Abstract
Ezh2, the methyltransferase within Polycomb Repressive II Complexes, was thought to be essential for all H3K27me3 marks in embryonic stem cells (ESCs). Recently in Molecular Cell, Shen and colleagues (2008) revealed that EZH2 is dispensable for ESC derivation and self-renewal, and that EZH1 may unexpectedly compensate for its loss.
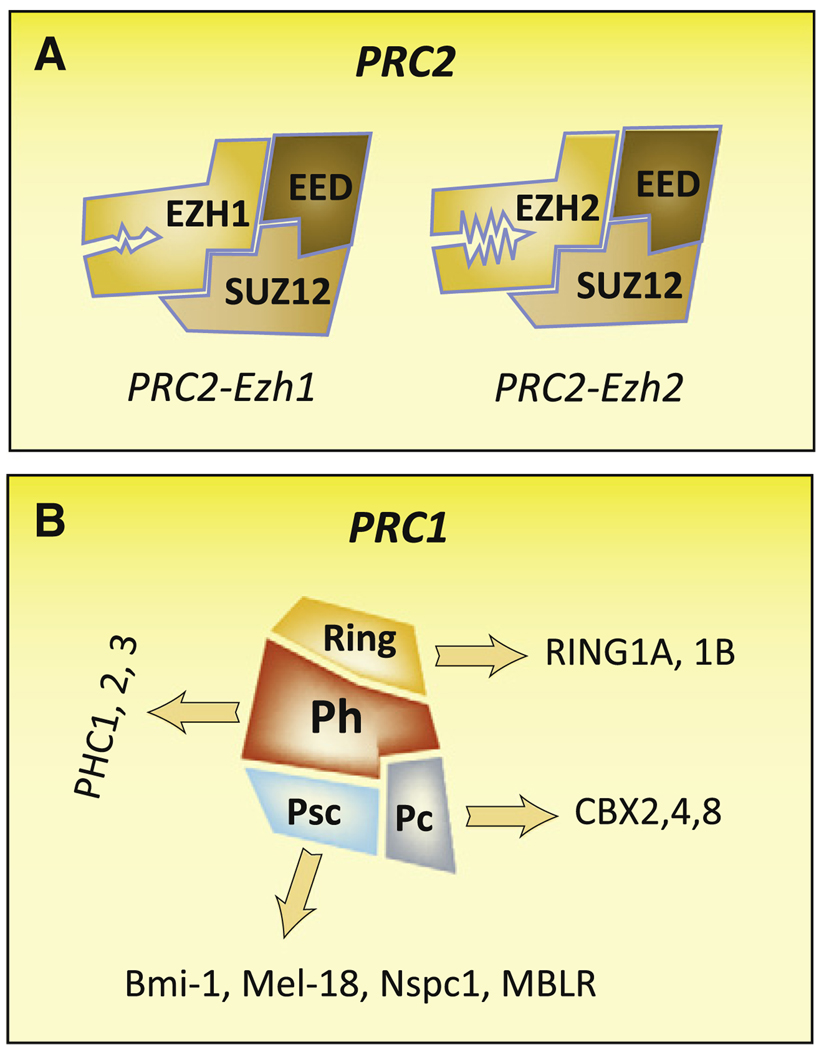
Polycomb proteins (PcG) are an evolutionarily conserved family of chromatin regulators known best for their function in establishing and maintaining epigenetic memory during development. Polycomb Repressive complex 2 (PRC2) consists of three core components: enhancer of zeste 2 (EZH2), embryonic ectoderm development (EED), and suppressor of zeste 12 (SUZ12) (Figure 1A). The catalytic subunit, EZH2, is a SET domain-containing methyltransferase that catalyzes the formation of the H3K27me3 mark, which forms the recruiting mark for Polycomb Repressive Complex 1 (PRC1) (Figure 1B) thought to be the effector of PcG-mediated long-term epigenetic memory (reviewed in Schuettengruber et al., 2007).
Figure 1. Alternative PRC1 and PRC2 Complexes Are Formed by Combinatorial Assembly.
(A) PRC2-Ezh2 and PRC2-Ezh1 complexes may differ in their intrinsic methyltransferase activity or mechanism of repression.
(B) A number of possible PRC1 complexes could be also be formed by the combinatorial assembly of a selection of alternative subunits.
The involvement of PcG proteins in maintaining ESC identity and pluripotency was first suggested by genome-wide studies that observed that PcG targets are highly enriched in genes involved in developmental patterning, morphogenesis, and organogenesis (Boyer et al., 2006). Loss of EED in ESCs leads to genome-wide and near total loss of H3K27me3 and, consequently, the derepression of PcG targets (Chamberlain et al., 2008). Despite this dramatic reduction in H3K27Me3, ESCs can be derived in the absence of EED. Embryos lacking individual components of the PRC2 complex, EED, EZH2, and SUZ12, survive past implantation and die from gastrulation defects 7 to 9 days postfertilization (Faust et al., 1995; O’Carroll et al., 2001; Pasini et al., 2007). The timing of fetal death suggests that developmental defects are responsible, rather than deficiencies in the formation and/or viability of early pluripotent cells. Consistent with these findings, ESCs have been derived from EED−/− and SUZ12−/− embryos (Chamberlain et al., 2008; Pasini et al., 2007) and are viable given the right culture conditions. So, while PcG proteins appear to be required for proper cell cycle in tumor cells and fibroblasts (Valk-Lingbeek et al., 2004), ESCs do not have this requirement and do not require H3K27me3 marks for both establishment and self-renewal. ESCs nonetheless require PcG proteins for stable maintenance of the self-renewing state, since EED−/− ESCs are prone to differentiation in culture (Boyer et al., 2006; Chamberlain et al., 2008). PcG proteins are also required for ESCs to be pluripotent in the strictest sense, i.e., give rise to all differentiated lineages in a cell-autonomous fashion, since EED−/− ESCs are unable to give rise to all cell types in in vitro differentiation assays, and high-contribution chimeras display developmental defects similar to knockout embryos (Chamberlain et al., 2008). In a recent issue of Molecular Cell, Orkin and colleagues demonstrate that similar to EED and SUZ12, EZH2 is dispensable for ESC derivation and maintenance (Shen et al., 2008). In addition, the authors show that similar to other mammalian complexes regulating chromatin, such as ATP-dependent chromatin remodeling complexes (Wang et al., 1996), PRC2 complexes are biochemically and possibly functionally diverse and that the diversity is based on subunit assembly (Figure 1A).
