Abstract
The pharmacokinetics (PK) and pharmacodynamics (PD) of orbifloxacin were studied in beagle dogs after intravenous (i.v.) and intramuscular (i.m.) administration at a dose of 2.5 mg/kg body weight. An absolute bioavailability of 100.1% ± 4.76%, a terminal half-life of 4.23 ± 0.2 h and 3.95 ± 0.15 h after i.v. and i.m. administration, a steady-state volume of distribution of 1.61 ± 0.13 liters/kg, and clearance of 0.31 ± 0.03 liters/h/kg were observed. Orbifloxacin showed rapid, concentration-dependent killing against the Escherichia coli, Staphylococcus aureus, Staphylococcus intermedius, and Proteus mirabilis clinical isolates. Computations based on PK-PD analysis indicated that the recommended dose is unlikely to be clinically effective against some strains like S. intermedius. Therefore, a higher dose of orbifloxacin would be worthy of consideration for treatment of certain bacterial infections in dogs.
Orbifloxacin, a synthetic antibacterial agent from the class of fluoroquinolone carboxylic acid derivatives, exhibits bactericidal activity against numerous gram-negative and gram-positive bacteria (9, 15, 24). It has been developed exclusively for use in veterinary medicine (20). In companion animals, orbifloxacin is available mainly as an oral preparation and as a topical preparation for local medication in some countries and is indicated for the treatment of various infections, including those of the urinary tract, skin, ear, and soft tissues (6, 9).
The pharmacokinetics (PK) of orbifloxacin have been evaluated in goats (17), in horses (6, 12), in pigs (19), in rabbits (18), in dogs (14, 15, 20), in cats (20), in camels (10), in cattle (7), and recently, in sheep (11). The in vitro activity and clinical efficacy of orbifloxacin against naturally occurring bacterial infections of the skin and soft tissues in dogs have also been evaluated by different researchers (8, 9, 16, 20, 25). An evolving appreciation of the relationship between antimicrobial PK in the target animal species and their action on target pathogens (pharmacodynamics [PD]) has led to greater sophistication in the design of dosage schedules, which in turn, has improved clinical response to therapy and reduced the selection pressure for resistance in antimicrobial therapy (23). Although the disposition kinetics for oral formulations of orbifloxacin and other fluoroquinolones have been investigated, there are few reports on the PK for the injectable formulation of orbifloxacin in dogs. In a comparative study with five fluoroquinolones, Boothe et al. (3) used bacterial pathogens isolated from dogs and cats and PK data of orbifloxacin from package inserts to assess whether the magnitude of the targeted indices, ≥10 for the maximum concentration of drug in serum (Cmax)/MIC and ≥125 for the area under the concentration-time curve (AUC)/MIC, could be achieved at the recommended low and high doses. Although it is generally accepted that these endpoints are the surrogates used as activity indicators for fluoroquinolones (6, 21, 26), various studies suggest that the optimal PD endpoint for fluoroquinolones varies by pathogen. For example, the AUC/MIC ratio associated with the prompt eradication of Streptococcus pneumoniae and most other gram-positive bacteria is typically within a range of 30 to 40 (22), and the ex vivo AUC/MIC ratio for danofloxacin for the elimination of Mannheimia haemolytica or Escherichia coli in different ruminant species ranged from 28.7 to 68.7 (1, 26). This suggests that distinct PK-PD endpoints are required for different levels of antibacterial activity, according to the host or pathogen. Therefore, the current study was conducted to simultaneously determine the serum levels and disposition kinetics of orbifloxacin after intravenous (i.v.) and intramuscular (i.m.) injections to beagle dogs and the antibacterial activity of the drug, in serum and broth, against clinical isolates of E. coli, Staphylococcus aureus, Staphylococcus intermedius, and Proteus mirabilis and to apply the PK-PD modeling approach as a basis of dosage optimization for orbifloxacin in dogs.
MATERIALS AND METHODS
Animals and experimental design.
Six healthy beagle dogs, each weighing between 8 and 10 kg and aged from 1 to 2 years, were purchased from KyeRyong Science Corp. (Daejeon, Korea). The animals were housed individually in stainless steel cages in climate-controlled rooms, with a 12-h light/12-h dark cycle. Dogs had ad libitum access to water and were fed a standard dry feed (Orient Bio Inc., Gyeonggi-do, Korea). A two-period cross-sectional study was conducted. In period 1, three dogs received orbifloxacin (Victas 50 injection; Samyang Anipharm Co., Ltd, Seoul, Korea) at an i.v. dosage of 2.5 mg/kg body weight (into the jugular vein), and the other three received the drug at the same dose rate administered i.m. (into the inner thigh muscle). A 15-day “wash out” period was allowed. In period 2, the routes of administration were reversed. The study was approved by the bioethical committee of Kyungpook National University (Korea).
