidromically elicited spike should have occurred. D: Example of collision from another neuron. In (i) and (ii) an orthodromically elicited spike can be seen prior to the stimulus artifact (black dot above) and antidromically elicited spike (black bar below). When the orthodromic spike occurs within a critical period of time prior to the stimulus it blocks propagation of the antidromic spike, which should have occurred at the arrow.
In 19 animals, we studied two individual neurons as described above. To ensure recordings were made from different units, the second neuron was isolated either from the other side of the spinal cord, or from the same side of the spinal cord but possessed a nonoverlapping receptive field.
Experimental Protocols
Neurons were initially located and characterized at 0.8 MAC; however, the neuronal response to heat was tested at 0.8 and 1.2 MAC. At each anesthetic concentration, the heat response was tested at least three times with 5 min between stimuli, and the responses averaged. One group of animals received halothane and a separate group received isoflurane. The order of delivery of anesthetic concentrations (0.8 and 1.2 MAC) was randomized with 15 min (isoflurane) or 20 min (halothane) of stable expired anesthetic concentration allowed after a change in anesthetic concentration.
In a third group, the animals were anesthetized with isoflurane and prepared as above. During neuronal recording, the isoflurane concentration was held constant at 0.8 MAC and the effects of 3 and 5 mg/kg of propofol (Abbott Laboratories, Chicago, IL) on noxious heat-evoked response were evaluated. Neuronal responses to noxious thermal stimulation were tested at 0.8 MAC isoflurane three times at 5-min intervals. A single dose of IV propofol was administered 4 min later and the heat response tested 1, 6, 11, 16, 21, and 26 min after propofol administration such that the heat stimuli remained at 5-min intervals. Each neuron was tested at both propofol doses and the order of administration was randomized. At least 30 min elapsed between propofol doses.
Data Analysis
The number of spikes in the 30 s before each stimulus application in the halothane and isoflurane groups, and the baseline and 1-min tests in the propofol groups, were counted to give spontaneous neuronal activity. The heat-evoked neuronal response included all spikes from that neuron in the 60 s from application of the heat stimulus. Data are presented as mean ± se. Paired Student’s t-test were used to compare the effect of anesthetic dose (0.8 vs 1.2 MAC) on the spontaneous activity and neuronal heat responses in the halothane and isoflurane antidromic stimulation (AD) and without (nAD) groups. For each propofol dose in either AD or nAD neurons, a two-way repeated measures ANOVA, for effects of neuron and time, followed by Fisher’s least significant difference test, was used to evaluate at what time points evoked neuronal responses were different from baseline. Paired Student’s t-test was used to compare spontaneous activity between average baseline and 1-min time points at both propofol doses in the AD and nAD groups. Significance level was set at P ≤ 0.05.
