Abstract
The MerTK plays several important roles in normal macrophage physiology, including regulation of cytokine secretion and clearance of apoptotic cells. Mer signaling in other cell types, including malignant cells that ectopically overexpress the RTK, leads to downstream prosurvival pathway activation. We explored the hypothesis that Mer has a prosurvival role in macrophages exposed to oxidative stress. H2O2 treatment of peritoneal exudate murine macrophages and J774 cells rapidly stimulated Mer phosphorylation in a concentration-dependent manner. Mer phosphorylation was dependent on the ligand Gas6, as treatment with warfarin or MerFc (a fusion protein of the extracellular domain of Mer and the Fc portion of human Ig), inhibitors of Gas6 activity, blocked H2O2-mediated activation of Mer. Antiapoptotic signals including pAkt and pErk 1/2 were increased dramatically (threefold and 4.5-fold, respectively) in WT Mer-positive macrophages compared with Mer KO macrophages stimulated with H2O2. In a consistent manner, Mer expression led to decreased cleavage of proapoptotic indicators PARP and Caspase-3. Furthermore, Mer provided up to twofold enhanced cellular survival to primary macrophages exposed to H2O2. These data represent the first report of Mer activation in response to oxidative stress and demonstrate the ability of Mer RTK to promote macrophage survival in disease states that involve an oxidative stress environment.
Keywords: hydrogen peroxide, receptor, leukocyte, antiapoptotic signaling
Introduction
The MerTK belongs to the TAM receptor subfamily [1,2,3,4]. The TAM family members have similar extracellular motifs (two Ig-like and two fibronectin III motifs), a transmembrane region, and an intracellular TK domain. The TAM family receptors share a common ligand, Gas6 [5, 6]. More recently, the anticoagulant protein S, which shares significant homology with Gas6, has also been confirmed to be a ligand for Mer and Tyro-3 [7]. Ligand interaction with TAM receptors leads to receptor phosphorylation and activation of downstream signaling pathways that affect cell survival, proliferation, cytoskeletal architecture/cellular shape, and cell migration [3].
Abnormal expression of Mer leads to a transformed phenotype in fibroblasts [8] and to cytokine-independent growth in lymphocytes [9]. In addition to the in vitro studies suggesting the transforming properties of Mer, abnormally increased Mer expression has been reported in multiple human cancer types including leukemias, lymphomas, gastric cancer, prostate cancer, breast cancer, pituitary adenoma, and rhabdomyosarcoma [3]. In leukemia cells, Mer activation results in reduced apoptosis without a change in proliferation [10]. The survival advantage from Mer signaling provides lymphoblasts a competitive advantage over noncancerous lymphocytes and may contribute to oncogenesis. Mer transgenic mice, which ectopically express Mer in thymocytes and lymphocytes in a similar manner as leukemia patient samples, develop lymphoblastic leukemia/lymphoma. Furthermore, lymphocytes from Mer transgenic mice demonstrate decreased cell death in response to steroid treatment, suggesting a possible role of Mer prosurvival signaling in cancer cell chemoresistance [11].
In addition to the abnormal expression and oncogenic role of Mer in cancer cells, biological roles for physiologic expression of the TAM family receptors have been investigated in cells of the nervous, reproductive, vascular, and immune systems. Within cells of the immune system, TAM receptor expression has been detected in NK cells, NKT cells, macrophages, and DC [12]. All three receptors are detected on NK cells and found to be essential for NK cell differentiation [13]. In DC, TAM receptors inhibit TLRs to decrease proinflammatory cytokine secretion and help regulate the immune response. TAM receptors are also responsible for attenuating the immune response of macrophages following an inflammatory response [14]. The role in dampening the macrophage immune response is evident in Mer KO mice, which are hypersensitive to LPS-induced endotoxic shock as a result of excessive production of TNF-α [15]. Mer KO mice have also been used to demonstrate the need for Mer expression in macrophages for the clearance of apoptotic cells [16].
In the current study, we evaluate whether Mer mediates a prosurvival function in macrophages under conditions of oxidative stress. We demonstrate Gas6-dependent Mer phosphorylation in response to H2O2 treatment. This activation of Mer leads to significantly increased downstream antiapoptotic signaling via Akt and Erk 1/2 and subsequent decreased PARP and Caspase-3 cleavage in WT Mer-positive macrophages compared with Mer KO macrophages. The antiapoptotic Mer signaling in response to oxidative stress results in increased macrophage survival. We thus describe a previously unrecognized physiologic role for Mer in macrophages, which allows these cells to survive and function in conditions and disease states that produce increased ROS.