Sample collection and analysis.
Blood samples were collected from the cephalic veins of the dogs. By alternating between the two forelegs, a total of 11 samples per animal were collected before and at 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 h after drug administration. Samples were stored immediately at room temperature for 20 min and then placed on ice to encourage clot retraction. Tubes were placed at 4°C for 12 h. After centrifugation at 2,000 × g for 10 min, the supernatant sera were pipetted and stored at −20°C until analysis. The orbifloxacin concentration in serum was measured by high-pressure liquid chromatography (HPLC), using a Hewlett-Packard 1100 system comprising an HPLC pump, a 5-μm HP Hypersil octyldecyl silane column (200 by 4.6 mm), an autoinjector, and an HP 1046A fluorescence detector. The mobile phase used was acetonitrile at 15% and 50 mmol potassium phosphate buffer at 85% (pH adjusted to 3 by adding hydrochloric acid). Acetonitrile (HPLC grade) was purchased from J. T. Baker (Phillipsburg, NJ), and dibasic potassium phosphate (reagent grade) was purchased from Sigma-Aldrich Corp. (St. Louis, MO). The flow rate used was 1 ml/min. The excitation and emission wavelengths used with the fluorescence detector were 287 nm and 470 nm, respectively. Treatment of samples and validation of the chromatographic method through the determination of specificity, linearity, accuracy, precision, detection, and quantitation limits were similar to those of our reported method (7). The retention time for orbifloxacin was 6.5 min. No interfering peaks in all blank samples were noted in the elution position of orbifloxacin. A linear relationship existed in the calibration curve at both low and high concentrations within the concentration range of the study. Orbifloxacin yielded a recovery from serum ranging from 98.7% ± 1.43% to 101.9% ± 0.35%. The repeatability and within-run precision (percent coefficient of variation) values across the range of tested concentrations were 2.5 to 7% and 3.2 to 6.1%, respectively. The limit of detection (LOD) and limit of quantitation were 0.01 and 0.02 μg/ml, respectively.
Determination of MIC.
The MIC of orbifloxacin was determined in both Mueller-Hinton broth (Difco Laboratories, Detroit, MI) and drug-free serum, according to the standard broth microdilution method (5). Four isolates of canine origin (S. intermedius, S. aureus, E. coli, and P. mirabilis) were obtained from Gyongsangbuk-Do Veterinary Service Laboratory (Daegu, Korea). The quality control organisms included in each MIC determination were E. coli ATCC 25922/KCCM 11234 and S. aureus ATCC 29213, purchased from the Korean Culture Center of Microorganisms (Seoul, Korea).
Ex vivo and in vitro bacterial killing curves.
Serum samples obtained from dogs before and at 1, 2, 4, 6, 8, 12, and 24 h after i.m. administration of orbifloxacin were used to determine ex vivo killing curves against an S. intermedius strain. The selection of this single strain was based on the fact that S. intermedius has been one of the most frequently isolated bacteria in clinical infections of the different body systems, including the urinary tract, skin, and ear of dogs, and on the difficulty of obtaining a large amount of serum samples from beagle dogs in order to undertake studies of many strains. Standard inoculum (100 μl) was added to 1 ml of each serum sample, giving a final concentration of approximately 5 × 105 CFU/ml, and incubated. Aliquots (100 μl) were withdrawn from each culture tube before and at 1, 3, 6, 9, 12, and 24 h after incubation and subjected to serial dilutions by 10-fold in saline. Twenty-five microliters of the suspensions was then dropped onto quadrants of Trypticase soy agar (Becton, Dickinson and Co., Sparks, MD). Once dry, the plates were incubated at 37°C for 24 h to determine viable counts. Results were expressed as CFU/ml.
In vitro bacterial killing curves were established as described above, using pooled serum samples harvested from six dogs prior to drug administration. In vitro killing curves were determined for all clinical isolates (S. intermedius, S. aureus, P. mirabilis, and E. coli), with orbifloxacin concentrations ranging from one-half to 32 times the MIC for each strain.
PK-PD integration.
The surrogate markers of antibacterial activity, including observed Cmax/MIC, AUC from 0 to 24 h (AUC0-24)/MIC, and the duration of time when the concentration of drug in serum exceeds the MIC (T > MIC), were determined using in vitro MIC data in serum and in vivo PK parameters obtained after both i.v. and i.m. dosing of orbifloxacin. The WinNonlin 5.2 standard computer program (noncompartmental analysis model 220) was used to determine T > MICs on the basis of all samples.
PK-PD analysis.