RESULTS
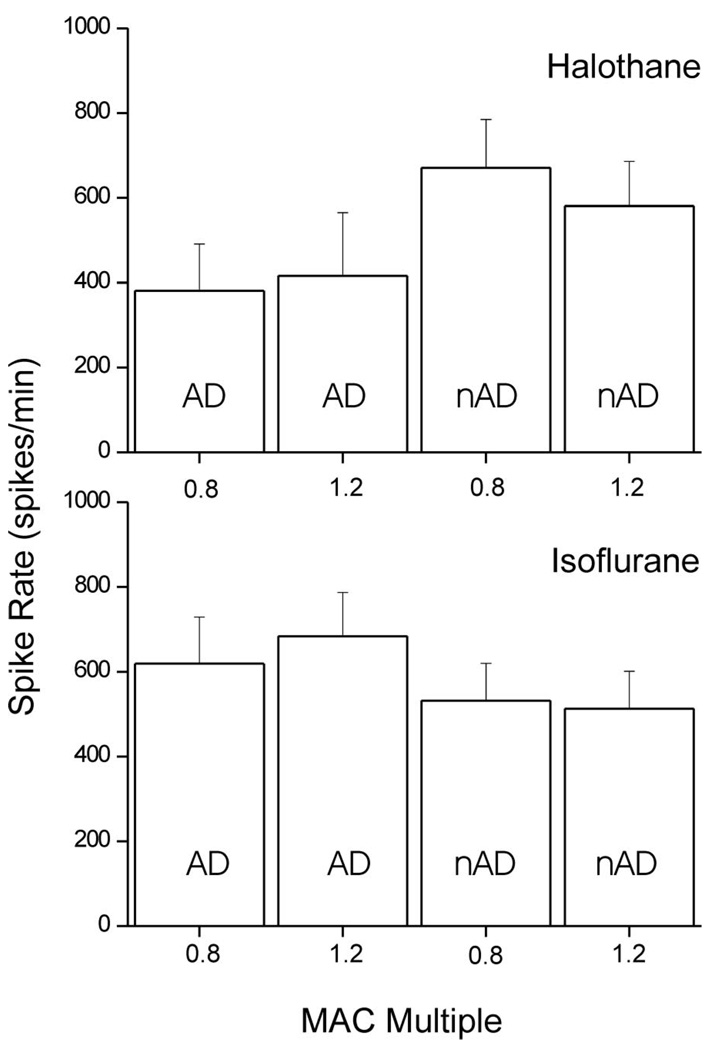
Thirteen rats were anesthetized with halothane; MAC was 1.0% ± 0.04% atm. Eight antidromically activated neurons were identified, of which seven were classified as WDR and one as NS. The recording depth was 580 ± 125 µm. The spontaneous activity of these neurons was 6 ± 2 and 5 ± 3 spikes/min at 0.8 and 1.2 MAC respectively. Spike rates in response to noxious heat were 381 ± 110 and 416 ± 149 spikes/min for 0.8 and 1.2 MAC respectively (Fig. 2). In the halothane group a further eight units were isolated, which could not be antidromically activated. All of these were characterized as WDR neurons and their average recording depth was 816 ± 142 µm. Spontaneous activity in these nAD neurons was 17 ± 5 and 6 ± 3 spikes/min at 0.8 and 1.2 MAC respectively (P < 0.05). The spike rates of these neurons in response to heat were 671 ± 114 and 581 ± 105 spikes/min for 0.8 and 1.2 MAC respectively (Fig. 2). Examples of recordings from individual neurons in response to noxious heat, typical for neurons with and without ascending projections, are shown in Figure 3. Increasing the halothane concentration from 0.8 to 1.2 MAC did not significantly alter noxious heat-evoked responses of either antidromically activated or nonantidromically activated neurons.
Figure 2.
Extracellular single unit recordings from lumbar dorsal horn neurons with ascending projections identified by antidromic stimulation (AD) and those without (nAD). Neuronal response during the 60 s from application of a noxious thermal stimulus to the hindpaw (54°C for 10 s). Mean responses (±se) are shown at 0.8 and 1.2 MAC doses of halothane (n = 8 AD and nAD) or isoflurane (n = 14 AD and nAD).
