Abstract
Valganciclovir (VGC) is an oral prodrug of ganciclovir (GCV) recently introduced for prophylaxis and treatment of cytomegalovirus infection. Optimal concentration exposure for effective and safe VGC therapy would require either reproducible VGC absorption and GCV disposition or dosage adjustment based on therapeutic drug monitoring (TDM). We examined GCV population pharmacokinetics in solid organ transplant recipients receiving oral VGC, including the influence of clinical factors, the magnitude of variability, and its impact on efficacy and tolerability. Nonlinear mixed effect model (NONMEM) analysis was performed on plasma samples from 65 transplant recipients under VGC prophylaxis or treatment. A two-compartment model with first-order absorption appropriately described the data. Systemic clearance was markedly influenced by the glomerular filtration rate (GFR), patient gender, and graft type (clearance/GFR = 1.7 in kidney, 0.9 in heart, and 1.2 in lung and liver recipients) with interpatient and interoccasion variabilities of 26 and 12%, respectively. Body weight and sex influenced central volume of distribution (V1 = 0.34 liter/kg in males and 0.27 liter/kg in females [20% interpatient variability]). No significant drug interaction was detected. The good prophylactic efficacy and tolerability of VGC precluded the demonstration of any relationship with GCV concentrations. In conclusion, this analysis highlights the importance of thorough adjustment of VGC dosage to renal function and body weight. Considering the good predictability and reproducibility of the GCV profile after treatment with oral VGC, routine TDM does not appear to be clinically indicated in solid-organ transplant recipients. However, GCV plasma measurement may still be helpful in specific clinical situations.
Valganciclovir (VGC), a prodrug ester of ganciclovir (GCV) and l-valine, has been developed to overcome the poor oral bioavailability of GCV, which limits its exposure after oral administration. GCV administered as VGC is characterized by a 10-fold-higher oral bioavailability (6, 10, 19, 24), with a VGC dose of 900 mg once daily providing a systemic exposure comparable to that of intravenous GCV at 5 mg/kg (6, 19, 24). The efficacy of GCV delivered as VGC has been established for the prevention of cytomegalovirus (CMV) disease in kidney, heart, and kidney-pancreas recipients at high risk for developing it (i.e., CMV-seropositive donor/CMV-seronegative recipient [D+/R−]) (21) and recently for the treatment of CMV disease in organ transplant recipients (2).
After administration, VGC is absorbed by peptide transporters through the intestinal epithelium and hydrolyzed into GCV, which is only 1 to 2% bound to plasma protein (5) and extensively eliminated through the kidney by both glomerular filtration and tubular secretion. Thus, in renal insufficiency, the dosage of VGC has to be adjusted to the estimated glomerular filtration rate (GFR) (6). GCV is secreted through the organic anion transporters (OAT) (14) and therefore at risk for drug interactions with transport inhibitors. Other factors could also influence VGC pharmacokinetics, including a patient's body weight (BW), gender, and comorbidities. Pharmacokinetic variability represents a potential nuisance for drug efficacy and safety, if it does not receive proper consideration on prescription. The potential burden of CMV infection plays a significant role after transplantation in terms of morbidity and mortality (8), and the incidence of infection is nonnegligible in CMV D+/R− patients in the absence of preventive treatment (45% according to Lowance et al. [18]). Thus, insufficient GCV exposure may lead to breakthrough viremia, especially in high-risk patients, or to the selection of resistant strains, as reported with oral GCV (16). On the other hand, overexposure enhances the risk of dose-dependent hematologic toxicity (28). The maintenance of circulating concentrations inside an effective and safe range is thus of therapeutic importance. This goal requires not only that dosages are adjusted to patient factors affecting VGC absorption and GCV disposition but also that VGC absorption and GCV disposition are sufficiently reproducible and predictable, knowing those factors. Otherwise, therapeutic drug monitoring (TDM) may represent a useful alternative to compensate for high interpatient variability (27).
The objectives of the present study were (i) to describe the population pharmacokinetic profile of GCV delivered as VGC and its variability in solid-organ transplant recipients receiving oral VGC for either oral VGC prophylaxis or treatment, (ii) to define clinical factors that could explain interpatient differences, and (iii) to explore the relation between GCV profile and efficacy and tolerability outcomes. Our findings thus help us to evaluate the usefulness of GCV TDM after VGC administration in solid-organ transplant patients.
MATERIALS AND METHODS
Patient population.
