Abstract
Injections of the angiotensin(1–7) [Ang(1–7)] antagonist [d-Ala7]-Ang(1–7) into the nucleus of the solitary tract (NTS) of Sprague–Dawley rats reduce baroreceptor reflex sensitivity (BRS) for control of heart rate by ~40%, whereas injections of the angiotensin II (Ang II) type 1 receptor antagonist candesartan increase BRS by 40% when reflex bradycardia is assessed. The enzyme angiotensin-converting enzyme 2 (ACE2) is known to convert Ang II to Ang(1–7). We report that ACE2 activity, as well as ACE and neprilysin activities, are present in plasma membrane fractions of the dorsomedial medulla of Sprague–Dawley rats. Moreover, we show that BRS for reflex bradycardia is attenuated (1.16±0.29 ms mmHg−1 before versus 0.33±0.11 ms mmHg−1 after; P < 0.05; n = 8) 30–60 min following injection of the selective ACE2 inhibitor MLN4760 (12 pmol in 120 nl) into the NTS. These findings support the concept that within the NTS, local synthesis of Ang(1–7) from Ang II is required for normal sensitivity for the baroreflex control of heart rate in response to increases in arterial pressure.
The baroreceptor reflex control of heart rate is regulated by the brain renin–angiotensin system. There is an improvement in the gain (sensitivity) of the baroreceptor reflex for control of heart rate in terms of the bradycardic response to increases in arterial pressure following microinjection of angiotensin(1–7) [Ang(1–7)] into the nucleus of the solitary tract (NTS) (Chaves et al. 2000). A reduction in reflex bradycardia occurs with the Ang(1–7) receptor antagonist [d-Ala7]-Ang(1–7) (Chaves et al. 2000; Sakima et al. 2005), and the antagonist blocks the improvements produced by exogenous Ang(1–7) in baroreflex function in response to increases in arterial pressure (Oliveira et al. 1996). The impairment in reflex function during Ang(1–7) receptor blockade is taken as evidence that endogenous brain Ang(1–7) provides tonic enhancement of the reflex gain, an effect opposite to that of Ang II (Sakima et al. 2005, 2007; Campagnole-Santos et al. 1988).
Angiotensin-converting enzyme (ACE) and neprilysin mRNAs are present in the dorsal medulla of Sprague–Dawley rats (Sakima et al. 2005, 2007), and these enzymes are localized to the NTS (Chevillard & Saavedra, 1982; Back&Gorenstein, 1989; Lasher et al. 1990). Angiotensin-converting enzyme is well known as the predominant enzyme catalysing the formation of angiotensin II (Ang II) from angiotensin I (Ang I), while neprilysin is a key enzyme contributing to the formation of Ang(1–7) from Ang I in the circulation (Yamamoto et al. 1992). There is a reciprocal relationship between the two enzymes, in that ACE is the main enzyme for the hydrolysis of Ang(1–7) and circulating neprilysin metabolizes Ang II to Ang(1–4) (Chappell et al. 1998; Vickers et al. 2002; Rice et al. 2004). Angiotensin-converting enzyme 2 (ACE2) has high catalytic activity for conversion of Ang II to Ang(1–7) (Vickers et al. 2002; Rice et al. 2004; Warner et al. 2004; Shaltout et al. 2007; Raizada & Ferreira, 2007; Chappell, 2007), and recent studies localized the ACE2 protein (Doobay et al. 2007) and its mRNA (Sakima et al. 2005; Sakima et al. 2007) to the dorsal medulla oblongata of mice and rats. Thus, the relative levels of Ang II and Ang(1–7) may reflect the activities of these enzymes in brain medullary tissue. Transgenic mice with overexpression of ACE2 behind a neuronal promoter (Feng et al. 2007) exhibit normal resting blood pressure but are protected from increases in blood pressure produced by intravenous infusions of Ang II. In contrast, overexpression of ACE2 in the ventral medulla of spontaneously hypertensive rats via gene transfer (Yamazato et al. 2007) is associated with a reduction in arterial pressure. However, functional data showing the impact of inhibition of endogenous ACE2 on baroreflex function and the relative activities of these key enzymes in dorsal medullary tissue are lacking. Therefore, we assessed both the activities of ACE, ACE2 and neprilysin in membrane homogenates prepared from the dorsal medulla and the effects of the specific ACE2 inhibitor MLN4760 in the NTS on baroreflex control of heart rate in Sprague–Dawley rats.