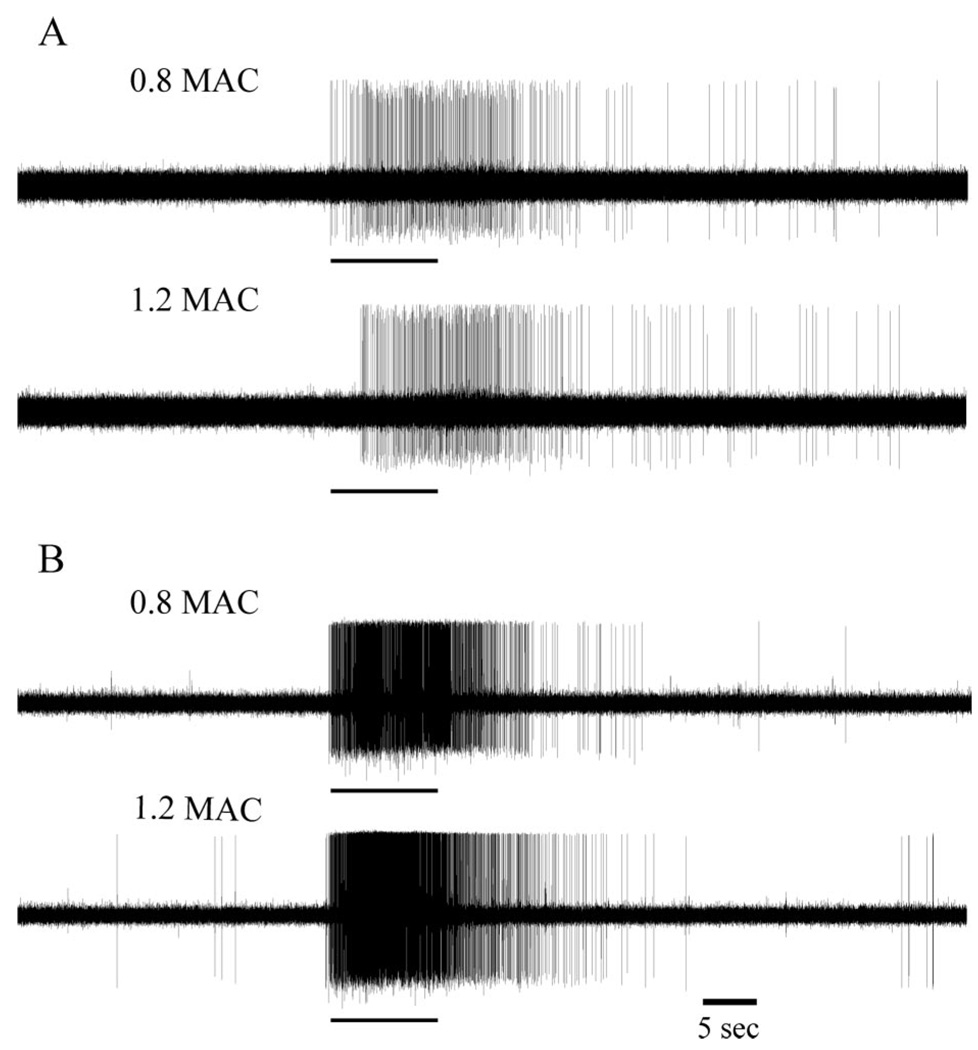
Figure 3.
Examples of extracellular single unit recordings from a neuron with identified ascending projection (A) and a neuron with no identifiable ascending projection (B) at 0.8 and 1.2 MAC of halothane. Tracings show 30 s of neuronal activity prior to, and 60 s after, application of noxious thermal stimulus (54°C for 10 s, indicated by the black bar below each tracing) to the receptive field of the neuron on the plantar aspect of the hind paw.
Sixteen rats were anesthetized with isoflurane; MAC was 1.2% ± 0.03% atm. Fourteen antidromically activated neurons were isolated at an average depth of 469 ± 59 µm, of which five were characterized as NS and nine as WDR. Spontaneous activity in this group of neurons at 0.8 and 1.2 MAC was 20 ± 18 and 16 ± 14 spikes/min respectively. Response to noxious heat stimulus was 619 ± 110 and 684 ± 103 spikes/min at 0.8 and 1.2 MAC respectively (Fig. 2). A further 14 nonantidromically activated neurons were isolated at an average recording depth of 411 ± 45 µm. One of these neurons was classified as NS and the remainder WDR. Spontaneous activity at 0.8 and 1.2 MAC was 34 ± 11 and 8 ± 3 spikes/min respectively (P < 0.05). Noxious heat-evoked responses for these neurons were 532 ± 88 and 513 ± 88 spikes/min at 0.8 and 1.2 MAC respectively (Fig. 2). Increasing the isoflurane dose from 0.8 to 1.2 MAC had no significant effect on neuronal responses to the thermal stimulus in either the antidromically or nonantidromically activated neurons.