This prospective observational study was conducted from November 2005 to January 2008 at the University Hospital of Lausanne (CHUV) and the University Hospital of Geneva (HUG) in Switzerland. Protocols were approved by local ethics committees. Adult solid-organ transplant patients at risk for CMV infection (donor or recipient CMV seropositive) receiving oral VGC prophylaxis, oral VGC treatment, or intravenous GCV treatment were enrolled consecutively after giving their written informed consent. Exclusion criteria were failure to provide informed consent or known intolerance to GCV or VGC. A 3-month course of VGC prophylaxis was administered from day 3 posttransplantation, except in lung transplant recipients that were donor seropositive and recipient seronegative who received VGC prophylaxis for 6 months. The VGC prophylactic dosage was 900 mg once daily in heart and lung recipients. Kidney transplant recipients, having on average a slight degree of renal impairment, received 450 mg once daily. Further dose adjustment to renal function was applied according to the manufacturer's recommendations. VGC therapeutic dosage for CMV disease (25) was 900 mg twice daily, adjusted to the renal function. Two patients had to receive intravenous GCV treatment. The dosage was of 5 mg/kg every 12 h, with further adjustment to renal function. GCV levels were measured monthly both at the trough point and ∼3 h after oral or intravenous administration during prophylaxis and at weekly intervals during treatment. During prophylaxis, the first sample was collected after at least 3 days of VGC administration, and the next one was given about 1 or 2 months later. Intensive pharmacokinetic data (rich data) obtained in two kidney recipients were also included in the analysis.
For each patient, the gender, height, age, graft type, CMV serostatus (both donor and recipient), and comorbidities were recorded. Samples were generally obtained under steady-state conditions (i.e., drug regimen unchanged for at least 3 days). However, when this condition was not reached, the detailed dosing schedule was recorded during the last 3 days. Actual dosing and sampling time information was carefully recorded on each sampling occasion, along with patient's BW, serum creatinine, concomitant medications, adverse events (nausea, diarrhea, skin toxicity, anemia, leucopenia, neutropenia, thrombocytopenia, and liver enzyme elevation). Adverse events were recorded as present or absent, including anemia (hemoglobin <10.4 g/liter [male] or <9.9 g/liter [female]), leucopenia (leukocytes <3.5 g/liter), neutropenia (neutrophils <2.0 g/liter), thrombopenia (platelets <140 g/liter), and liver enzyme elevation (aspartate aminotransferase and alanine aminotransferase, >1.1 upper limit of normal range).
Analytical method.
Blood samples (5.5 ml) were collected into Monovettes (Sarstedt, Nümbrecht, Germany), with K-EDTA as an anticoagulant. The samples were sent without delay to the laboratory, and plasma was isolated by centrifugation and stored at −20°C to ensure stability up to the time of analysis. Plasma GCV levels were determined by reversed-phase high-performance liquid chromatography coupled with spectrofluorimetric detection according to a validated method (22). The calibration curve is linear between 0.1 and 10 mg/liter. The mean absolute recovery of GCV was 100.3% ± 2.5%. The method is precise (with mean interday coefficients of variation [CVs] within 1.2% to 3.5%) and accurate (range of interday deviations, −0.4% to +1.4%).
Model-based pharmacokinetic analyses.
The analysis was performed by using the NONMEM computer program written in FORTRAN 77 (version VI, with NM-TRAN version II) (3) running on a mainframe station (Sun Fire 3800 server with UltraSPARC III processors; Sun Santa Clara). It uses mixed (fixed and random) effects regression to estimate population means and variances of the pharmacokinetic parameters and to identify factors that influence them.
Structural model.
The following stepwise procedure was used (see model selection below): first, one- and two-compartment models with first-order absorption from the gastrointestinal tract for oral VGC were compared based on the data from the two patients who underwent intensive kinetic investigation (rich data) and from the entire population (sparse data). The estimated parameters were the systemic clearance (CL), the intercompartmental clearance (Q), the central volume of distribution (V1), the peripheral volume of distribution (V2), and the absorption rate constant (ka). Since GCV was administered intravenously only in two patients, the bioavailability (F) could not be estimated with sufficient accuracy and was fixed at 0.6 according to previous studies (6, 10, 19, 24). Derived parameters were the absorption half-life [t1/2a = ln(2)/ka] and the elimination half-life [t1/2β = ln(2)/λβ, with λβ derived from CL, Q, V1, and V2].
Statistical model.
Exponential errors following a log-normal distribution were assumed for the description of interpatient variability of the pharmacokinetic parameters and were of the form θj = θeηj, where θj is the individual pharmacokinetic parameter value in the jth individual, θ is the population parameter estimate, and ηj is the random effect value, which is independently and normally distributed with a mean of 0 and variance ω. Considering potential modifications in the patients’ condition over time as a consequence of changes in pathophysiological processes (blood samples were drawn a few days to many months after transplantation), an interoccasion variability (12) was assigned on clearance that accounted for three different occasions: up to month 1, up to month 2, and up to month 6 or more of the posttransplantation period. A specific model for time-varying creatinine clearance (31) was also tested. Proportional and combined proportional-and-additive error models were compared to describe intrapatient (residual) variability.