Methods
Microinjection studies
Male Hannover Sprague–Dawley rats, obtained from the Hypertension & Vascular Research Center Animal Core, were used at 10–20 weeks of age according to procedures approved by the Institutional Animal Care and Use Committee. Animals were anaesthetized with a mixture of chloralose and urethane (35 and 750 mg kg−1, respectively) and instrumented as previously reported for blood pressure and heart rate recordings and microinjections via glass micropipettes into the NTS (Diz et al. 1984; Campagnole-Santos et al. 1988; Sakima et al. 2005; Sakima et al. 2007). After a stabilization period of at least 30 min, three doses (2, 5 and 10 µg kg−1) of phenylephrine were injected intravenously at ~5 min intervals via the femoral vein to produce graded increases in arterial pressure. The bradycardia elicited by the increases in pressure was converted to pulse interval, and the slope of the relationship between phenylephrine-induced increase in blood pressure and change in pulse interval was used as the index of the sensitivity of the baroreceptor reflex control of heart rate, since the effects of either Ang II or Ang(1–7) predominate on this arm of the reflex (Campagnole-Santos et al. 1988, 1992; Sakima et al. 2005, 2007). The baroreceptor reflex sensitivity for the bradycardic response to increases in pressure (BRS) was assessed before, at 30 or 60 min, and once again between 90 and 180 min after bilateral microinjection into the NTS of a selective inhibitor of ACE2 (Shaltout et al. 2007), MLN4760 (12 pmol in 120 nl of CSF; gift from Millenium Pharmaceuticals, Cambridge, MA, USA). To determine whether there was any residual action of Ang(1–7) as well as to assess the specificity of the effects of the ACE2 inhibitor, the Ang(1–7) receptor antagonist [d-Ala7]-Ang(1–7) (144 fmol in 120 nl of CSF) was injected bilaterally into the NTS ~50 min following the MLN4760 and the reflex retested within 5 min in five rats at a dose effective in eliciting decreases in BRS in Sprague–Dawley rats in previous studies (Sakima et al. 2005, 2007; Arnold et al. 2008). At the end of the study, rats were decapitated while anesthetized for brain removal and verification of injection sites.
Enzyme activity in solubilized membranes
The dorsal medulla from a separate group of Sprague–Dawley rats (removed immediately following decapitation and stored at −80°C) was weighed, immersed in reaction buffer (25mm Hepes, 125mm NaCl, 10 µm ZnCl2, pH 7.4), and homogenized using a Qiagen Tissue Lyser for 1 min at 25 Hz. The homogenate was centrifuged (28 000g, 10 min) to separate the supernatant (native soluble) from the membranes. The pellet (native membrane) was resuspended in 1 ml of reaction buffer and isolated by recentrifugation (28 000g, 10 min). The resulting supernatant was discarded and the remaining pellet (native membrane) was resuspended in reaction buffer (10 µl mg−1 of original dorsal medulla). Three hundred and sixty microlitres of the native membrane solution was mixed with 40 µl of Triton X-100 and left to incubate on ice overnight, after which the soluble portion of the native membrane was separated from the insoluble portion by centrifugation (28 000g, 5 min). As previously published (Shaltout et al. 2007), four combinations of enzyme inhibitors were used: (1) all inhibitors, which included the ACE inhibitor lisinopril, the ACE2 inhibitor MLN4760, the neprilysin inhibitor SCH39370, the aminopeptidase inhibitors amastatin and bestatin, the chymase inhibitor chymostatin and the cysteine protease inhibitor p-chloromercuribenzoate; (2) all inhibitors except SCH39370 to reveal neprilysin activity; (3) all inhibitors except lisinopril to reveal ACE activity; and (4) all inhibitors except MLN4760 to reveal ACE2activity. For the activity assay, the final concentration of each of the inhibitors listed above was 10 µm (except amastatin at 2 µm) and these were incubated with 125I-Ang I or 125I-Ang II (2 × 106 c.p.m.) and the soluble portion of the Triton X-100-treated native membranes. The reaction was incubated at 37°C for up to 2 h. At 1 and 2 h, 200 µl of the reaction vessel contents were removed and mixedwith 1% phosphoric acid to terminate the process. The mixture was subjected to centrifugation (~10 000g, 1 min) and placed on ice until subsequent analysis by HPLC. Separation conditions for the HPLC analysis and the quantification of enzyme activities were as previously described (Shaltout et al. 2007). Enzyme activity was expressed as femtomoles of product generated per minute per milligram protein of the solubilized membranes (fmol min−1 mg−1). The protein content for each sample was determined using aBio-Rad Protein Assay kit (Hercules, CA, USA).
Statistics
All data are presented as means±s.e.m., and data analysis was by one-way analysis of variance followed by comparisons with the baseline control responses using Dunnett’s post hoc analysis. P < 0.05 was the criterion for statistical significance.