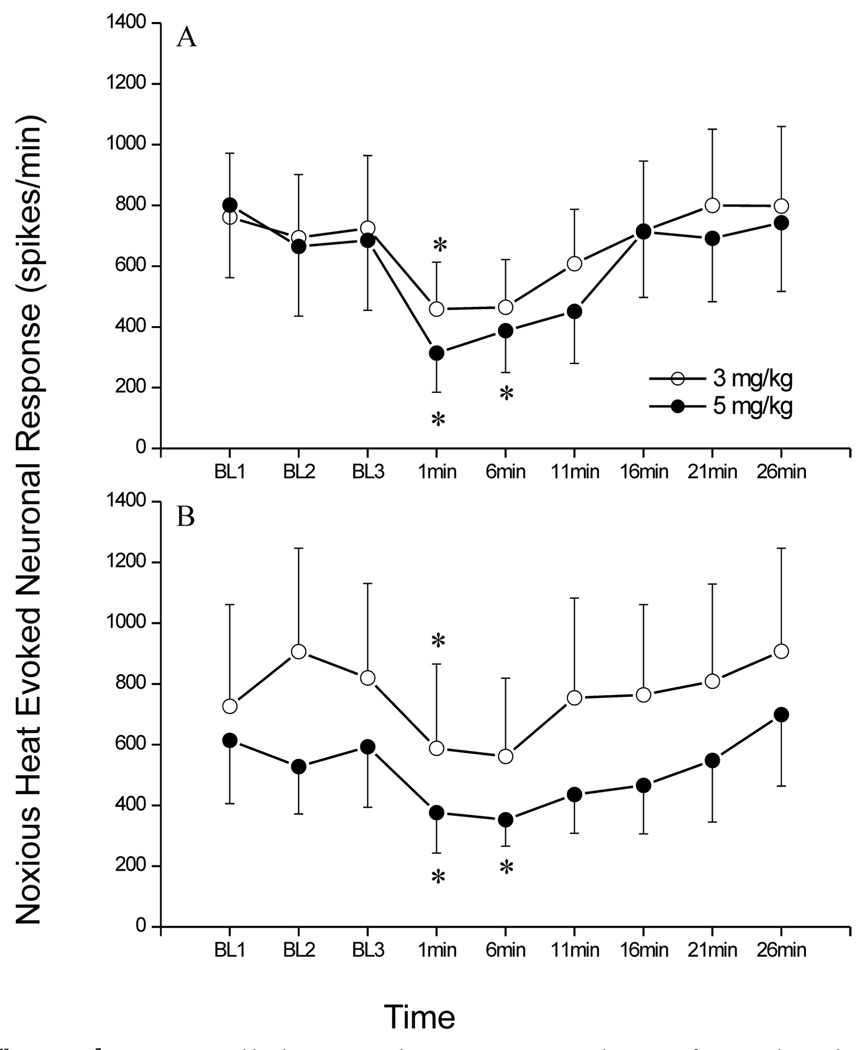
In the propofol group, six each antidromically and nonantidromically activated neurons were studied from eight rats on a background of 0.8 MAC isoflurane (isoflurane MAC was 1.3 ± 0.05% atm in these animals). Of the antidromically activated neurons, four were classified as WDR and two as NS with an average recording depth of 563 ± 185 µm. Spontaneous activity in these neurons was 24 ± 20 spikes/min at baseline and 2 ± 2 spikes/min 1 min after administration of 3 mg/kg propofol, and 21 ± 20 spikes/min at baseline and 1 ± 1 spike/min after 5 mg/kg propofol. The average response for these neurons before propofol administration (baseline) was 727 ± 119 and 717 ± 127 spikes/min for the 3 and 5 mg/kg treatment conditions respectively. One minute after propofol administration at 3 or 5 mg/kg the heatevoked response was 459 ± 154 (P = 0.05) and 314 ± 129 (P < 0.05) spikes/min respectively. This corresponds to reductions of 40% ± 18% and 61% ± 14% of control respectively for 3 and 5 mg/kg propofol doses. In the nonantidromically activated group there were five WDR neurons and one NS neuron recorded at a depth of 499 ± 86 µm. Spontaneous activity in these neurons was 25 ± 11 spikes/min at baseline and 17 ± 12 spikes/min 1 min after administration of 3 mg/kg propofol, and 44 ± 26 spikes/min at baseline and 38 ± 29 spike/min after 5 mg/kg propofol. Baseline heatevoked responses were 822 ± 179 and 578 ± 103 spikes/min for the 3 and 5 mg/kg conditions respectively. One minute after propofol administration heatevoked spike rates were 588 ± 277 (P < 0.05) and 376 ± 133 (P < 0.05) spikes/min for the 3 and 5 mg/kg propofol treatments respectively. This corresponds to reductions in neuronal response of 54% ± 14% and 50% ± 10% of control by 3 and 5 mg/kg of propofol respectively. Neuronal heat-evoked responses, at baseline conditions and after administration of 3 and 5 mg/kg of propofol, are shown in Figure 4. Administration of 3 mg/kg propofol reduced MAP from 105 ± 8 to a nadir of 67 ± 7 mm Hg. By 1 min after propofol average MAP had increased to 79 ± 7 mm Hg. The 5 mg/kg dose of propofol reduced MAP from 106 ± 7 to a nadir of 51 ± 2 mm Hg, which returned to 68 ± 5 mm Hg by 1 min.
Figure 4.