The PK parameters of orbifloxacin following both i.v. and i.m. administration to all animals were analyzed by noncompartmental methods using the WinNonlin Professional program (version 5.2; Pharsight Corporation). Mean absorption time (MAT) following i.m. injection was calculated as the difference between i.m. mean residence time (MRTi.m.) and MRTi.v.. The bioavailability following i.m. administration was calculated as the ratio of the total AUC from the i.m. dose to the total AUC from the i.v. injection.
For time-kill studies, the change in log10 colony count at each sampling point from the starting inoculum, or log10 reduction, was calculated. Time-kill curves were constructed by plotting the log10 colony count versus time. The ex vivo antibacterial effect of orbifloxacin in serum after i.m. administration was quantified by applying the sigmoid log10 difference in bacterial counts between 0 and 24 h in the control sample (Emax) equation to calculate the AUC0-24/MIC for bacteriostatic action (no change in bacterial count, E = 0), the AUC0-24/MIC ratio for 50% reduction in bacterial count, the AUC0-24/MIC ratio for bactericidal action (99% reduction in bacterial count, E = −3), and the AUC0-24/MIC ratio for elimination of bacteria (i.e., the lowest AUC0-24/MIC ratio, which produced a reduction in bacterial count to the LOD [40 CFU/ml]). The log10 difference between bacterial count (CFU/ml) after 24-h incubation and the initial inoculum bacterial count was fitted against the ex vivo AUC0-24/MIC ratio (2, 13). The ex vivo AUC0-24/MIC ratio for this fitting was estimated by multiplying the measured serum concentrations in samples collected between 1 and 24 h, following i.m. administration of orbifloxacin by the incubation period of 24 h, and then dividing the latter value by the MIC determined in serum. The basic equation used in this estimation procedure was
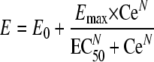 |
where E is the antibacterial effect, measured as the change in bacterial counts (in log CFU/ml) in the serum sample after 24 h of incubation compared to the initial log10 CFU/ml; Emax is the log10 difference in bacterial counts between 0 and 24 h in the control sample (when no drug is present); E0 is the log10 difference in bacterial counts in the test sample containing orbifloxacin after 24 h of incubation, when the LOD of 40 CFU/ml is reached; Ce is the AUC0-24/MIC ratio in the effect compartment (serum); EC50 is the AUC0-24/MIC of drug producing 50% of the maximal antibacterial effect; and N is the Hill coefficient, which describes the steepness of the AUC0-24/MIC effect curve. Because the drug is inhibitory in this investigation, Emax represents the baseline bacterial count, and E0 is the maximal effect (1). These PD indices were calculated by using the WinNonlin nonlinear regression program.
RESULTS
No adverse effects from drug administration were noted during this study. The relevant PK parameters derived from noncompartmental analysis of the i.v. and i.m. data are summarized in Table 1. Mean serum concentrations obtained are shown in Fig. 1. A rapid and nearly complete absorption was observed, with a MAT of 0.51 h and mean absolute bioavailability of 100.1%. The Cmax of 1.15 ± 0.14 μg/ml was reached at 1.15 ± 0.37 h. Orbifloxacin was eliminated, with elimination half-lives of 4.23 ± 0.2 h and 3.95 ± 0.15 h after i.v. and i.m. administration, respectively.
TABLE 1.
PK parameters of orbifloxacin after a single i.v. and i.m. injection at a dose of 2.5 mg/kg to beagle dogsa
| Parameterb | Orbifloxacin injection via:
|
|
|---|---|---|
| i.v. route | i.m. route | |
| Tmax (h) | 1.15 ± 0.37 | |
| Cmax (μg/ml) | 1.15 ± 0.14 | |
| AUC0-24 (μg·h/ml) | 8.07 ± 0.69 | 8.37 ± 0.94 |
| AUC0-∞ (μg·h/ml) | 8.21 ± 0.6 | 8.49 ± 0.93 |
| λz (1/h) | 0.17 ± 0.01 | 0.17 ± 0.01 |
| t1/2 λz (h) | 4.23 ± 0.2 | 3.95 ± 0.15 |
| MRT0-24 (h) | 4.71 ± 0.13 | 5.31 ± 0.17 |
| MRT0-∞ (h) | 5.13 ± 0.13 | 5.69 ± 0.11 |
| Vλz (liters/kg) | 1.95 ± 0.23 | |
| Vss (liters/kg) | 1.61 ± 0.13 | |
| CL (liters/kg/h) | 0.31 ± 0.03 | |
| MAT (h) | 0.51 ± 0.15 | |
| F (%) | 100.1 ± 4.76 | |
Values are means ± standard errors of the means; n = 6 beagle dogs.