Results
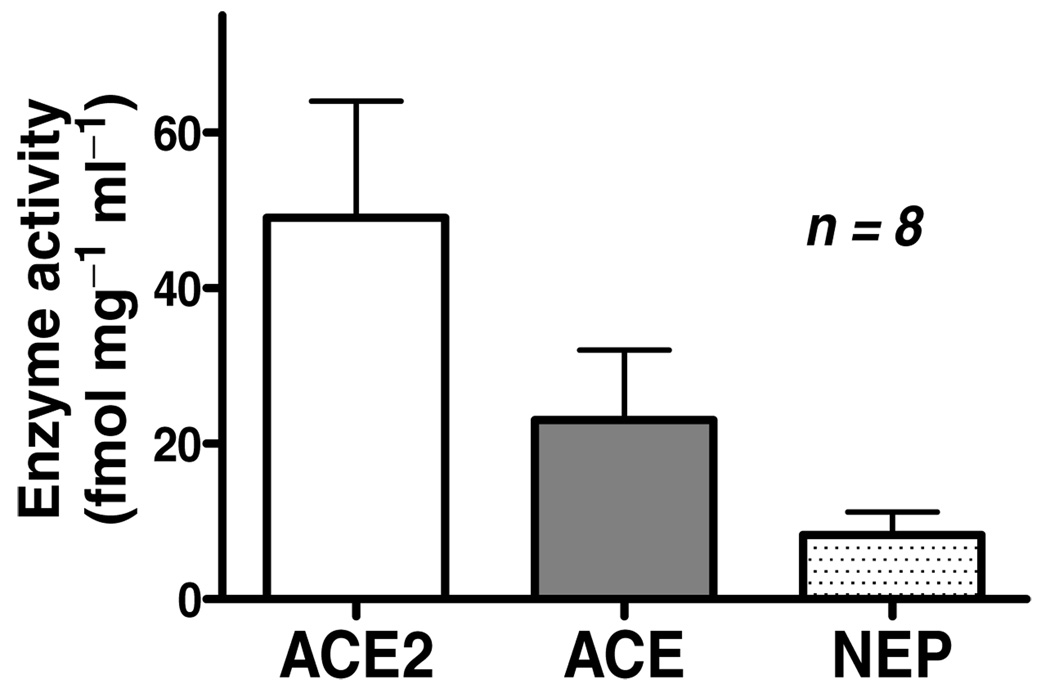
Consistent with previous reports of the presence of mRNA for each of the enzymes in brain medullary tissue (Sakima et al. 2005, 2007), we detected activity of ACE, ACE2 and neprilysin in solubilized membranes obtained from homogenates of dorsal medulla oblongata of Sprague–Dawley rats (Fig. 1).
Figure 1. Activity for ACE, ACE2 and neprilysin (NEP) in membrane homogenates from rat brain dorsal medulla.
Activities of ACE and neprilysin were determined with Ang I, and activity of ACE2 was determined with Ang II as the substrate. Of the two Ang(1–7) forming enzymes, ACE2 activity is sixfold greater than NEP. Values for each enzyme were greater than zero, indicating that significant activity was detected for all three enzymes.
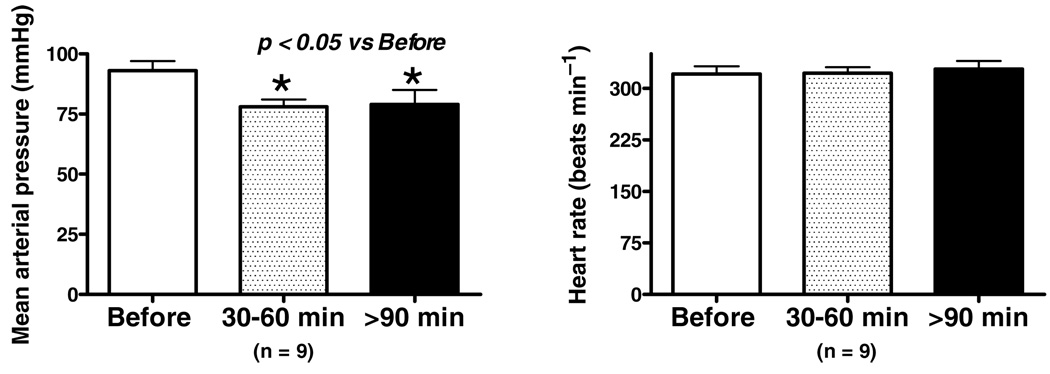
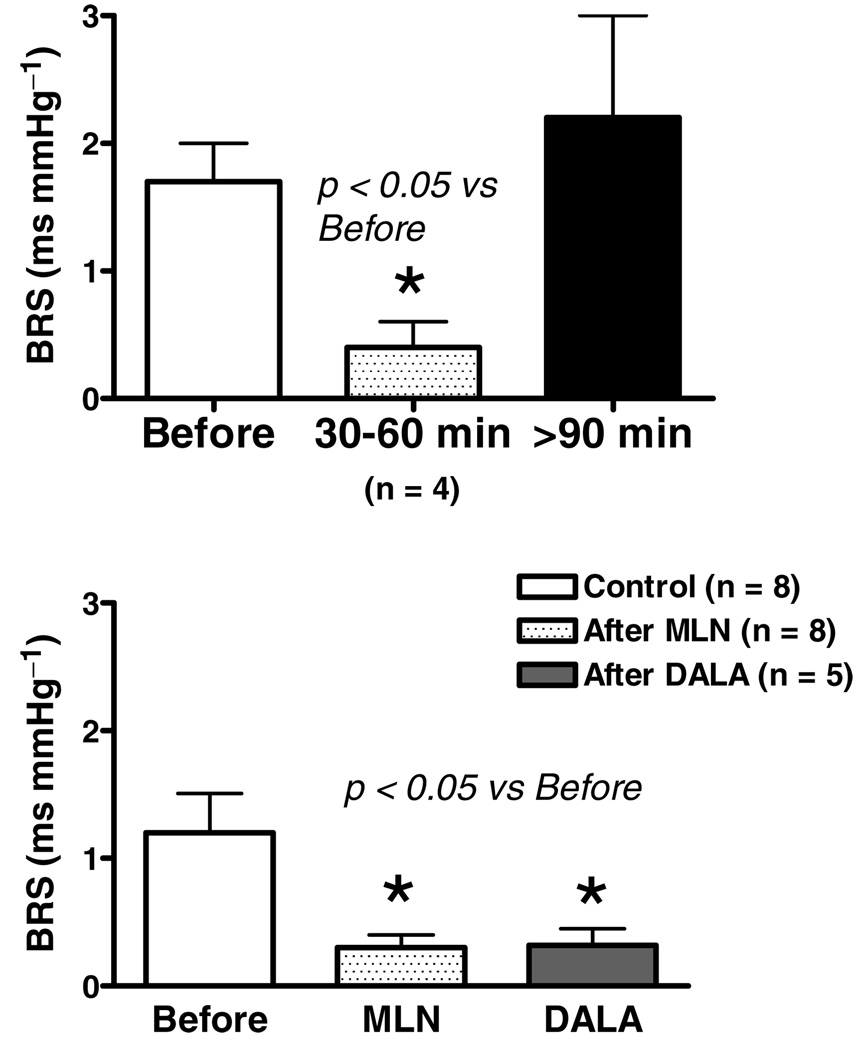
Baseline mean arterial pressure and heart rate are shown in Fig. 2. Resting arterial pressure was significantly lower 30–60 min following administration of the ACE2 inhibitor MLN4760 into the NTS, and this persisted at the later time points tested (Fig. 2, left panel). The fall in blood pressure was not accompanied by any change in heart rate (Fig. 2, right panel). The BRS for control of heart rate was attenuated by ~70% in Sprague–Dawley rats treated with MLN4760 (Fig. 3, top panel) when tested 30–60 min following injection of the ACE2 inhibitor. In four animals, the recovery of the BRS was assessed and found to return towards control levels over the next 90–180 min following application of the enzyme inhibitor. The BRS was determined within 5 min of injection of the Ang(1–7) receptor antagonist [d-Ala7]-Ang(1–7) into the NTS approximately 50 min after injection of MLN4760 in five rats (Fig. 3, lower panel). The BRS after [d-Ala7]-Ang(1–7) injection was similar to the level seen after the MLN4760 alone. The lack of an additional effect ofAng(1–7) receptor blockade in the presence of the ACE2 inhibitor suggested that there was no residual Ang(1–7) contributing to the BRS.
Figure 2. Effects of ACE2 inhibition on mean arterial pressure and heart rate at time points coincident with reflex testing.
There was a significant reduction in mean arterial pressure within the first 30–60 min of the response to NTS injection of MLN4760 and this persisted for 90–180 min. There were no differences in heart rate throughout the study. *P < 0.05 versus before.
Figure 3. Baroreceptor reflex sensitivity for control of heart rate in response to increases in pressure produced by phenylephrine before and after NTS injections of MLN4760.