Covariate model.
The covariate analysis was performed by using a stepwise insertion/deletion approach. Visual inspection of the correlation between post hoc individual parameter estimates and the available covariates (demographic characteristics, comorbidities, and concomitant medications) was first conducted by graphical exploration. Potentially influential covariates were then incorporated sequentially into the pharmacokinetic model. The typical value of a given parameter θ (e.g., CL) was modeled to depend either linearly on the covariate X (general equation: θ = θa·X, where θa is the estimated coefficient) or as a power function for categorical covariates (general equation: θ = θa·θbX, where θa is the estimate of the basal value and θb is the contribution of the factor X). Covariates (X) evaluated for inclusion during the model building process were gender (sex), age, BW, height, GFR, comorbidities, and concomitant medications. Concomitant medications included the presence or absence of calcineurin inhibitors (ICAL) (tacrolimus or cyclosporine), mycophenolate (MMF), cotrimoxazole (COTM), and OAT inhibitors (14). Two different equations for the estimation of GFR were compared: (i) the traditional Cockroft-Gault equation, GFRC-G = [(150 − age) × BW]/Crs × 1.1 (if male) or × 0.9 (if female) (4), and (ii) the four-variable modification of diet in renal disease (MDRD) formula with individual body surface area, GFRMDRD = 175 × (Crs/88.4)−1.154 × age−0.203 × 0.742 (if female) (15), where Crs is the standardized serum creatinine value expressed in μmol/liter and the age is given in years. This simplified four-variable MDRD formula was shown to accurately predict GFR in kidney transplant patients (7).
At the end of the analysis, all patient characteristics showing an influence on the parameters were again confirmed by comparing the full model (with all factors included) to models from which each of the factors was removed sequentially.
Model selection and parameter estimation.
The models were fitted by use of the first-order conditional method (and three significant digits) with the subroutine ADVAN 4, TRANS 4. Goodness-of-fit statistics and graphical displays were used to compare models on each step of model building. The goodness-of-fit criterion was the change in the objective function (ΔOF) resulting from the addition of one covariate, which approximates a χ2 distribution and can be regarded as statistically significant (P < 0.05) if it exceeds 3.8. A simulation based on the final pharmacokinetic estimates was performed with NONMEM using 1,000 individuals to calculate 95% prediction intervals of the concentrations versus time curve. Those individuals were taken as 70-kg male kidney transplant patients with GFRMDRD of 50 ml/min/1.73 m2. The figures were generated with GraphPad Prism (version 4).
Concentration-effect analyses.
Individual Bayesian estimates of the GCV trough concentration (Ctrough) and the area under the curve (AUC) obtained through NONMEM were used to explore the relationship with prophylaxis outcomes (breakthrough viremia during prophylaxis and 3 months beyond) and tolerability (nausea, diarrhea, skin toxicity, anemia, leucopenia, neutropenia, thrombocytopenia, or liver enzyme elevation on sampling time). CMV viremia was detected by CMV DNA PCR (20) and recorded as either negative (limit of detection = 100 to 1,000 copies/ml depending on the cell count) or positive (limit of quantification = 1,000 copies/ml). Adverse events were recorded as present or absent based on the criteria presented above.
The relationship between GCV AUC and Ctrough and those outcomes was assessed by using logistical regression analyses. A sample-level analysis (individual estimates) was complemented with a patient-level analysis (mean estimates) when significant, with a statistical significance level assigned at P ≤ 0.05 for model improvement by the pharmacokinetic predictor (chi-square test, one-tailed distribution). Statistical analyses were performed using STATA software (version 8.2).
RESULTS
Data.
A total of 437 GCV plasma samples from 65 solid organ transplant patients were included in the population analysis (41 kidney, 10 heart, 12 lung, and 2 liver recipients). Blood samples were drawn from 55 patients receiving oral VGC prophylaxis (n = 330), from 5 patients receiving oral VGC treatment for CMV infection or disease (n = 52), from 3 patients receiving both successive regimens (n = 23 and 26, respectively), and from 2 patients receiving intravenous GCV treatment for CMV disease (n = 6). Eight patients were not enrolled due to transfer to another hospital or refusal. A median (range) of 6 (1 to 22) samples per subject were available. In addition to this sparse sampling data set, four full concentration-time profiles at steady state were available from two patients under VGC 450 mg once daily (n = 26, six to seven time points per profile before the dose and from 2 to 24 h after drug intake: rich data set). Among the 437 GCV samples used for model building, 197 (45%) were collected up to 6 h after dosing, 46 (11%) were obtained between 6 and 14 h, 168 (38%) were obtained between 14 and 26 h after dosing, and the remaining 26 (6%) were collected later than 26 h after drug intake (with a maximum of 75 h). Table 1 lists the patients’ characteristics.