Tmax, time of maximum observed concentration; λz, first-order rate constant associated with the terminal portion of the curve; t1/2 λz, terminal half-life; Vλz, apparent volume of distribution; Vss, volume of distribution at steady state; CL, total body clearance; F, bioavailability.
FIG. 1.
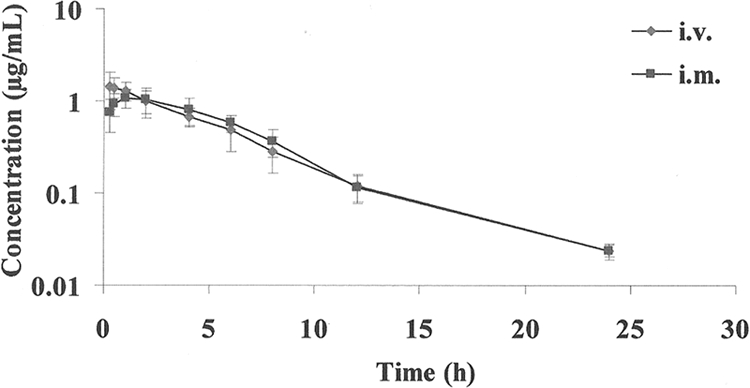
Semilogarithmic plot of serum concentration versus time of orbifloxacin in beagle dogs (n = 6), following a single i.v. or i.m. dose of 2.5 mg/kg. Bars represent standard deviations.
The MIC of orbifloxacin was determined in both broth and serum against clinical isolates from dogs and ATCC strains. No difference was observed between the two fluids. The MIC of orbifloxacin for both strains of E. coli was 0.06 μg/ml. S. aureus and S. intermedius isolates both had an orbifloxacin MIC of 0.25 μg/ml. The ATCC strain of S. aureus had a higher MIC value of 0.5 μg/ml, and the MIC of P. mirabilis was 0.5 μg/ml.
The PK-PD parameters obtained by integration of the in vivo PK data and in vitro MICs for the clinical strains are presented in Table 2. The AUC0-24/MIC values obtained for the E. coli test strain were 134.6 h and 129.8 h for i.v. and i.m. injections, respectively. The i.m. Cmax/MIC ratio obtained was 19.1. The orbifloxacin concentration remained above the MIC of the E. coli test strain for 21.1 h and 21.3 h following i.v. and i.m. injection, respectively. Owing to their similar MICs, similar in vivo serum AUC0-24/MIC ratios of 32.3 h and 31.1 h following i.v. and i.m. injection, respectively, and an i.m. Cmax/MIC ratio of 4.61 were obtained for both S. aureus and S. intermedius test strains. The T > MICs obtained were 8.75 h and 9.15 h after i.v. and i.m. injections, respectively. The corresponding AUC0-24/MICs obtained for the P. mirabilis strain were 16.1 h and 15.5 h. The i.m. Cmax/MIC ratio obtained was 2.3. The orbifloxacin concentration rapidly decreased to less than the MIC of P. mirabilis (5.6 h and 6.5 h after i.v. and i.m. injections, respectively).
TABLE 2.
PD predictors of antimicrobial activity based on in vitro data, following i.m. and i.v. administration of 2.5 mg/kg orbifloxacin to beagle dogs
| Clinical isolate | PD predictors used for determination of antimicrobial activitya
|
||||
|---|---|---|---|---|---|
| AUC0-24/MIC (h)
|
T > MIC (h)
|
Cmax/MIC
|
|||
| i.v. route | i.m. route | i.v. route | i.m. route | i.m. route | |
| E. coli | 134.6 ± 11.5 | 129.8 ± 16.1 | 21.1 ± 0.25 | 21.3 ± 0.54 | 19.1 ± 1.9 |
| S. aureus | 32.3 ± 2.7 | 31.1 ± 3.9 | 8.75 ± 0.53 | 9.15 ± 0.55 | 4.61 ± 0.46 |
| S. intermedius | 32.3 ± 2.7 | 31.1 ± 3.9 | 8.75 ± 0.53 | 9.15 ± 0.55 | 4.61 ± 0.46 |
| P. mirabilis | 16.1 ± 1.3 | 15.5 ± 1.9 | 5.6 ± 0.47 | 6.5 ± 0.41 | 2.3 ± 0.23 |
Values are means ± standard errors of the means; n = 6 beagle dogs.