MATERIALS AND METHODS
Animals
WT C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mer KO mice, generated by deletion of exon 17 of the TK domain [15] and lacking expression of Mer protein, were kindly provided by Drs. Glenn Matsushima and H. Shelton Earp (University of North Carolina, Chapel Hill, NC, USA). The care of animals and experimental procedures were in accordance with the guidelines of the University of Colorado Center for Comparative Medicine (Aurora, CO, USA).
Cell culture
The mouse macrophage cell line J774 was obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM supplemented with 10% FBS. Macrophages were incubated under conditions of 10% CO2. To recruit peritoneal exudate macrophages, WT and Mer KO mice were injected i.p. with 1.5 ml 3% thioglycollate. After 72 h, mice were killed, and cells were harvested from the peritoneal cavity. Macrophages and J774 cells were plated in DMEM with 10% FBS at a density of 2 × 106 cells/well in a 12-well plate for Mer activation experiments/immunoblots or 4 × 105 cells/well in a 12-well plate for cell survival experiments and were incubated overnight at 37°C prior to treatment.
Cell treatments
To stimulate cells with H2O2 or Gas6, J774 cells or peritoneal exudate macrophages were plated at a concentration of 2–2.5 × 106 cells/well in 12-well plates and starved in serum-free media for 2 h. Cells were stimulated with 100–1000 μM H2O2 or 200 nM mouse rGas6 (R&D Systems, Minneapolis, MN, USA) for 10 min at 37°C prior to preparation of whole cell lysates. We have demonstrated previously that this concentration of Gas6 induces phosphorylation and activation of Mer in J774 cells [17].
To evaluate the effect of warfarin on Mer activation, mouse peritoneal exudate macrophages were cultured in DMEM containing 10% FBS and 1 μM warfarin (Sigma Chemical Co., St. Louis, MO, USA) for 44 h. Experimental and control (nonwarfarin-treated) cells were placed in serum-free medium, with or without 1 μM warfarin for an additional 3 h and treated with 800 μM H2O2 for 5 min. Cells were lysed, and Mer activation was analyzed by immunoprecipitation and immunoblotting with anti-pMer antibody.
Immunoblotting
Cells were lysed in buffer [50 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM EDTA, 10% v/v glycerol, 1% v/v Triton X-100, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, and protease inhibitors (Roche, Nutley, NJ, USA)] and placed on ice for 10 min. Protein supernatant was collected by centrifugation.
For analysis of proteins in the conditioned medium, cell culture media were collected after treatment and concentrated using a Millipore Amicon (Bedford, MA, USA) centrifugal filter (molecular weight cutoff, 30,000). Immunoprecipitation was performed on cell lysates obtained from exposure to H2O2, with and without treatment with warfarin to ensure detection of the potentially low-concentration pMer protein. Cell lysates were incubated with mouse Mer antibody (R&D Systems) on ice for 30 min. Protein G beads (Invitrogen, Carlsbad, CA, USA) were added, and the combination was rotated for 1 h at 4°C. Supernatant was discarded, and bead complexes were washed with lysis buffer twice. Protein was denatured from the bead/antibody complex with SDS containing sample buffer and separated by SDS-PAGE as described below.
Cell lysates, conditioned medium, or immunoprecipitated proteins were resolved on 8% SDS-PAGE gels and transferred to nitrocellulose membranes. The immunoblots were probed with the following antibodies at concentrations recommended by the manufacturer protocols: pMer antibody (FabGennix, Frisco, TX, USA); pAKT (Ser473), AKT, pERK 1/2 (Thr202/Tyr204), ERK 1/2, PARP, and Caspase-3 antibodies (Cell Signaling Technology, Danvers, MA, USA); mouse Mer and Gas6 antibodies (R&D Systems); actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Proteins were visualized by luminol-based ECL detection (Pierce, Rockford, IL, USA). Reactive bands were quantified by densitometry using AlphaEase v. 5.5 from Alpha Innotech Corp. (San Leandro, CA, USA).
ELISA analysis
To measure Gas6 secretion, J774 cells were incubated 3 or 6 h in serum-free medium, and 800 μM H2O2 was added during the last 15 min of incubation. Gas6 in the culture supernatant was measured using a Gas6 ELISA (R&D Systems), according to the manufacturer’s instructions. Experiments were performed in triplicate.