There is a significant reduction in BRS within the first 30–60 min following injection of the ACE2 inhibitor MLN4760 relative to values before treatment (upper panel), which recovered to control levels 90–180 min later. In 5 rats, the reflex was tested after MLN4760 (bottom panel) and again ~50 min later within 5 min of [d-Ala7]-Ang(1–7) (DALA), the Ang(1–7) receptor antagonist, injected bilaterally into the NTS. The BRS after [d-Ala7]-Ang(1–7) was not different from that after MLN4760 alone. *P < 0.05 versus before.
Discussion
Novel findings of this study indicate that ACE2 activity in the membrane fraction of dorsal medulla oblongata of Sprague–Dawley rats may account forAng(1–7) formation in this brain area. Consistent with earlier observations (Chevillard & Saavedra, 1982; Back & Gorenstein, 1989; Lasher et al. 1990), both neprilysin and ACE activities are also present in this brain region. The BRS for control of heart rate in response to increases in arterial pressure was impaired by injection into the NTS of the ACE2 inhibitor MLN4760, and there was no further reduction in the BRS with Ang(1–7) receptor blockade in the presence of the ACE2 inhibitor. In addition, resting arterial pressure under anaesthesia was reduced by MLN4760. On the basis of these findings, we suggest that ACE2 contributes to local production of Ang(1–7) in the NTS, and this peptide is involved in control of arterial pressure and enhancement of the BRS for control of heart rate.
The formation and metabolism of Ang(1–7) has been characterized in pulmonary, cardiac and renal tissues (Allred et al. 2000; Ferrario et al. 2005; Trask et al. 2007; Chappell, 2007; Chappell et al. 1998), but processing of these hormones in the medulla oblongata has yet to be fully characterized. Reverse transcriptase-polymerase chain reaction analysis of medullary tissue reveals the presence of mRNA for ACE, ACE2 and neprilysin (Sakima et al. 2005, 2007).The fact that ACE2 activity is detected suggests that conversion of Ang II to Ang(1–7) would occur in this brain region, consistent with the functional assessment of the BRS in the presence of the ACE2 inhibitor. While neprilysin activity is sixfold lower, its presence in this brain region indicates potential involvement in the generation of Ang(1–7) from Ang I, as well as degradation of Ang II into Ang(1–4) (Chappell et al. 1998; Allred et al. 2000; Shaltout et al. 2007). Given its high catalytic efficiency for metabolism of Ang(1–7), ACE would be expected to participate in degradation of the peptide in this brain region (Chappell et al. 1998; Allred et al. 2000; Shaltout et al. 2007). However, the functional outcome of blockade of these two enzymes on the baroreflex was not tested directly in our studies.
Blockade of Ang(1–7) receptors with [d-Ala7]-Ang(1–7) in the NTS leads to a reduction in BRS for control of heart rate of approximately 40% (Sakima et al. 2005, 2007; Arnold et al. 2008). The ACE2 inhibitor MLN4760 reduced baroreflex function in a manner qualitatively similar to Ang(1–7) receptor blockade with [d-Ala7]-Ang(1–7) in the present study. However, the magnitude (70%) of the reduction in BRS appeared to be greater with ACE2 blockade than with blockade of the Ang(1–7) receptor. The greater suppression of the reflex may reflect the combined actions of ACE2 inhibition to elevate Ang II as it lowers Ang(1–7), since Ang II attenuates BRS via actions at Ang II type 1 (AT1) receptors within the NTS (Averill&Diz, 2001; Diz et al. 2001; Sakima et al. 2005, 2007;Arnold et al. 2008). The lack of effect of [d-Ala7]-Ang(1–7) in the presence of MLN4760 suggests that there is no additional source of Ang(1–7) with acute exposure to the ACE2 inhibitor.
It is well known that injections of exogenous Ang II or Ang(1–7) in this brain region result in decreases in arterial pressure (Diz et al. 1984, 2001;Campagnole-Santos et al. 1989; Averill & Diz, 2001). Since Ang(1–7) might be expected to decrease following the ACE2 inhibitor, an increase in Ang II with the ACE2 blocker provides a potential mechanism for the reduction in resting arterial pressure in response to MLN4760 in these studies. While the depressor effects of Ang II are mediated by substance P (Diz et al. 1997, 1998), substance P is not a likely substrate for ACE2 (Vickers et al. 2002; Warner et al. 2004). In contrast to the reduction in arterial pressure with the ACE2 inhibitor given into the NTS of the normotensive Sprague–Dawley rats in our study, overexpression of the enzyme behind the synapsin promoter to target expression to brain neurons did not alter resting arterial pressure in mice (Feng et al. 2007). The overexpression of the enzyme did, however, provide protection from pressor actions of exogenously administered Ang II (Feng et al. 2007). Overexpression of ACE2 by gene transfer in ventrolateral medulla is associated with a fall in blood pressure in spontaneously hypertensive rats (Yamazato et al. 2007). Increased expression of ACE2 in neurons of the ventrolateral medulla by the transfection restores the deficit in ACE2 protein documented to accompany the hypertension in these rats (Yamazato et al. 2007). In the ventrolateral medulla, both Ang II and Ang(1–7) act to increase blood pressure in hypertension of genetic or stress-induced origin (Fontes et al. 1993; Dampney et al. 1996; Ford et al. 1997; Potts et al. 2000, 2004; Mayorov & Head, 2003), implicating the reduction in Ang II in this brain region as the likely mechanism for the observed antihypertensive effect.