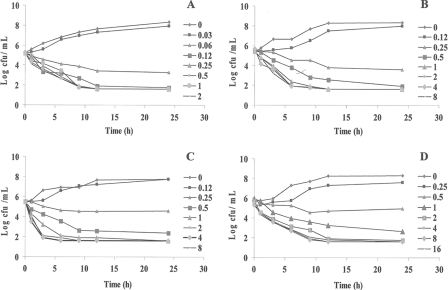
The in vitro killing curves against the E. coli, S. aureus, S. intermedius, and P. mirabilis clinical strains are presented in Fig. 2. The killing profile of orbifloxacin against all strains was concentration dependent, increasing the drug concentration leading to more-rapid killing. At 2× MIC and all higher concentrations, either bactericidal activity or elimination of E. coli was observed after 6 h of incubation. At 2× MICs of the S. aureus, S. intermedius, and P. mirabilis strains, bactericidal action was observed after 6 to 9 h of incubation, whereas only 6 h of incubation was sufficient to kill or eliminate all bacteria (LOD, 40 CFU/ml) at all higher concentrations tested.
FIG. 2.
In vitro killing curves for orbifloxacin against E. coli (A), S. aureus (B), S. intermedius (C), and P. mirabilis (D) clinical isolates. Numerical values on the right for each panel are orbifloxacin concentrations (μg/ml).
The ex vivo antibacterial activity of orbifloxacin was determined in serum against the S. intermedius test strain at predetermined time points using serum samples collected before and at 1, 2, 4, 6, 8, 12, and 24 h after i.m. administration (when mean orbifloxacin concentrations were 1.09, 1.02, 0.81, 0.58, 0.37, 0.11, and 0.02 μg/ml, respectively) (Fig. 3). A rapid bactericidal action was exerted, and no bacteria was detected (LOD, 40 CFU/ml) after 24 h for all samples collected at time points between 1 and 6 h. Samples collected at 8 h exerted bactericidal action, and a small number of bacteria (102) remained after 24 h of incubation. No inhibition of growth occurred for samples collected at 12 and 24 h.
FIG. 3.
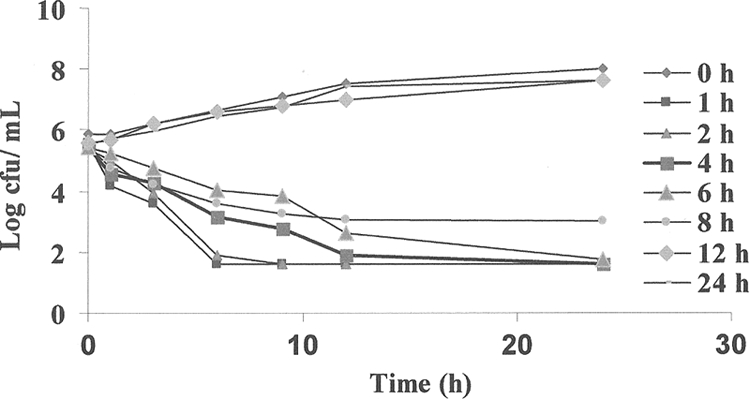
Ex vivo antibacterial activity of orbifloxacin against S. intermedius after i.m. administration at a dose rate of 2.5 mg/kg. Values are the means from six dogs. Samples were harvested at predetermined times between 0 and 24 h. Data for standard error of the mean values were excluded for clarity. At 0 h, the control serum contains no orbifloxacin.
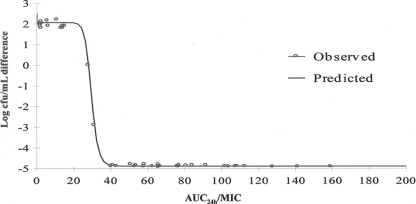
Integration of the serum PK and ex vivo data obtained for the S. intermedius strain, by using the inhibitory form of the sigmoid Emax equation, provided numerical values of ex vivo AUC0-24/MIC required for various degrees of bacterial inhibition (Table 3). The relationship between bacterial counts and AUC0-24/MIC ratios is presented in Fig. 4. The calculated mean AUC0-24/MIC ratios for serum that produced bacteriostasis (no change in the number of bacteria), bactericidal activity (a log3 reduction in the bacterial count), and elimination of bacteria (a reduction in the bacterial count to 40 CFU/ml) were 26.7, 31.8, and 40 h, respectively. The mean slope of the curve of the AUC0-24/MIC ratio versus the bacterial count was 11.7, which explains the relatively close bacteriostatic and bactericidal concentrations.
TABLE 3.