Survival analysis
Primary peritoneal exudate mouse macrophages from WT or Mer KO mice were starved in serum-free DMEM, with or without 200 nM MerFc (a fusion protein of the extracellular domain of Mer and the Fc portion of human Ig) or RetFc (a commercially available fusion protein of the extracellular domain of the Ret receptor and the Fc portion of human Ig; R&D Systems), for 2 h and then stimulated with 400 μM H2O2 for 3 h at 37°C. Cells were rinsed with PBS and stained with 500 μl trypan blue, digital images were taken, and cells were assessed for trypan blue exclusion to determine viability. Cell counting was done in triplicate, and the survival assays were performed in three independent experiments.
Statistical analysis
All graphs and statistical analyses were performed using Prism v. 4.0a (GraphPad Software, San Diego, CA, USA). Densitometry analysis involved a one-tailed paired t-test. Survival analysis used a two-tailed paired t-test.
RESULTS
MerTK is activated by H2O2 in a concentration-dependent manner
H2O2, in concentrations ranging from 20 μm to 5 mM, will activate several RTKs, including EGFR [18], platelet-derived growth factor receptor [19], and Axl [20, 21]. Based on this known concentration range of H2O2 used to activate other RTKs, we tested a range of 200 μM to 1 mM H2O2 to discern the concentration that sufficiently activates MerTK in the mouse macrophage cell line J774 and in primary exudate mouse peritoneal macrophages. In J774 cells, pMer was detected over a range of 400–1000 μM H2O2, with a maximum Mer activation at 800 μM (Fig. 1A). Treatment with H2O2 did not alter total Mer expression. These data represent the first report of MerTK activation in response to oxidative stress.
Figure 1.
MerTK activation is induced by H2O2 in a dose-dependent manner. (A and B) J774 mouse macrophage cells (A) or primary peritoneal murine macrophages (B) were serum-starved and stimulated with the indicated concentrations of H2O2 for 10 min. Whole cell lysates were immunoblotted with an anti-pMer (p-Mer) antibody or an anti-Mer (Mer) antibody. Actin (43 kD) was used as a loading control. (C) WT or Mer KO peritoneal exudate macrophages were treated with 400 μM H2O2 for 10 min, and cell lysates were immunoblotted with pMer antibody or total Mer antibody. Actin (43 kD) was used as a loading control. The data shown are representative of three independent experiments.
In thioglycollate-elicited peritoneal exudate macrophages, pMer was detected first at a concentration of 200 μM with substantial Mer activation at 400–600 μM H2O2 (Fig. 1B). Thus, for all further experiments, 800 μM H2O2 was used for activation of Mer in J774 cells, and 400 μM H2O2 was used for activation of Mer in peritoneal exudate macrophages. To demonstrate specificity of pMer detection, peritoneal exudate macrophages from WT and Mer KO mice were treated with 400 μM H2O2. pMer was detected in the macrophages from WT mice but not Mer KO mice (Fig. 1C). The Mer and pMer proteins detected in J774 cells and peritoneal exudate macrophages were 200–205 kDa, consistent with the reported size of Mer in macrophages [17]. Mer activation at these concentrations was found 1–30 min after addition of H2O2, and maximal Mer phosphorylation was detected 5–10 min after addition of H2O2 (unpublished results). A 5- to 10-min H2O2 treatment time has also been selected in experiments, demonstrating activation of other RTKs [19, 20]. Based on these data, 10 min was selected as the treatment time for H2O2 stimulation of Mer.
H2O2 activation of Mer is mediated by Gas6
In J774 macrophages, pMer was detected after treatment with 200 nM Gas6 for 10 min (Fig. 2A) in a similar manner as was noted with treatment of the J774 macrophages with 800 μM H2O2. As reported previously, phosphorylation of Mer mediated by the ligand Gas6 can be blocked via ligand sequestration using MerFc [17]. Interestingly, MerFc was also able to abrogate pMer in response to H2O2 (Fig. 2A), suggesting a role for Gas6 in Mer activation following macrophage exposure to H2O2.
Figure 2.