TABLE 1.
Characteristics of patients evaluated in the population pharmacokinetic analysis of VGC
| Patients (n = 65) | Value | % of total or range |
|---|---|---|
| Baseline characteristics | ||
| Sex (men/women) (no.) | 45/20 | 69/31 |
| Median age (yr) | 55 | 18-70 |
| Median body wt (kg) | 72 | 46-115 |
| Median ht (cm) | 172 | 147-192 |
| Median creatinine (μmol/liter) | 108 | 29-691 |
| Graft type (no. of patients) | ||
| Kidney | 41 | 63 |
| Heart | 10 | 15 |
| Lung | 12 | 18 |
| Liver | 2 | 3 |
| CMV serostatus (no. of patients) | ||
| D+/R− | 22 | 34 |
| D+/R+ | 28 | 43 |
| D−/R+ | 15 | 23 |
| Comorbidity (no. of patients) | ||
| Cardiopathy | 19 | 29 |
| Overweight | 9 | 14 |
| Cystic fibrosis | 1 | 2 |
| Samples (n = 437) | ||
| Drug (no. of samples) | ||
| VGC: CMV prophylaxis | 353 | 81 |
| VGC: CMV therapy | 78 | 18 |
| GCV: CMV therapy | 6 | 1 |
| Concomitant medications | ||
| Tacrolimus/cyclosporine (ICAL) | 347/90 | 79/21 |
| Mycophenolate (MMF) | 374 | 86 |
| Cotrimoxazole (COTM) | 321 | 74 |
| OAT inhibitors | 354 | 73 |
Population pharmacokinetic analyses.
The model-building process (structure, variability) is shown in Table 2. A two-compartment model with first-order absorption from the gastrointestinal tract appropriately described the data (both rich and sparse) (ΔOF = −37.5). Since GCV is known to be almost exclusively eliminated by the kidney, GFRC-G was introduced as a covariate on CL at an early step, improving significantly the description of the data (ΔOF = −200.9). An interpatient variability was best assigned to both CL and V1. The use of a proportional plus additive error model for the residual intrapatient variability was the most satisfactory at this early step.
TABLE 2.
Summary of the model-building steps of the population pharmacokinetic analysis of VGCa
| Hypothesis | Model | θa | θb | θc | θd | θe | θf | ΔOFb | OFc |
|---|---|---|---|---|---|---|---|---|---|
| Model structure | |||||||||
| One-compartment, 1st order | ω(CL) | −4349.5 | |||||||
| Two-compartment, 1st order | ω(CL) | −37.5 | −4386.9 | ||||||
| Model variability (ω) | |||||||||
| GFRC-G on CL, variability on CL | CL = θa·GFRC-G ω(CL) | 1.41 | −200.9 | −4587.8 | |||||
| and variability on V1 | CL = θa·GFRC-G ω(CL, V1) | 1.41 | 21.6 | −20.3 | −4608.2 | ||||
| and variability on V1 and Q | CL = θa·GFRC-G ω(CL, V1, Q) | 0.83 | 8.6 | 69.3 | −4538.8 | ||||
| and variability on V1 and V2 | CL = θa·GFRC-G ω(CL, V1, V2) | 1.32 | 14.5 | −3.3 | −4611.5 | ||||
| Covariate analysis | |||||||||
| Four-variable MDRD estimated GFR | CL = θa·GFRMDRD | 1.52 | −2.9 | −4899.7 | |||||
| Extrarenal CL? | CL = θa·GFRC-G + θb | 1.35 | 0.015 | +0.8 | −4896.0 | ||||
| Does gender influence CL (GFRC-G)? | CL = θa·GFRC-G ·θbsex (sex = 0 if male, sex = 1 if female) | 1.27 | 1.23 | −10.5 | −4907.3 | ||||
| Does gender influence CL (GFRMDRD)? | CL = θa·GFRMDRD·θbsex (sex = 0 if male, sex = 1 if female) | 1.43 | 1.24 | −16.4 | −4913.2 | ||||
| Does ICAL influence CL? | CL = θa·GFRC-G·θbICAL | 1.40 | 0.79 | −16.6 | −4913.4 | ||||
| Does MMF influence CL? | CL = θa·GFRC-G·θbMMF | 1.36 | 0.99 | 0.0 | −4896.8 | ||||
| Does IOAT influence CL? | CL = θa·GFRC-G·θbIOAT | 1.37 | 0.98 | −0.3 | −4897.1 | ||||
| Does COTM influence CL? | CL = θa·GFRC-G·θbCOTM | 1.30 | 1.06 | −3.4 | −4900.2 | ||||
| Does Card influence CL? | CL = θa·GFRC-G·θbCard | 1.33 | 1.05 | −0.5 | −4897.3 | ||||
| Does graft type influence CL? | CL = θgraft·GFRC-G (K: θa, H: θb, Lu/Li: θc) | 1.50 | 0.90 | 1.31 | −34.1 | −4930.9 | |||
| Does BW influence V1? | V1 = θd·(BW/70) | 30.0 | −30.4 | −4927.2 | |||||