PK-PD integration of ex vivo data after i.m. administration of 2.5 mg/kg orbifloxacin
| Parametera | Ex vivo data after i.m. administration of orbifloxacinb
|
|
|---|---|---|
| Mean | SEM | |
| Log Emax (CFU/ml) | 2.06 | 0.05 |
| Log E0 (CFU/ml) | −4.87 | 0.04 |
| Log Emax − log E0 (CFU/ml) | 6.94 | 0.07 |
| AUC0-24/MIC for bacteriostatic action (h) | 26.7 | 1.1 |
| AUC0-24/MIC50 (h) | 29 | 0.22 |
| AUC0-24/MIC for bactericidal action (h) | 31.8 | 2.42 |
| AUC0-24/MIC for bacterial elimination (h) | 40 | 2.5 |
| Slope (N) | 11.7 | 1.43 |
PD data of the S. intermedius clinical isolate were used to compute all parameters. Emax, the difference in the number of bacteria (CFU/ml) in the control sample (absence of orbifloxacin) between 0 and 24 h; E0, the difference in the number of bacteria (CFU/ml) in the sample incubated with orbifloxacin between 0 and 24 h, when the LOD (40 CFU/ml) is reached; N, the Hill coefficient.
n = 6 beagle dogs.
FIG. 4.
Sigmoidal Emax relationship for bacterial count (log10 CFU/ml) versus the ex vivo AUC0-24/MIC ratio for S. intermedius in serum of dogs. The curve represents the line of predicted values, based on the sigmoid Emax equation, and the circles are the values for the individual animals.
DISCUSSION
A rapid absorption with complete i.m. bioavailability was observed for orbifloxacin in dogs. Similar values of i.m. bioavailability for orbifloxacin were reported in goats (105.01%), rabbits (109.87%), camels (97.47%), Korean Hanwoo cows (101.4%), and sheep (114%) (7, 10, 11, 17, 18). However, Davis et al. (6) reported a lower oral bioavailability (68.35%) for orbifloxacin in horses. A Cmax value of 1.15 μg/ml was achieved rapidly (1.15 h) following i.m. administration of orbifloxacin in dogs, and the value of Cmax obtained in this study was fairly comparable with results obtained for other species. The terminal half-lives of orbifloxacin after i.v. and i.m. injections were almost similar (harmonic means, 4.19 and 3.93 h, respectively), which indicates that absorption does not seem to affect the elimination of orbifloxacin in dogs. However, in a previous study, we observed absorption-dependent elimination (flip-flop kinetics) in cattle (7). The i.v. terminal half-life of orbifloxacin for this study was longer than those reported for goats (1.84 h), rabbits (2.5 h), sheep (3.16 h), and cows (3.2 h) (7, 17, 11, 18) but is lower than those reported for horses (5.08 h) and camels (5.74 h) (6, 11). The longer terminal half-life (7.1 h) after oral administration of orbifloxacin in dogs (14) compared to that for our study may be due to the different route of administration, analytical method (microbiological assay), or commercial preparation used in the study.
The AUC0-24 values after i.v. and i.m. injections of orbifloxacin in dogs were fairly close (8.07 and 8.37 μg·h/ml, respectively), indicative of approximate exposure following the two routes. These are within the range of AUC0-24 values reported for other species in the studies cited above, the lowest being for rabbits (5.74 and 6.75 μg·h/ml) and the highest for camels (10.34 and 10.45 μg·h/ml) for i.v. and i.m. routes, respectively. The apparent volume of distribution at steady state of 1.61 liters/kg in dogs suggests good penetration of orbifloxacin through biological membranes. This is in agreement with the reported wide body distribution of orbifloxacin and other fluoroquinolones in different species.
As presented in Table 2, the desired endpoints (a AUC0-24/MIC ratio of greater than 100 to 125 h or a Cmax/MIC ratio of greater than 8 to 10) (6, 21, 26) were reached only for the E. coli strain, whereas owing to their higher MICs (0.25 to 0.5 μg/ml), this dose is not likely to be adequate for the treatment of infections associated with the S. aureus, S. intermedius, and P. mirabilis strains tested here.
The in vitro and ex vivo killing studies indicate concentration-dependent activity of orbifloxacin; increasing the drug concentration led to more-rapid killing of all tested bacterial strains. By applying the inhibitory sigmoid Emax equation, the lowest effective ex vivo AUC0-24/MIC ratios required for different levels of antibacterial activity were determined in serum. At a dose of 2.5 mg/kg, the in vivo AUC0-24/MIC ratios achieved following both routes of administration (32.3 h and 31.1 h after i.v. and i.m. routes, respectively) were higher than the ex vivo AUC0-24/MIC ratio required for bacteriostatic action against S. intermedius (26 h) and comparable with the ex vivo AUC0-24/MIC ratio required for bactericidal action (31.8 h). However, it is lower than the corresponding value required to ensure elimination of the S. intermedius strain (40 h), suggesting the inadequacy of the experimental dose in treating infections associated with the S. intermedius strain tested here.