Whatever the mechanism for the change in resting arterial pressure following administration of the ACE2 inhibitor in the NTS in our study, it is likely to be different from the mechanisms involved in the modulation of the baroreflex (Averill & Diz, 2001; Diz et al. 2001; Sakima et al. 2007; Arnold et al. 2008). The time course of the reduction in arterial pressure differs from that of the reduction in BRS, and a reduction in arterial pressure would be more likely to contribute to an improvement or no change in reflex function rather than the reduction in BRS observed (Krieger, 1970;Moreira et al. 1990; Farah et al. 2001). In addition, previous studies indicate that the source of the angiotensinogen for the Ang II responsible for attenuation of the BRS appears to derive from glia, while that for Ang(1–7) is derived from a non-glial source for enhancement of the BRS (Sakima et al. 2007; Arnold et al. 2008).Thus, the two peptidesmay arise fromdifferent cellular sources (Campagnole-Santos et al. 1990; Sakima et al. 2007;Arnold et al. 2008), and the different time course of actions on the BRS versus resting pressure may reflect different rates of recovery of these sources from the effects of the ACE2 inhibitor.
The pathways by which the angiotensin peptides are created and destroyed in the medulla are of particular interest because the balance of Ang II and Ang(1–7) is important to regulation of the baroreceptor reflex. Angiotensin-converting enzyme 2 in glial cells in culture is downregulated by Ang II, effects that are prevented by Ang(1–7) (Gallagher et al. 2006). In ASrAogen rats with low glial angiotensinogen, tissue levels of Ang I are lower than in Sprague–Dawley control rats, with a similar trend for Ang II (Huang et al. 2001); however, there are no differences in the mRNA for these enzymes (Sakima et al. 2007). Angiotensin peptides in the neuronal pathways of the ASrAogen rats appear intact (Vinsant et al. 2005), and the Ang(1–7) acting on the BRS or resting pressure is of non-glial origin (Sakima et al. 2007; Arnold et al. 2008). Thus, the maintenance of normal levels of the enzymes in the ASrAogen rats may indicate that the regulation of the processing enzymes is dependent upon peptides in neuronal or vascular rather than glial elements.Consistent with the above observations, ACE2 is localized in neuronal cells within this brain region (Doobay et al. 2007). In the present study, the ACE2 inhibitor is lipophilic, and therefore an intracellular site of action for reducing Ang(1–7) release from neuronal fibre terminals is possible.
Regulation of the processing enzymes and the functional consequences in brain with respect to changes in BRS is reported. Neprilysin mRNA at ~18 months of age in Sprague–Dawley rats is lower in brain medullary tissue than in younger animals of the same strain (Sakima et al. 2005, 2007; Arnold et al. 2008). The age-related reduction in mRNA is accompanied by a lower BRS in conscious or anaesthetized ~18 month old animals (Sakima et al. 2005; Diz et al. 2007). The decline in BRS is associated with loss of Ang(1–7) endogenous tone (Sakima et al. 2005). Thus, functional studies are necessary to confirm the extent to which alterations in the expression or activity of each of the enzymes impact on the overall levels of the peptides and their actions.