| Does gender influence V1? | V1 = θd·θesex (sex = 0 if male, sex = 1 if female) | 28.7 | 0.64 | −16.0 | −4912.8 | ||||
| Do BW and gender influence V1? | V1 = θd·(BW/70)·θesex (sex = 0 if male, sex = 1 if female) | 27.7 | 0.79 | −5.3 | −4932.4 | ||||
| Does HGT influence V1? | V1 = θd·(HGT/170) | 29.2 | −6.7 | −4903.5 | |||||
| Do BW and HGT influence V1? | V1 = θd·(BW/70)·(HGT/170) | 26.4 | −1.5 | −4928.6 | |||||
| Does graft type influence V1? | V1 = θgraft·(BW/70) (K: θd, H: θe, Lu/Li: θf) | 30.1 | 29.6 | 30.2 | −30.4 | −4927.2 | |||
| Simple model | CL = θa·GFRC-G | 1.35 | −4927.2 | ||||||
| V1 = θd·(BW/70) | 30.0 | ||||||||
| Interoccasion variability (IOV) on CL | CL = θa·GFRC-G | 1.39 | −62.1 | −4989.3 | |||||
| V1 = θd·(BW/70) | 23.2 | ||||||||
| Final model with IOV | CL = θgraft·GFRMDRD (K: θa, H: θb, Lu/Li: θc)·θdsex | 1.68 | 0.86 | 1.17 | 1.21 | −66.4 | −5055.7 | ||
| V1 = θe·(BW/70)·θfsex | 24.0 | 0.72 |
GFRC-G, GFR estimated with Cockroft-Gault formula (liters/h); ICAL (tacrolimus = 0, cyclosporine = 1); IOAT, OAT inhibitors; Card, cardiopathy; K, kidney recipients; H, heart recipients; IOV, interoccasion variability; Lu/Li, lung and liver recipients; GFRMDRD, GFR estimated with four-variable MDRD formula (liters/h); BW, body weight (in kg); HGT, height (in cm).
ΔOF, difference in the NONMEM objective function compared to the best previous model.
OF, NONMEM objective function.
The pharmacokinetic estimates and the variability (CV%) of the population model, with only GFRC-G as covariate on CL, were as follows: CL = 1.35·GFR liters/h (26%), Q = 3.1 liters/h, V1 = 28 liters (28%), V2 = 19.5 liters, ka = 0.65 h−1, and Ffixed = 0.6.
The model-building steps for the covariate analysis are detailed in Table 2. A time-varying creatinine clearance (31) did not improve the model. Inclusion of the covariates sex (ΔOF = −10.5) and graft type (ΔOF = −34.1) as modifiers of CL in addition to GFRC-G significantly improved the model. CL differed between female and male patients by 23%, which fairly corresponds to the correction factor for sex included in the Cockroft-Gault formula. The assignment of GFRMDRD rather than GFRC-G with the addition of the covariate sex on CL reduced the objective function (ΔOF = −16.4), with a difference of 24% remaining between females and males. The type of graft had a significant influence on CL, showing a 40 and 13% lower elimination of GCV in heart and lung/liver transplant patients, respectively, compared to kidney transplant recipients. Among the concomitant medications potentially influencing CL, only ICAL (ΔOF = −16.6) significantly improved the fit, showing a reduction in CL of 20% in patients receiving cyclosporine versus those under tacrolimus treatment.
Inclusion of the demographic covariates (BW, sex, height) on V1 significantly improved the pharmacokinetic model with a predominant effect from weight (ΔOF = −30.4). Multivariate confirmation of the significant covariates showed that only GFR, sex, and graft type remained statistically significant regarding CL (ΔOF = −46.7), and only BW and sex remained statistically significant regarding V1 (ΔOF = −35.6).
The introduction of a supplemental term for interoccasion variability, accounting for changing clearance over time from the graft, improved significantly the fit (ΔOF = −62.1). The proportional interpatient and interoccasion variability terms were 23 and 14%, respectively. At this last step, only the proportional error remained significant, while the additive component vanished out.