H2O2 activation of Mer is a Gas6-dependent process. (A) Serum-starved J774 cells were untreated (Untx; Lane 1) or stimulated with 800 μM H2O2 (Lane 2) or 200 nM Gas6 for 10 min (Lane 3). Alternatively, starved cells were pretreated with 200 nM MerFc and then stimulated with H2O2 or Gas6 (Lanes 4 and 5). Whole cell lysates were immunoblotted with an anti-pMer antibody or an anti-Mer antibody. Actin (43 kD) was used as a loading control. (B) Serum-starved J774 cells were treated with 1 μM warfarin for 44 h prior to stimulation with 800 μM H2O2. Whole cell lysates were immunoprecipitated with anti-mouse Mer antibody and immunoblotted with anti-pMer antibody or anti-Mer antibody. (C) J774 cells were untreated or stimulated with 800 μM H2O2 as indicated. Conditioned media were collected at the indicated time-points, and an ELISA assay was performed for the presence of Gas6. The data shown are representative of three independent experiments.
These results are consistent with previous reports that have established a direct effect of H2O2 to enhance TK activity via reversible inactivation of protein tyrosine phosphatases [22, 23]. The inactivation of protein tyrosine phosphatases, likely via cysteine oxidation of the phosphatases [24, 25], allows the balance of phosphorylation/dephosphorylation to be tipped in favor of increased TK activation. Thus, in our system, the presence of a Mer ligand, such as Gas6, may be necessary to initiate the activation of Mer, and H2O2 likely sustains Mer activation via phosphatase inhibition. Removal of the available Gas6 with MerFc blocks the initiation of Mer activation and negates any potential effect on Mer activation by H2O2.
Additional support for the role of Gas6 in Mer activation following macrophage exposure to H2O2 was provided by pretreating J774 cells with warfarin prior to H2O2 stimulation. Warfarin inhibits γ-glutamyl carboxyltransferase, which is necessary for the activation of vitamin K-dependent proteins, including Gas6. Other reports have confirmed that Gas6, in the presence of warfarin for 2–5 days, can be rendered incapable of activating TAM family receptors [20, 26, 27]. We found that pretreatment of J774 cells with warfarin for 44 h inhibited the activation of Mer in response to H2O2 stimulation (Fig. 2B). Although warfarin treatment is not specific to Gas6, these data are consistent with the observation that functional Gas6 is necessary to initiate Mer activation in this system, and the likely role of H2O2 is to sustain the activation provided by the ligand.
In addition to the known effect of H2O2 on phosphatase inhibition, a potential, direct effect of H2O2 on Gas6 ligand availability was addressed. Gas6 is secreted continuously by J774 macrophages into conditioned media; however, the level of Gas6 secreted by the macrophages was not enhanced by H2O2 at 3 and 6 h following H2O2 administration (Fig. 2C). In a similar manner, we did not detect increased levels of Gas6 from conditioned media of H2O2-treated primary exudate macrophages or J774 cells by Western blot analysis (data not shown). These data further support the hypothesis that the primary effect of H2O2 on Mer activation in macrophages is via the known inhibitory effect of H2O2 on phosphatases rather than a direct effect on the initiation of receptor activation.
H2O2 treatment of primary macrophages leads to Mer-dependent phosphorylation of Akt and Erk 1/2
The effect of H2O2 treatment on sustained Mer activation and downstream signaling in macrophages was evaluated using primary thioglycollate-recruited peritoneal macrophages from WT mice and Mer KO mice lacking Mer protein (Mer KO) on the macrophage surface. Peritoneal exudate macrophages from WT C57BL/6 mice expressed Mer as shown previously (Fig. 1B). H2O2 treatment of the Mer-expressing macrophages significantly increased activation of pAkt and pErk 1/2, which are known downstream targets of Mer (Fig. 3) [9,10,11]. In contrast, the effect of H2O2 on pAkt and pErk 1/2 was diminished significantly in Mer KO macrophages (Fig. 3A). Quantitative densitometric analysis revealed that H2O2-mediated phosphorylation of Akt was 67% lower in Mer KO macrophages relative to WT macrophages (P=0.035; Fig. 3B). Similarly, H2O2-mediated pErk was 78% percent lower in Mer KO macrophages relative to WT macrophages (P=0.0003; Fig. 3C). This dramatic reduction in pAkt and pErk 1/2 in in the absence of Mer suggests a predominant role of Mer in activating these antiapoptotic pathways in macrophages exposed to oxidative stress.
Figure 3.