Extracellular single unit recordings from lumbar dorsal horn neurons with ascending projections (AD, n = 6) and those without (nAD, n = 6). Noxious thermal stimuli (54°C for 10 s) were delivered three times prior to IV administration of propofol, at 3 or 5 mg/kg, after which noxious thermal stimuli were delivered at 1 min, and every 5 min thereafter up to 26 min postpropofol and are summarized as mean ± se. Asterisk denotes activity at that time point is different from baseline (P ≤ 0.05).
DISCUSSION
Increasing anesthetic dose from 0.8 to 1.2 MAC for either halothane or isoflurane did not significantly alter response to noxious thermal stimulation in dorsal horn neurons with or without identified ascending projections. IV propofol depressed the neuronal response to noxious thermal stimulation to similar extents in both groups of neurons.
Increasing the halothane dose from 0.8 to 1.2 MAC has been reported to depress the firing rate of lumbar dorsal horn neurons in response to noxious thermal stimulation.9 Although overall this same trend was seen in our data in neurons without identified ascending projections, individual cell responses were variable. Some neurons were depressed by higher doses of halothane whereas responses of others were facilitated. The differences between the present data and those of Jinks et al.9 may relate to different stimulus paradigms. In the previous study, a series of thermal stimuli of increasing intensity was used, with the greatest neuronal depression being seen in the moderate thermal stimulus intensity range (~48°C). We used a single intense noxious thermal stimulus of 54°C for which a minimal depression was seen in both the previous and present study.
Propofol has been shown to depress dorsal horn responses to noxious stimulation.12,13 We had hypothesized that the depressant effects of an anesthetic such as propofol, which acts primarily via GABAA receptors,8 may depress neurons with ascending projections to a greater degree, as suggested by Soja et al.’s3 work in cats. In our study the depressant effects of propofol on response to noxious heat were similar in neurons with and without identifiable ascending projections to the cervical cord, similar to the findings of Paik et al.4 who reported no difference in the depressant effect of sodium pentobarbital on cat dorsal horn neurons, regardless of whether they could be antidromically activated from the thoracic spinal cord.