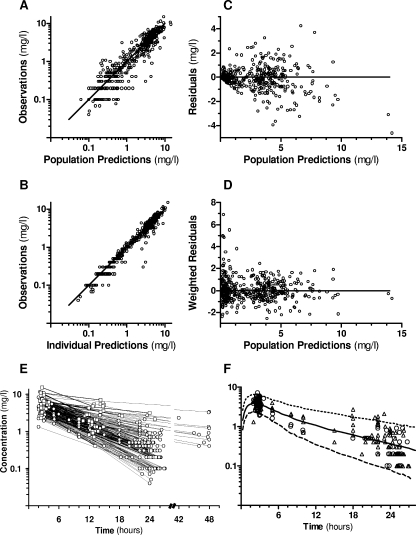
The parameter estimates for the final model are given in Table 3. Derived parameters were an absorption half-life (t1/2a) of 1.2 h and a median elimination half-life (t1/2β) of 8 h (range, 5 to 68 h). Figure 1 shows the overall goodness-of-fits plots and the concentration-time plot of the 36 kidney transplant recipients under VGC at 450 mg once daily, along with the average population prediction and 95% prediction interval for male kidney transplant patients receiving VGC at 450 mg once daily (GFRMDRD of 50 ml/min, body surface of 1.73 m2, and BW of 70 kg).
TABLE 3.
Population pharmacokinetic parameter estimates of VGC
| Parametera | Mean estimate (SE)
|
|
|---|---|---|
| Population (%)b | % Variabilityc | |
| F | 0.6 (fixed) | |
| ka (h−1) | 0.56 (19) | |
| CL (liters/h) = θGraftType·GFRMDRD (liters/h)·θfemalesex | 26 (54)* | |
| θkidney | 1.68 (5.5) | |
| θheart | 0.86 (14) | |
| θlung/liver | 1.17 (9.0) | |
| θfemale (male: sex = 0, female: sex = 1) | 1.21 (8.3) | |
| V1 (liters) = θBW·[BW (kg)/70 kg]·θfemalesex | 20 (75)* | |
| θBW | 24 (12) | |
| θfemale (male: sex = 0, female: sex = 1) | 0.78 (9.7) | |
| Q (liters/h) | 4.1 (19) | |
| V2 (liters) | 22 (7.4) | |
| IOV (CV %) | 12 (54)† | |
| σprop (CV %) | 21 (41)‡ | |
F, bioavailability; GFRMDRD four-variable MDRD estimated GFR; BW, body weight. The other abbrviations are as defined in the text. The IOV on CL is expressed as the CV (%). The residual variability (σprop) in the GCV plasma concentrations was associated with the proportional error term and is expressed as CV (%).
The standard errors (SE) of the estimates, calculated as SEestimate/estimate, expressed as a percentage, are given in parentheses.
Estimates of variability are expressed as the CV (%). *, Interpatient; †, interoccasion; ‡, residual. Standard errors (SE) of the variance components, calculated as √SEestimate/estimate, are expressed as a percentage.
FIG. 1.
Goodness-of-fit plots of the final model for VGC. (A) Log-log plot of observed concentrations versus population predictions. The line indicates the line of identity. (B) Log-log plot of observations versus individual predictions. The line indicates the line of identity. (C) Population residuals versus population predictions. The line is at ordinate value zero. (D) Population-weighted residuals versus population predictions. The dotted line is at ordinate value zero. (E) Plasma concentrations in 65 solid-organ transplant recipients receiving VGC prophylaxis (○) or treatment (□) of CMV infection/disease. (F) Pharmacokinetic profile of ganciclovir in a selection of 36 kidney transplant recipients under VGC at 450 mg once daily (men, ▵; women, ○) with average population prediction (solid line) and a 90% prediction interval (dashed lines) for male kidney transplant patients (GFR of 50 ml/min, body surface area of 1.73 m2, BW of 70 kg).
Prophylactic efficacy and tolerability.
Viremia was monitored during the 3-month prophylaxis and a further 3-month follow-up in 49 and 41 patients, respectively. During prophylaxis, CMV viremia was detected in three (6%) patients (one lung [D+/R−] and two heart [D+/R− and D+/R+] recipients); however, this did not exceed a low level (<100 copies/ml). No association between estimates of mean GCV exposure (AUC) or trough concentration and breakthrough viremia was noticed (P = 0.4 and = 0.2, respectively [chi-square test]). During the 3 months after prophylaxis cessation, CMV viremia was detected in 13 of 41 (32%) transplant recipients, including 2 patients (D+/R−) with CMV disease who were treated with oral VGC. The remaining 11 patients had low-grade viremia and were not treated with VGC. No association was observed either between estimates of GCV AUC or Ctrough and after prophylaxis viremia (P = 0.6 and = 0.7, respectively).