Based on the calculated PK values and PK-PD values generated from the inhibitory sigmoid Emax equation, an optimal dosage that provides a specific desired effect could be calculated using this equation described elsewhere (27):
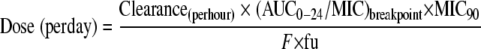 |
From different animal studies, the protein binding of orbifloxacin is estimated to be 20% (6, 7, 10). Therefore, the free/unbound fraction of orbifloxacin (fu) would be 0.8. For the i.m. bioavailability (F) of 1, ex vivo AUC0-24/MIC ratio of 40 h (bacterial elimination), and the MIC of the S. intermedius strain of 0.25 μg/ml obtained in this study, the calculated daily dose for orbifloxacin is 3.9 mg/kg. However, this value is based upon a single strain. Rather, it would be preferable to base a dose upon estimates of MIC90 values to accommodate the potential needs of the entire patient population. The MIC90 of the S. intermedius isolates from dogs has been reported to be 0.5 μg/ml (8, 9, 15). Accordingly, the calculated daily dose of orbifloxacin against S. intermedius for the MIC90 of 0.5 μg/ml would be 7.7 mg/kg, which is higher than the recommended range for dogs (2.5 to 7.5 mg/kg). Considering the values of the Cmax/MIC ratio (19.1) and the in vivo AUC0-24/MIC ratio (129.8 h) obtained in this study, which are well above the generally recommended cutoff values for fluoroquinolones, one could predict that orbifloxacin at an i.m. dose of 2.5 mg/kg per day would be effective against strains of E. coli likely to be encountered under clinical field conditions.
Repeated exposure to suboptimal antibiotic concentrations is the most important risk factor for the development of bacterial resistance to anti-infective agents (4). The experimental dosage used here seems to be sufficient for some strains like E. coli; however, it is far from reaching the cutoff values for the activity of fluoroquinolones for Staphylococcus and Proteus test strains due to their higher MICs. This is in agreement with a previous finding by Boothe et al. (3), in which recommended doses of orbifloxacin failed to achieve targeted indices of predicted efficacy. Therefore, a higher dose of orbifloxacin would be worthy of consideration for treatment of certain bacterial infections in dogs.
Acknowledgments
We are grateful to Seung-Hee Jang at the veterinary pharmacology and toxicology laboratory of Kyungpook National University for his assistance. The kind provision of bacterial strains by Kim Young Hoan is gratefully acknowledged.
This work was supported by the Korea Research Foundation grant funded by the Korean Government (KRF-2008-521-E00146).
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Aliabadi, F. S., B. H. Ali, M. F. Landoni, and P. Lees. 2003. Pharmacokinetics and PK/PD modeling of danofloxacin in camel serum and tissue cage fluids. Vet. J. 165:104-118. [DOI] [PubMed] [Google Scholar]
- 2.Aliabadi, F. S., and P. Lees. 2002. Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of marbofloxacin in calf serum, exudate and transudate. J. Vet. Pharmacol. Ther. 25:161-174. [DOI] [PubMed] [Google Scholar]
- 3.Boothe, D. M., A. Boeckh, R. B. Simpson, and K. Dubose. 2006. Comparison of pharmacodynamic and pharmacokinetic indices of efficacy of 5 fluoroquinolones toward pathogens of dogs and cats. J. Vet. Intern. Med. 20:1297-1306. [DOI] [PubMed] [Google Scholar]
- 4.Burgess, D. S. 1999. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest 115:19S-23S. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved guideline M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Davis, J. L., M. G. Papich, and A. Weingarten. 2006. The pharmacokinetics of orbifloxacin in the horse following oral and intravenous administration. J. Vet. Pharmacol. Ther. 29:191-197. [DOI] [PubMed] [Google Scholar]
- 7.Elias, G., J.-S. Lee, M.-H. Hwang, Y.-S. Park, K.-H. Cho, Y.-H. Kim, and S.-C. Park. 2009. Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of orbifloxacin in Korean Hanwoo cattle. J. Vet. Pharmacol. Ther. 32:219-228. [DOI] [PubMed] [Google Scholar]
- 8.Fujii, T., S. Takatsu, M. Stegemann, M. Ogata, and S. I. Kamata. 2007. Clinical efficacy and safety of an injectable formulation of cefovecin in the treatment of bacterial skin infections of dogs. Jpn. J. Vet. Dermatol. 13:81-88. [Google Scholar]