In the Orkin study, EZH2−/− ESCs were successfully derived from the inner cell mass of EZH2−/− embryos, and while these cells displayed global loss of H3K27me2 and -me3, the repression of developmental genes that are known targets of PcG, evidenced by their derepression in EED−/− ESCs, was surprisingly intact (Shen et al., 2008). Finer analysis of these key target genes revealed that H3K27me3 was selectively maintained at a group of key developmental targets of PcG. This residual H3K27me3 activity was unexpected, since EZH2 had been thought to be the sole methyltransferase in PRC2. In addition, the observed loss of H3K27Me3 marks is less severe than in the case of EED deficiency. The more severe effect apparent in the absence of EED may be because it is the sole gene encoding the subunit at this position in the PRC2 complex (Figure 1). Using a proteomic approach, Orkin and colleagues identified noncanonical PRC2 complexes that contain EZH1, a homolog of EZH2. Shen et al. found that EZH1 exhibited methyltransferase activity in vitro and colocalized with EED at PcG targets. EZH1-containing complexes appear to be selectively targeted to key developmental genes, the repression of which may be critical for preventing ESC differentiation. This unanticipated capacity of EZH1 may, thus, allow it to complement the function of EZH2-containing complexes in repression of crucial functional genes. Indeed, depletion of EZH1 in EZH2−/− ESCs leads to the loss of H3K27me3 on the selective PcG targets that had retained the mark in the absence of EZH2, demonstrating the functional complementation of the two proteins. However, EZH1 and EZH2 do not appear to be completely redundant, since EZH1 cannot complement the pluripotency defects seen in EZH2−/− ESCs, and EZH2−/− embryos do not survive past gastrulation. This distinction is consistent with another recent finding that the two genes have very different patterns of expression, in that EZH2, but not EZH1, is present in actively dividing cells (Margueron et al., 2008). In addition, it is possible that the two proteins utilize distinct mechanisms for mediating gene repression, since the methyltransferase activity of EZH1-PRC complexes was shown to be very weak in comparison to that of EZH2-PRC. Instead, EZH1-PRC complexes appear to utilize an independent mechanism for compacting and repressing chromatin that is independent of its histone methyltransferase activity (Margueron et al., 2008). However, more rigorous genetic studies are essential in order to test the functional differences between the two complexes, such as complementation of null mutants with properly expressed transgenes or insertions of EZH1 into the EZH2 locus.
The findings of Shen et al. demonstrate the existence of diversity in mammalian PcG complexes via combinatorial assembly of homologous subunits, which is an emerging trend for multisubunit complexes that regulate chromatin and transcription. Orkin and colleagues also confirm the dispensability of PcG proteins in the establishment of pluripotent cells and in the maintenance of ESC self-renewal in culture.
The presence of noncanonical PRC2 complexes that functionally complement canonical complexes, but only at a selective subset of genes, raises the question of targeting mechanisms. How are EZH1-containing complexes selectively targeted to specific developmental genes whose repression is crucial for preventing premature differentiation? Do noncanonical complexes contribute to later developmental events and, if so, to what extent? Most provocatively, ESCs lacking Eed1 or both Ezh2 and Ezh1 have almost no detectable H3K27Me3. Without this modification, mutant ESCs would be unlikely to exhibit bivalent marks (i.e., the coincidence of the repressing mark, H3K27Me3, and the activating mark, H3K4Me3) thought to be important to maintain genes in a poised state for expression or repression in more differentiated progeny. If not via bivalent histone marks, what mechanisms allow genes to remain poised in pluripotent cells for action at a later time? The discovery that ESCs can be generated in the virtual absence of H3K27Me3, and bivalent marks should clear the stage for new developments in the study of the role of chromatin in pluripotency.
REFERENCES
- Boyer L, Plath K, Zeitlinger J, Brambrink T, Medeiros L, Lee T, Levine S, Wernig M, Tajonar A, Ray M, et al. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, Yee D, Magnuson T. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust C, Schumacher A, Holdener B, Magnuson T. Development. 1995;121:273–285. doi: 10.1242/dev.121.2.273. [DOI] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. Mol. Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. Mol. Cell. Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. Mol. Cell. Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Shen X, Liu Y, Hsu Y-J, Fujiwara Y, Kim J, Mao X, Yuan G-C, Orkin SH. Mol. Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR. Genes Dev. 1996;10:2117–2130. doi: 10.1101/gad.10.17.2117. [DOI] [PubMed] [Google Scholar]