H2O2-induced Mer phosphorylation leads to activation of downstream antiapoptotic pathways. (A) Serum-starved WT and KO peritoneal exudate mouse macrophages were treated with 400 μM H2O2 for 10 min. Whole cell lysates were evaluated by immunoblot with antibodies for total and pAkt, as well as total and pErk 1/2. pAkt (B) and pErk 1/2 (C) were quantified by densitometry. To reflect relative phosphorylation, the IDV of the phospho band was divided by the IDV of the total protein band, and WT H2O2 was normalized to 100% for each experiment. Data shown are the mean ± sem from three independent experiments.
Mer protects primary macrophages from H2O2-induced apoptosis
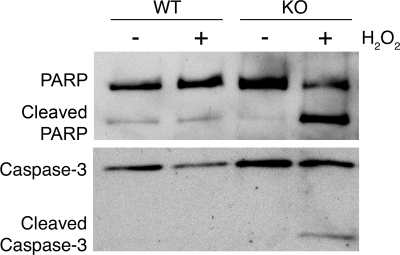
Cleavage of PARP and Caspase-3 is the final step in cell signaling leading to apoptotic cell death. Although macrophage exposure to H2O2 rapidly stimulates activation of apoptotic pathways, the effect of an apoptotic trigger on PARP and Caspase-3 cleavage occurs hours later. PARP and Caspase-3 cleavage was detected as early as 3 h in Mer KO macrophages treated with H2O2. However, macrophages from WT mice, which express Mer and activate antiapoptotic proteins such as Akt and Erk 1/2 in response to H2O2, had less PARP and Caspase-3 cleavage (Fig. 4). The decreased PARP and Caspase-3 cleavage detected in Mer-expressing macrophages suggests that MerTK mediates protection from apoptotic cell death when macrophages are exposed to oxidative stress.
Figure 4.
Mer decreases PARP and Caspase-3 cleavage in response to H2O2 stimulation. WT and Mer KO peritoneal exudate mouse macrophages were harvested, serum-starved for 2 h, and treated with 400 μM H2O2 for 3 h. Whole cell lysates were immunoblotted with antibodies for PARP and Caspase-3, which detect unprocessed and cleaved forms. The data shown are representative of three independent experiments.
Mer provides a functional survival advantage to macrophages treated with H2O2
To extend the findings suggesting activation of antiapoptotic signaling pathways in Mer-expressing macrophages exposed to oxidative stress, experiments were conducted to test for a direct functional effect of Mer expression on macrophage cell survival in the presence of H2O2. Peritoneal exudate macrophages from WT mice were serum-starved for 2 h and treated with H2O2 for 3 h. Cell survival was then determined by trypan blue exclusion. The Mer-expressing WT macrophages were treated with H2O2 in the presence and absence of MerFc (Fig. 5). In the presence of the Mer inhibitor, H2O2 reduced survival of WT macrophages 48% relative to untreated control. However, in the absence of Mer inhibition, H2O2 reduced survival of WT macrophages only 25% relative to untreated control. This increase in macrophage survival with a functional MerTK was statistically significant (P=0.01). There was no significant difference between H2O2 alone and H2O2 following pretreatment with the negative control molecule, RetFc.
Figure 5.
Mer signaling provides a functional survival advantage for macrophages exposed to H2O2. WT and Mer KO peritoneal exudate mouse macrophages were harvested and serum-starved for 2 h. Prior to stimulation with 400 μM H2O2, macrophages were incubated in serum-free media, with or without 200 nM MerFc or RetFc for 2 h as indicated. Cultures were treated with H2O2 for an additional 3 h and then stained with trypan blue and counted in triplicate to determine cell viability. Survival of WT-untreated cells was normalized to 100% for each experiment. Data shown are the mean ± sem from three independent experiments.
To confirm the observation of decreased cell survival with MerFc inhibition of Mer, cell survival assays were performed with primary macrophages isolated from Mer KO mice, in which H2O2 reduced survival of macrophages 63% relative to untreated control WT macrophages (Fig. 5). However, the presence of Mer in the WT mice restricted the H2O2-mediated reduction in cell survival to 23% of untreated WT macrophages. The increase in macrophage survival as a result of the presence of Mer was statistically significant (P=0.03). Thus, the presence of a functional MerTK enhanced survival significantly in response to oxidative stress.
DISCUSSION
This report represents the first observation that the MerTK is activated in response to oxidative stress. Specifically, macrophages activate Mer upon exposure to H2O2, which occurs in the human body under a variety of physiologic conditions and disease states. Phagocytic cells produce superoxide anion and H2O2 via reduction of O2 by a NADPH oxidase complex. In addition, many cell types produce low levels of superoxide anion and H2O2 in response to extracellular stimuli, including cytokines and growth factors [24]. Increased ROS are noted in diseases such as Alzheimer’s disease, Parkinson’s disease, diabetes, cancer, cerebral and myocardial ischemia, and atherosclerosis. ROS are also found in conditions of inflammation, sepsis, or treatment with chemotherapeutic agents [28].