In this study the effect of propofol on neuronal firing rate to noxious heat stimulus was evaluated against a background of isoflurane anesthesia. We cannot exclude that isoflurane and propofol may have had interacting effects on neuronal responses to noxious stimulation. Widely variable kinetics, including nonlinear kinetics, have been reported for propofol in rats.14–17 This, in combination with the length of the recording period, made it problematic to study propofol alone at defined plasma concentrations. We have previously reported that etomidate, which, like propofol, has predominant GABAergic effects, did not preferentially depress spinal dorsal horn neuronal responses to noxious stimulation in isoflurane-free, decerebrate rats when compared to intact rats anesthetized with isoflurane.18 Thus, although we cannot exclude the possibility of an isofluranepropofol interaction, we think it unlikely.
The present study had several limitations. Neurons identified by antidromic stimulation of the contralateral C1 spinal cord may have terminated in the C1 segment or projected to one of several sites, including reticular formation, cerebellum, thalamus, or other sites known to receive direct spinal projections. No attempt was made to further characterize the destination of their projections, so we cannot exclude the possibility that a specific subset of neurons, for example spinothalamic tract cells, may have responded differently to increasing anesthetic concentrations. Attempts were made to ensure that neurons characterized as not having ascending projections were indeed not able to be antidromically activated by stimulation of the cervical cord. Moving the stimulating electrode throughout the dorsoventral extent of the contralateral cervical cord, and increasing the stimulus intensity to several mA was performed to accomplish this. It is possible some neurons which could not be antidromically activated may have had an ascending axon, for example in the ipsilateral spinal cord,19,20 which would have been missed in our paradigm. Another possibility is that there is a differential anesthetic sensitivity between projection neurons and interneurons only at low anesthetic concentrations (<0.8 MAC), but not in the concentration range within which we tested responses of dorsal horn neurons. The anesthetic doses used in this study were selected as those that bracket the production of immobility. If anesthetic effect on a population of neurons was critical to immobility it would be expected that these neurons would be active at 0.8 MAC, when animals would move in response to noxious stimulation, and substantially less active at 1.2 MAC when movement would be abolished. Responses of dorsal horn neurons isolated at zero anesthetic in decerebrate rats have been shown to be depressed, by up to 50%, but not totally abolished, at 0.8 MAC.21 This would argue against the likelihood that, by selecting neurons at 0.8 MAC, we would have preselected anesthetic-insensitive neurons. Furthermore, we observed significant variability in neuronal responses, and cannot exclude the possibility that a small difference between neurons with ascending projections and those without may have been missed. We investigated only response to acute noxious thermal stimulus and do not know if these findings extend to other types of stimuli, such as wind-up.
The findings of our study do not support any preferential effect of increasing doses of halothane, isoflurane, or propofol in the immobilizing range on nociceptive responses of lumbar dorsal horn neurons with identifiable ascending projections to or beyond the first cervical cord segment. This suggests that anesthetics equally affect transmission of nociceptive information locally within a reflex pathway and to sites more rostral within or beyond the spinal cord.
Acknowledgments
Supported by NIH Grant GM 61283 (to J.F.A).