Finally, ACE2, ACE and neprilysin do not selectively metabolize peptides of the renin–angiotensin system. Angiotensin-converting enzyme 2 may also metabolize apelin, neurotensin and dynorphin within the medulla (Vickers et al. 2002; Warner et al. 2004). In the present study, the absence of an additional effect of the Ang(1–7) antagonist following the ACE2 inhibitor suggests that the decline in BRS does not result from inhibition of formation or metabolism of other peptides. While neurotensin and apelin are known to have actions on sympathetic outflow, arterial pressure or regulation of BRS (Seagard et al. 2000; Seyedabadi et al. 2002; Kagiyama et al. 2005; Kleinz & Davenport, 2005), dynorphin(1–13) is devoid of cardiovascular activity at the NTS (May et al. 1989). Neurotensin lowers blood pressure, but has no effect on BRS (Seagard et al. 2000). Apelin-13 has no effect on blood pressure when given intracerebroventricularly (Reaux et al. 2001), but increases blood pressure following injection into the NTS (Seyedabadi et al. 2002; Kleinz & Davenport, 2005; Kagiyama et al. 2005). The available evidence would not readily support involvement of these other neuropeptides in the effects of ACE2 inhibitor on the BRS in our studies, although testing exactly as we did here has not been performed. In contrast, only neurotensin would be expected to produce a depressor effect if elevated in the NTS, suggesting that apelin-13 and dynorphin(1–13) are not likely candidates for the reduction in arterial pressure.
In conclusion, the present study indicates that an ACE2 inhibitor injected into the NTS attenuates the function of the baroreflex for heart rate control in response to increases in arterial pressure. We demonstrate the presence of ACE2 activity in homogenates of membranes from this brain area, consistent with prior demonstrations of protein (Yamazato et al. 2007; Feng et al. 2007) and mRNA (Sakima et al. 2005) for the enzyme. The data on BRS following application of the ACE2 inhibitor are consistent with the interpretation that a reduction in formation of Ang(1–7) or an increase in Ang II occurs. Regulation of ACE2, in concert with neprilysin and ACE in this brain area, may influence neural control of the circulation and resting arterial pressure via an impact on peptides of the renin– angiotensin system.
Acknowledgements
The authors wish to acknowledge support from NIH, National Heart, Lung and Blood Institute grants HL-51952, HL-079498 and HL-56973. Partial support from Unifi, Inc., Greensboro, NC,USA and Farley-Hudson Foundation, Jacksonville, NC, USA is also appreciated. The MLN4760 was supplied by Millenium Pharmaceuticals. M.A.G-E. was supported by a COSEHC Warren Trust Fellowship.
References
- Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841–F850. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.107.106005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2001;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Back SA, Gorenstein C. Histochemical visualization of neutral endopeptidase-24.11 (enkephalinase) activity in rat brain: cellular localization and codistribution with enkephalins in the globus pallidus. J Neurosci. 1989;9:4439–4455. doi: 10.1523/JNEUROSCI.09-12-04439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreflex reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension. 1988;11 Suppl. I:I167–I171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Ferrario CM. Actions of angiotensin peptides after partial denervation of the solitary tract nucleus. Hypertension. 1990;15 Suppl. I:I34–I39. doi: 10.1161/01.hyp.15.2_suppl.i34. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Santos RAS, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol. 1989;257:H324–H329. doi: 10.1152/ajpheart.1989.257.1.H324. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol Regul Integr Comp Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)–MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1–7) by angiotensin converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- Chevillard C, Saavedra JM. Distribution of angiotensin-converting enzyme activity in specific areas of the rat brain stem. J Neurochem. 1982;38:281–284. doi: 10.1111/j.1471-4159.1982.tb10883.x. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Hirooka Y, Potts PD, Head GA. Functions of angiotensin peptides in the rostral ventrolateral medulla. Clin Exper Pharmacol Physiol Suppl. 1996;3:S105–S111. [PubMed] [Google Scholar]
- Diz DI, Barnes KL, Ferrario CM. Hypotensive actions of microinjections of angiotensin II into the dorsal motor nucleus of the vagus. J Hypertens Suppl. 1984;2:S53–S56. [PubMed] [Google Scholar]
- Diz DI, Fantz DL, Benter IF, Bosch SM. Acute depressor actions of angiotensin II in the nucleus of the solitary tract are mediated by substance P. Am J Physiol Regul Integr Comp Physiol. 1997;273:R28–R34. doi: 10.1152/ajpregu.1997.273.1.R28. [DOI] [PubMed] [Google Scholar]
- Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exper Pharmacol Physiol. 2001;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin-angiotensin system: insights from studies in transgenic rats. Cleveland Clin J Med. 2007;74:S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- Diz DI, Westwood B, Bosch SM, Ganten D, Ferrario C. NK1 receptor antagonist blocks angiotensin II responses in renin transgenic rat medulla oblongata. Hypertension. 1998;31:473–479. doi: 10.1161/01.hyp.31.1.473. [DOI] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah VM, Moreira ED, Irigoyen MC, Krieger EM. Baroreflex depression persists in the early phase after hypertension reversal. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1620–R1626. doi: 10.1152/ajpregu.2001.280.6.R1620. [DOI] [PubMed] [Google Scholar]
- Feng Y, Halabi C, Sigmund CD, Lazartigues E. Brain-selective overexpression of angiotensin-converting enzyme 2 prevents Ang II-mediated pressor effects in transgenic mice. Hypertension. 2007;50:e83. [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of Angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fontes MAP, Silva LCS, Campagnole-Santos MJ, Khosla MC, Guertzenstein PG, Santos RAS. Evidence that angiotensin-(1–7) plays a role in the central control of blood pressure at the ventrolateral medulla acting through specific receptors. Brain Res. 1994;665:175–180. doi: 10.1016/0006-8993(94)91171-1. [DOI] [PubMed] [Google Scholar]
- Ford WR, Clanachan AS, Jugdutt BI. Opposite effects of angiotensin AT1 and AT2 receptor antagonists on recovery of mechanical function after ischemia-reperfusion in isolated working rat hearts. Circulation. 1997;96:2747–2748. doi: 10.1161/01.cir.94.12.3087. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- Huang BS, Ganten D, Leenen FH. Responses to central Na+ and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension. 2001;37:683–686. doi: 10.1161/01.hyp.37.2.683. [DOI] [PubMed] [Google Scholar]
- Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regul Pept. 2005;125:55–59. doi: 10.1016/j.regpep.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Krieger EM. Time course of baroreceptor resetting in acute hypertension. Am J Physiol. 1970;218:486–490. doi: 10.1152/ajplegacy.1970.218.2.486. [DOI] [PubMed] [Google Scholar]
- Lasher RS, Lutz EM, Mulholland F, Sanderson R, Stewart JM, Bublitz C. Immunocytochemical localization of endopeptidase-24.11 in the nucleus tractus solitarius of the rat brain. Neurosci Lett. 1990;117:43–49. doi: 10.1016/0304-3940(90)90117-r. [DOI] [PubMed] [Google Scholar]
- May CN, Dashwood MR, Whitehead CJ, Mathias CJ. Differential cardiovascular and respiratory responses to central administration of selective opioid agonists in conscious rabbits: correlation with receptor distribution. Br J Pharmacol. 1989;98:903–913. doi: 10.1111/j.1476-5381.1989.tb14620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorov DN, Head GA. AT1 receptors in the RVLM mediate pressor responses to emotional stress in rabbits. Hypertension. 2003;41:1168–1173. doi: 10.1161/01.HYP.0000064574.29317.45. [DOI] [PubMed] [Google Scholar]
- Moreira ED, Ida F, Oliveira VL, Krieger EM. Rapid resetting of the baroreceptors in renal hypertensive rats. Hypertension. 1990;15:I40–I44. doi: 10.1161/01.hyp.15.2_suppl.i40. [DOI] [PubMed] [Google Scholar]
- Oliveira DR, Santos RA, Santos GF, Khosla M, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension. 1996;27:1284–1290. doi: 10.1161/01.hyp.27.6.1284. [DOI] [PubMed] [Google Scholar]
- Potts PD, Allen AM, Horiuchi J, Dampney RA. Does angiotensin II have a significant tonic action on cardiovascular neurons in the rostral and caudal VLM? Am J Physiol Regul Integr Comp Physiol. 2004;279:R1392–R1402. doi: 10.1152/ajpregu.2000.279.4.R1392. [DOI] [PubMed] [Google Scholar]
- Potts PD, Horiuchi J, Coleman MJ, Dampney RA. The cardiovascular effects of angiotensin-(1–7) in the rostral and caudal ventrolateral medulla of the rabbit. Brain Res. 2000;877:58–64. doi: 10.1016/s0006-8993(00)02626-3. [DOI] [PubMed] [Google Scholar]
- Raizada MKP, Ferreira AJP. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortes C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats. Role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glial-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Neurochemical transmission of baroreceptor input in the nucleus tractus solitarius. Brain Res Bull. 2000;51:111–118. doi: 10.1016/s0361-9230(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci. 2002;101:32–38. doi: 10.1016/s1566-0702(02)00178-9. [DOI] [PubMed] [Google Scholar]
- Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa J, Diz DI, Rose J, Chappell M. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Vinsant S, Chappell MC, Ferrario CM, Ganten D, Diz DI. Low glial angiotensinogen is not associated with deficits in angiotensin peptides in neuronal pathways in transgenic ASrAogen rats. FASEB J. 2005;19:A1188. [Google Scholar]
- Warner FJ, Smith AI, Hooper NM, Turner AJ. Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell Mol Life Sci. 2004;61:2704–2713. doi: 10.1007/s00018-004-4240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]