The primary effect of H2O2 on Mer activation is likely through transient and reversible inhibition of phosphatase activity, an effect of H2O2 reacting with the redox-sensitive invariant cysteine residue in the active site consensus motif of tyrosine phosphatases. Other groups [25, 29, 30] have previously established this effect of H2O2 on the inhibition of phosphatase activity. Although the specific phosphatase that acts upon Mer has not been identified, the putative intracellular phosphatase, C1 domain-containing phosphatase and tensin homologue, has been localized with the related Axl protein [31]. The effect of H2O2 on phosphatase activity alone is not sufficient to account for H2O2-mediated activation of Mer, as we demonstrated through the lack of Mer phosphorylation when macrophages were exposed to H2O2 in the presence of MerFc (to sequester Gas6) or warfarin (to render Gas6 nonfunctional). These data suggest that H2O2-mediated potentiation of Mer requires the presence of a functional Mer ligand for initial receptor activation.
ROS can pose a threat to macrophage survival through damage to DNA, protein, and lipids. Cellular survival depends on the ability of cells to adapt to or resist the stress. As part of an adaptive process, cells respond to H2O2 by activation of cell signaling pathways. The activation of TKs has been shown to be responsible for cellular survival responses to oxidative stress. For example, in the cervical cancer HeLa cell line, activation of Akt in response to H2O2 was mediated predominantly via EGFR signaling [18]. Furthermore, the Axl RTK was critical for Akt signaling when vascular smooth muscle cells were treated with H2O2 [20]. In a similar manner, we have found that pAkt and pErk 1/2 appear to occur primarily through a Mer-dependent process in macrophages exposed to H2O2. The H2O2-dependent pAkt and pErk 1/2 in Mer KO macrophages was reduced to 33% and 22%, respectively, of that observed in WT H2O2-treated macrophages (Fig. 3). The increased activation of antiapoptotic pathways such as Akt and Erk 1/2 led to decreased apoptotic cell death, as evidenced by the decreased Caspase-3 cleavage and PARP cleavage in Mer-expressing macrophages as compared with Mer KO macrophages exposed to H2O2 (Fig. 4). These findings were confirmed further by the functional observations of a significant increase in cell survival following H2O2 treatment of Mer-expressing macrophages compared with macrophages with inhibited Mer signaling, whether through pretreatment with MerFc or use of Mer KO macrophages (Fig. 5). This significant dependence on the presence of Mer suggests a predominant role for this TK in macrophage survival in the presence of oxidative stress.
Although we have confirmed that Gas6 is present and functional in our system, it is also possible that protein S may play a role as an alternative ligand for Mer activation in macrophages. Both ligands can activate Mer and can bind MerFc [17, 32]. Futhermore, warfarin has been shown to affect the activity of Gas6 via decarboxylation [26], and warfarin also decreases the production and specific activity of protein S [33, 34]. A lack of existence of necessary murine protein S reagents precludes our ability to decipher the role of protein S in our system at the current time, but this should be evaluated further in future studies.
In addition to the role of Mer in macrophage survival, Mer signaling has been implicated in macrophage clearance of apoptotic cells. Although Mer KO macrophages bind apoptotic cells appropriately, Mer is required for engulfment and efficient clearance of apoptotic cells [16, 35]. The Mer ligands Gas6 and protein S are also important in the process of apoptotic cell clearance. The N-terminal region of Gas6 and protein S contains a γ-carboxyglutamic acid domain that binds phosphatidylserine residues on the apoptotic cell [36], and the C-terminal region of the ligands binds the TAM receptors [37, 38]. Thus, the Gas6 and protein S ligands serve effectively as a bridge between the apoptotic cell and the MerTK. For protein S to stimulate phagocytosis effectively via Mer, auto-oxidation and oligomerization of protein S must first occur, a process enabled by interaction of protein S with phosphatidylserine on the apoptotic cell [39]. In a similar manner to the use of MerFc in this report to block Mer activation and thus, impact macrophage survival, Mer inhibition with MerFc has also been shown to block macrophage clearance of apoptotic cells [17]. The inefficient clearance of apoptotic cells in Mer KO mice leads to the presence of autoantibodies and the development of signs of autoimmunity [16, 40]. The degree of autoimmunity is enhanced in mice lacking all three TAM receptors on the macrophages [41].