REFERENCES
- 1.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Soja PJ, Taepavarapruk N, Pang W, Cairns BE, McErlane SA, Fragoso MC. Transmission through the dorsal spinocerebellar and spinoreticular tracts: wakefulness versus thiopental anesthesia. Anesthesiology. 2002;97:1178–1188. doi: 10.1097/00000542-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Paik KS, Nam SC, Chung JM. Different classes of cat spinal neurons display differential sensitivity to sodium pentobarbital. J Neurosci Res. 1989;23:107–115. doi: 10.1002/jnr.490230114. [DOI] [PubMed] [Google Scholar]
- 5.Kus L, Saxon D, Beitz AJ. NMDA R1 mRNA distribution in motor and thalamic-projecting sensory neurons in the rat spinal cord and brain stem. Neurosci Lett. 1995;196:201–204. doi: 10.1016/0304-3940(95)11878-z. [DOI] [PubMed] [Google Scholar]
- 6.Kondo E, Kiyama H, Araki T, Shida T, Ueda Y, Tohyama M. Coexpression of GABAA receptor gamma 1 and gamma 2 subunits in the rat trigeminal ganglion. Brain Res Mol Brain Res. 1994;21:363–367. doi: 10.1016/0169-328x(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 7.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. Faseb J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 9.Jinks SL, Martin JT, Carstens E, Jung SW, Antognini JF. Peri-MAC depression of a nociceptive withdrawal reflex is accompanied by reduced dorsal horn activity with halothane but not isoflurane. Anesthesiology. 2003;98:1128–1138. doi: 10.1097/00000542-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox GL, Giesler GJ., Jr An instrument using a multiple layer Peltier device to change skin temperature rapidly. Brain Res Bull. 1984;12:143–146. doi: 10.1016/0361-9230(84)90227-2. [DOI] [PubMed] [Google Scholar]
- 12.Jewett BA, Gibbs LM, Tarasiuk A, Kendig JJ. Propofol and barbiturate depression of spinal nociceptive neurotransmission. Anesthesiology. 1992;77:1148–1154. doi: 10.1097/00000542-199212000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Antognini JF, Wang XW, Piercy M, Carstens E. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth. 2000;47:273–279. doi: 10.1007/BF03018926. [DOI] [PubMed] [Google Scholar]
- 14.Ihmsen H, Tzabazis A, Schywalsky M, Schwilden H. Propofol in rats: testing for nonlinear pharmacokinetics and modelling acute tolerance to EEG effects. Eur J Anaesthesiol. 2002;19:177–188. doi: 10.1017/s0265021502000327. [DOI] [PubMed] [Google Scholar]
- 15.Naguib M, Baker MT, Spadoni G, Gregerson M. The hypnotic and analgesic effects of 2-bromomelatonin. Anesth Analg. 2003;97:763–768. doi: 10.1213/01.ANE.0000074796.10856.1F. [DOI] [PubMed] [Google Scholar]
- 16.Cox EH, Knibbe CA, Koster VS, Langemeijer MW, Tukker EE, Lange R, Kuks PF, Langemeijer HJ, Lie A, Huen L, Danhof M. Influence of different fat emulsion-based intravenous formulations on the pharmacokinetics and pharmacodynamics of propofol. Pharm Res. 1998;15:442–448. doi: 10.1023/a:1011980432646. [DOI] [PubMed] [Google Scholar]
- 17.Dutta S, Ebling WF. Formulation-dependent pharmacokinetics and pharmacodynamics of propofol in rats. J Pharm Pharmacol. 1998;50:37–42. doi: 10.1111/j.2042-7158.1998.tb03302.x. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuyo T, Antognini JF, Carstens E. Etomidate depresses lumbar dorsal horn neuronal responses to noxious thermal stimulation in rats. Anesth Analg. 2006;102:1169–1173. doi: 10.1213/01.ane.0000204764.13637.d4. [DOI] [PubMed] [Google Scholar]
- 19.Dutton RC, Carstens MI, Antognini JF, Carstens E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res. 2006;1119:76–85. doi: 10.1016/j.brainres.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 20.Miller KE, Douglas VD, Richards AB, Chandler MJ, Foreman RD. Propriospinal neurons in the C1–C2 spinal segments project to the L5–S1 segments of the rat spinal cord. Brain Res Bull. 1998;47:43–47. doi: 10.1016/s0361-9230(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuyo T, Dutton RC, Antognini JF, Carstens E. The differential effects of halothane and isoflurane on windup of dorsal horn neurons selected in unanesthetized decerebrated rats. Anesth Analg. 2006;103:753–760. doi: 10.1213/01.ane.0000230605.22930.52. [DOI] [PubMed] [Google Scholar]