- 9.Ganière, J. P., C. Mèdaille, and F. Etorè. 2004. In vitro antimicrobial activity of orbifloxacin against Staphylococcus intermedius isolates from canine skin and ear infections. Res. Vet. Sci. 77:67-71. [DOI] [PubMed] [Google Scholar]
- 10.Goudah, A., and K. Abo-El-Sooud. 2008. Pharmacokinetics and milk penetration of orbifloxacin after intravenous and intramuscular injections to dromedary lactating camels (Camelus dromedaries). J. Vet. Pharmacol. Ther. 31:276-280. [DOI] [PubMed] [Google Scholar]
- 11.Goudah, A., H. J. Cho, H. C. Shin, J. H. Shim, N. L. Regmi, M. Shimoda, and A. M. Abd El-Aty. 2008. Pharmacokinetics and milk distribution characteristics of orbifloxacin following intravenous and intramuscular injection in lactating ewes. J. Vet. Pharmacol. Ther. doi: 10.1111/j.1365-2885.2008.01046.x. [DOI] [PubMed]
- 12.Haines, G. R., M. P. Brown, R. R. Gronwall, K. A. Merritt, and L. K. Baltzley. 2001. Pharmacokinetics of orbifloxacin and its concentration in body fluids and in endometrial tissues of mares. Can. J. Vet. Res. 65:181-187. [PMC free article] [PubMed] [Google Scholar]
- 13.Haritova, A. M., N. V. Rusenova, P. R. Paranov, L. D. Lashev, and J. Fink-Gremmels. 2006. Pharmacokinetic-pharmacodynamic modelling of danofloxacin in turkeys. Vet. Res. Commun. 30:775-789. [DOI] [PubMed] [Google Scholar]
- 14.Heinen, E. 2002. Comparative serum pharmacokinetics of the fluoroquinolones enrofloxacin, difloxacin, marbofloxacin, and orbifloxacin in dogs after single oral administration. J. Vet. Pharmacol. Ther. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 15.Iherke, P. J., M. G. Papich, and T. C. Demanuelle. 1999. The use of fluoroquinolones in veterinary dermatology. Vet. Dermatol. 10:193-204. [DOI] [PubMed] [Google Scholar]
- 16.Kay-Mugford, P. A., A. J. Weingarten, M. Ngoh, R. Zolynas, A. White, T. Katz, R. Simmons, and K. J. Varma. 2002. Determination of plasma and skin concentrations of orbifloxacin in dogs with clinically normal skin and dogs with pyoderma. Vet. Ther. 3:402-408. [PubMed] [Google Scholar]
- 17.Marin, P., E. Escudero, E. Fernandez-Varon, and C. M. Carceles. 2007. Pharmacokinetics and milk penetration of orbifloxacin after intravenous, subcutaneous, and intramuscular administration to lactating goats. J. Dairy. Sci. 90:4219-4225. [DOI] [PubMed] [Google Scholar]
- 18.Marin, P., E. Fernandez-Varon, E. Escudero, and C. M. Carceles. 2008. Pharmacokinetic-pharmacodynamic integration of orbifloxacin in rabbits after intravenous, subcutaneous and intramuscular administration. J. Vet. Pharmacol. Ther. 31:77-82. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto, S., M. Nakai, M. Yoshida, and H. Katae. 1998. Absorption, distribution and excretion of orbifloxacin in swine and calves. J. Jpn. Vet. Med. Assoc. 51:13-18. (In Japanese with English abstract.) [Google Scholar]
- 20.Matsumoto, S., M. Takahashi, N. Kitadai, and H. Katae. 1998. A study of metabolites isolated from the urine samples of cats and dogs administered orbifloxacin. J. Vet. Med. Sci. 60:1259-1261. [DOI] [PubMed] [Google Scholar]
- 21.Mckellar, Q. A., S. F Sanchez Bruni, and D. G. Jones. 2004. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 27:503-514. [DOI] [PubMed] [Google Scholar]
- 22.McKinnon, P. S., and S. L. Davis. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur. J. Clin. Microbiol. Infect. Dis. 23:271-288. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, M., A. de la Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papich, M. G., and J. E. Riviere. 2001. Fluoroquinolone antimicrobial drugs, p. 898-917. In H. R. Adams (ed.), Veterinary pharmacology and therapeutics, 8th ed. Iowa State University Press, Ames.
- 25.Scott, D. W., J. Peters, and W. H. Miller, Jr. 2006. Efficacy of orbifloxacin tablets for the treatment of superficial and deep pyoderma due to Staphylococcus intermedius infection in dogs. Can. Vet. J. 47:999-1002. [PMC free article] [PubMed] [Google Scholar]
- 26.Toutain, P. L., J. R. E. Del Castillo, and A. Bousquet-Mélou. 2002. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 73:105-114. [DOI] [PubMed] [Google Scholar]
- 27.Toutain, P. L., A. Bousquet-Mélou, and M. Martinez. 2007. AUC/MIC: a PK/PD index for antibiotics with time dimension or simply a dimensionless scoring factor. J. Antimicrob. Chemother. 60:1185-1188. [DOI] [PubMed] [Google Scholar]