An additional role of Mer in macrophage function is to prevent potentially harmful sustained inflammatory responses by regulating the release of proinflammatory cytokines. The role of Mer in proinflammatory cytokine secretion was reported in studies using LPS stimulation of Mer KO monocytes/macrophages. Mer KO macrophages produced elevated levels of TNF-α and had increased NF-κB nuclear translocation in response to LPS. Furthermore, Mer KO mice died of increased proinflammatory secretion/toxic shock following LPS challenge, and the control WT mice survived [15]. These observations suggest a function for Mer in the attenuation of release of proinflammatory cytokines following exposure to bacterial endotoxin.
The recognized contributions of Mer in macrophage clearance of apoptotic cells, release of proinflammatory cytokines, and now, macrophage survival in response to oxidative stress demonstrate Mer’s role in macrophage physiologic functions. Other immune cells, including DC [42,43,44] and NK cells [13], are dependent on TAM TK signaling for critical cellular responses. Thus, the activation and downstream signaling of TAM receptors are important in the innate immune response and the ability to help prevent disease progression. The ability of Mer to promote enhanced macrophage survival in an oxidative stress environment permits the macrophage to persist in critical tasks of modulating the host response to disease.
ACKNOWLEDGMENTS
D. K. G. is the Damon Runyon-Novartis Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-39-07). This work was also supported by National Institutes of Health grants HL81151 and GM61031 (P. M. H.). A. K. K. is The St. Baldrick’s Foundation Scholar, and this work was supported in part by the St. Baldrick’s Foundation. The authors thank Dr. James DeGregori and Dr. Deborah DeRyckere for their insightful discussions and comments about this manuscript.
Footnotes
Abbreviations: DC=dendritic cell(s), EGFR=epidermal growth factor receptor, Gas6=growth arrest-specific 6, H2O2=hydrogen peroxide, IDV=integrated density value, KO=knockout, MerTK=Mer receptor tyrosine kinase, p=phosphorylated, PARP=polyADP-ribose polymerase, ROS=reactive oxygen species, RTK=receptor tyrosine kinase, TAM=Tyro-3, Axl, Mer, WT=wild-type
References
- Graham D K, Dawson T L, Mullaney D L, Snodgrass H R, Earp H S. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- Lai C, Gore M, Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9:2567–2578. [PubMed] [Google Scholar]
- Linger R M A, Keating A K, Earp H S, Graham D K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan J P, Frye R A, Cogswell P C, Neubauer A, Kitch B, Prokop C, Espinosa R, III, Le Beau M M, Earp H S, Liu E T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Stitt T N, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies D R, Jones P F. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Prasad D, Rothlin C V, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ling L, Kung H J. Mitogenic signals and transforming potential of Nyk, a newly identified neural cell adhesion molecule-related receptor tyrosine kinase. Mol Cell Biol. 1995;15:6582–6592. doi: 10.1128/mcb.15.12.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu M M, Kirsch K H, Shishido T, Zong C, Hanafusa H. Biological effects of c-Mer receptor tyrosine kinase in hematopoietic cells depend on the Grb2 binding site in the receptor and activation of NF-κB. Mol Cell Biol. 1999;19:1171–1181. doi: 10.1128/mcb.19.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge K L, Luft J C, Dawson T L, Kozlowska E, Mahajan N P, Varnum B, Earp H S. Mer receptor tyrosine kinase signaling: prevention of apoptosis and alteration of cytoskeletal architecture without stimulation or proliferation. J Biol Chem. 2002;277:24057–24066. doi: 10.1074/jbc.M112086200. [DOI] [PubMed] [Google Scholar]
- Keating A K, Salzberg D B, Sather S, Liang X, Nickoloff S, Anwar A, Deryckere D, Hill K, Joung D, Sawczyn K K, Park J, Curran-Everett D, McGavran L, Meltesen L, Gore L, Johnson G L, Graham D K. Lymphoblastic leukemia/lymphoma in mice overexpressing the Mer (MerTK) receptor tyrosine kinase. Oncogene. 2006;25:6092–6100. doi: 10.1038/sj.onc.1209633. [DOI] [PubMed] [Google Scholar]