Regarding VGC prophylaxis and tolerability, nausea/vomiting was reported in 7% of patients, diarrhea was reported in 18% of patients, skin toxicity (nonserious) was reported in 9% of patients, anemia in 67%, leucopenia was reported in 14% of patients (neutropenia in 9% of patients), thrombocytopenia was reported in 16% of patients, and liver enzyme elevation was reported in 25% of patients. Per-sample analyses indicated a significant association between estimates of GCV AUC and the occurrence of anemia, neutropenia, and leucopenia and between Ctrough and diarrhea (P = 0.004 [chi-square test]). However, these associations did not retain a statistically significant level in per-patient analyses, except Ctrough and diarrhea (P = 0.009). Considering only patients under VGC prophylaxis, no significant association remained between Ctrough and the occurrence of diarrhea.
DISCUSSION
Oral VGC is currently supplanting GCV in most indications, including the prophylaxis of CMV infection in solid-organ transplant patients and the treatment of overt CMV infection. According to the manufacturer, VGC (similar to GCV) is to be adjusted to renal function. However, graft recipients most often receive the standard dosage regimen without regard to the type of organ transplant, BW, sex, associated comorbidities, and medications. Notably, a normal renal function will never be fully restored in most kidney transplant recipients. The characterization of VGC pharmacokinetic profile, the identification of influential covariates beyond GFR and the quantification of interpatient and intrapatient variability are important elements for evaluating the potential usefulness of more elaborated dosage adjustment recommendations, including a TDM strategy.
The pharmacokinetic results of our population analysis are in agreement with other recently reported estimates (1, 32). Derived parameters such as absorption and elimination half-life are also congruent with previous observations (1, 6, 32). Our population of patients is characterized by a wide range of renal function (GFRC-G range, 10 to 170 ml/min), leading to a comparable range of elimination half-life values.
The dominant influence of renal function on GCV clearance has been reported in previous population analyses (1, 6, 32). GFR can be estimated with the traditional Cockroft-Gault formula and by the MDRD formula, which has shown some advantages in renal transplant patients (26). A difference in clearance between male and female patients was observed beyond the factor of correction for sex included in both formulas and the calculation for individual body surface area. The higher clearance observed in females could be due to sex-related differences in OAT expression, as reported in the rat and mouse (9, 17). Our model includes a small but significant difference in the correlation between GFR and GCV clearance according to graft type. This effect could be explained by the difference in the patients’ drug regimens between types of transplantation. For example, the administration of an ICAL affected clearance significantly in the univariate analysis, but no more than when it was combined with graft type; noticeably, cyclosporine was almost exclusively received by heart transplant recipients, this drug being known to decrease effective renal plasma flow to a slightly larger extent than tacrolimus, for the same degree of immunosuppressive effect (13). Regarding concomitant medication, both MMF and trimethoprim have been shown to reduce GCV renal clearance (11, 34). Although both drugs were administered to a large proportion of our population of patients (in 86 and 73% of samples, respectively), no effect of MMF on the GCV pharmacokinetic profile could be detected. COTM, which was specifically investigated in two kidney transplant recipients, also did not show such an effect (data not shown). A large proportion of our patients received various agents reported as OAT inhibitors (such as omeprazole, acetylsalicylic acid, etc. [14]) that did not affect GCV CL in our analysis, probably because several redundant anion transporter systems exist in the kidney (29).
The interpatient variability in GCV clearance and the volume of distribution estimated in the present study are in accordance with recently reported data (1, 32), despite the inclusion of an interoccasion variability term in our model. An interoccasion variability was justified considering the sampling schedule for patients under VGC prophylaxis, with a first blood sample collected during the first week posttransplant and subsequent samples collected after 1 and 2 months.
Interpatient differences in oral absorption were not specifically identified during the NONMEM analysis and were therefore combined with interpatient variability in clearance and volume (since bioavailability was fixed in our model). The variability in both of those parameters remained limited, however (26 and 20%, respectively), meaning that the absorption and disposition profile of VCG was fairly reproducible and predictable in our population of transplant recipients.
Our pharmacodynamic analysis did not reveal any significant association between GCV exposure (AUC) or trough concentration and the occurrence of breakthrough viremia during prophylaxis or during the following 3 months after prophylaxis discontinuation. In a previous population pharmacokinetic-pharmacodynamic study including 240 D+/R− solid-organ transplant recipients, GCV systemic exposure appeared to correlate with antiviral activity in terms of the incidence of developing CMV viremia during prophylaxis (3 months posttransplantation) and for the following month (33). Among our population of D+/R− patients, 14% developed detectable low-grade CMV viremia during prophylaxis (compared to 12% reported by Wiltshire et al. [33]). Despite this comparable incidence, our analysis was certainly limited by the small number of patients. GCV needs to be bioactivated in cells into GCV triphosphate to inhibit virus replication. The GCV plasma concentration is thus only a surrogate of the actual active-form concentration, explaining the loose concentration-effect relation.