- Behrens E M, Gadue P, Gong S Y, Garrett S, Stein P L, Cohen P L. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur J Immunol. 2003;33:2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- Caraux A, Lu Q, Fernandez N, Riou S, Di Santo J P, Raulet D H, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003;15:31–36. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Camenisch T D, Koller B H, Earp H S, Matsushima G K. A novel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- Scott R S, McMahon E J, Pop S M, Reap E A, Caricchio R, Cohen P L, Earp H S, Matsushima G K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Sather S, Kenyon K D, Lefkowitz J B, Liang X, Varnum B C, Henson P M, Graham D K. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCullough K D, Franke T F, Holbrook N J. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Saito S, Frank G D, Mifune M, Ohba M, Utsunomiya H, Motley E D, Inagami T, Eguchi S. Ligand-independent trans-activation of the platelet-derived growth factor receptor by reactive oxygen species requires protein kinase C-δ and c-Src. J Biol Chem. 2002;277:44695–44700. doi: 10.1074/jbc.M208332200. [DOI] [PubMed] [Google Scholar]
- Konishi A, Aizawa T, Mohan A, Korshunov V A, Berk B C. Hydrogen peroxide activates the Gas6-Axl pathway in vascular smooth muscle cells. J Biol Chem. 2004;279:28766–28770. doi: 10.1074/jbc.M401977200. [DOI] [PubMed] [Google Scholar]
- Valverde P. Effects of Gas6 and hydrogen peroxide in Axl ubiquitination and downregulation. Biochem Biophys Res Commun. 2005;333:180–185. doi: 10.1016/j.bbrc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- Lee S R, Kwon K S, Kim S R, Rhee S G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Meng T C, Fukada T, Tonks N K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Rhee S G, Bae Y S, Lee S R, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Rhee S G, Chang T S, Bae Y S, Lee S R, Kang S W. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- Hasanbasic I, Rajotte I, Blostein M. The role of γ-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3:2790–2797. doi: 10.1111/j.1538-7836.2005.01662.x. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Arai H, Nakano T, Ohashi K, Mizuno K, Fukatsu A, Doi T, Kita T. Gas6 induces mesangial cell proliferation via latent transcription factor STAT3. J Biol Chem. 2001;276:42364–42369. doi: 10.1074/jbc.M107488200. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook N J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Alindri F, Karlsson R, Dahlback B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun. 2002;299:793–800. doi: 10.1016/s0006-291x(02)02718-3. [DOI] [PubMed] [Google Scholar]
- Liao D, Wang X, Li M, Lin P H, Yao Q, Chen C. Human protein S inhibits the uptake of AcLDL and expression of SR-A through Mer receptor tyrosine kinase in human macrophages. Blood. 2009;113:165–174. doi: 10.1182/blood-2008-05-158048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D S, Marlar R A, Levin E G. Human endothelial cells synthesize protein S. Blood. 1986;67:1168–1171. [PubMed] [Google Scholar]
- Ogura M, Tanabe N, Nishioka J, Suzuki K, Saito H. Biosynthesis and secretion of functional protein S by a human megakaryoblastic cell line (MEG-01) Blood. 1987;70:301–306. [PubMed] [Google Scholar]
- Todt J C, Hu B, Curtis J L. The receptor tyrosine kinase MerTK activates phospholipase C γ2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol. 2004;75:705–713. doi: 10.1189/jlb.0903439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem. 1997;272:29411–29414. doi: 10.1074/jbc.272.47.29411. [DOI] [PubMed] [Google Scholar]
- Mark M R, Chen J, Hammonds R G, Sadick M, Godowsk P J. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271:9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Knyazev P G, Clout N J, Cheburkin Y, Gohring W, Ullrich A, Timpl R, Hohenester E. Structural basis for Gas6-Axl signaling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H, Shacter E. Auto-oxidation and oligomerization of protein S on the apoptotic cell surface is required for Mer tyrosine kinase-mediated phagocytosis of apoptotic cells. J Immunol. 2008;180:2522–2530. doi: 10.4049/jimmunol.180.4.2522. [DOI] [PubMed] [Google Scholar]
- Cohen P L, Caricchio R, Abraham V, Camenisch T D, Jennette J C, Roubey R A, Earp H S, Matsushima G, Reap E A. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- Lemke G, Rothlin C V. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin C V, Ghosh S, Zuniga E I, Oldstone M B, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Seitz H M, Camenisch T D, Lemke G, Earp H S, Matsushima G K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]