Our analysis did not detect significant relationships between GCV exposure or trough concentration and the occurrence of adverse events. Wiltshire et al. (33) reported a weak tendency toward increased neutropenia and leucopenia with high GCV exposure, but no association with anemia. Here again, our study population was probably inadequate to assess concentration-toxicity relationships. The identification of diarrhea as a concentration-related side effect was probably associated with patients treated for CMV disease (including some with CMV colitis) with a high dose of VGC. The occurrence of diarrhea may be related to a high dose of VGC and/or CMV colitis, a confounding factor that could not be circumvented. Taking into account all these elements, a routine clinical pharmacokinetic monitoring of VGC in solid-organ transplant recipients cannot be expected to be of much benefit. Nevertheless, in our experience, selective TDM of VGC appeared to be useful in specific clinical cases (e.g., unstable or not assessable renal function or continuous renal replacement therapy [23], iterative hemodialysis, and an unexplained absence of treatment response).
In conclusion, the pharmacokinetic parameters of VGC in a population of solid-organ transplant patients were adequately described by our population model, confirming a predominant role for renal function in clearance and for BW in the central volume of distribution. The type of transplant and gender also influenced clearance and the central volume of distribution, respectively. No drug interactions were found to impact VGC disposition. Only a limited degree of interpatient variability remained unexplained, which suggests a minor effect of additional unidentified covariates (e.g., genetic influences and absorption issues). Residual variability was moderate as well. Efficacy outcomes, as well as the occurrence of adverse events, did not correlate with drug exposure. This analysis highlights the importance of thorough adjustment of VGC dosage to renal function and BW. Considering the good predictability and reproducibility of the GCV profile after the administration of oral VGC in solid-organ transplant recipients, routine TDM does not appear to be clinically indicated. However, GCV plasma measurement may still be helpful in specific clinical situations.
Acknowledgments
Support for this study was partly provided by internal funds and partly by an unrestricted research grant from Roche (Switzerland) for studies related to transplantation. The CHUV transplantation center was supported by Institutional Strategic Plan 2004-2007.
The funding source had no role in the analysis and reporting of data or in the decision to submit the manuscript for publication. No potential financial conflict of interest is to be reported.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Armendariz, Y., A. Caldes, H. Colom, S. Gilvernet, C. Peraire, J. Domenech, J. M. Grinyò, J. J. Wilkins, and M. O. Karlsson. 2006. Abstr. 15th PAGE Meet., abstr. 1012. http://www.page-meeting.org/?abstract=1012.
- 2.Åsberg, A., A. Humar, H. Rollag, A. G. Jardine, H. Mouas, M. D. Pescovitz, D. Sgarabotto, M. Tuncer, I. L. Noronha, and A. Hartmann, on behalf of the VICTOR study group. 2007. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am. J. Transplant. 7:2106-2113. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., L. B. Sheiner, and A. J. Boeckmann (ed.). 1989-2006. NONMEM user guides. Icon Development Solutions, Ellicott City, MD.
- 4.Cockroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 5.Cvetković, R. S., and K. Wellington. 2005. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs 65:859-878. [DOI] [PubMed] [Google Scholar]
- 6.Czock, D., C. Scholle, F. M. Rasche, D. Schaarschmidt, and F. Keller. 2002. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin. Pharmacol. Ther. 72:142-150. [DOI] [PubMed] [Google Scholar]
- 7.Estrada-Zambarano, A., C. Biosca-Adzet, B. Bayés-Genís, M. Dolade-Botias, R. Lauruzica-Valmemoros, and R. Romero-González. 2007. Evaluation of equations to estimate the glomerular filtration rate in kidney transplant recipients. Transplant. Proc. 39:2210-2213. [DOI] [PubMed] [Google Scholar]
- 8.Fishman, J. A., and R. H. Rubin. 1998. Infection in organ-transplant recipients. N. Engl. J. Med. 338:1741-1751. [DOI] [PubMed] [Google Scholar]
- 9.Groves, C. E., W. B. Suhre, N. J. Cherrington, and S. H. Wright. 2006. Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J. Pharmacol. Exp. Ther. 316:742-752. [DOI] [PubMed] [Google Scholar]
- 10.Jung, D., and A. Dorr. 1999. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J. Clin. Pharmacol. 39:800-804. [DOI] [PubMed] [Google Scholar]
- 11.Jung, D., M. H. AbdelHameed, J. Hunter, P. Teitelbaum, A. Dorr, and K. Griffy. 1999. The pharmacokinetics and safety profile of oral ganciclovir in combination with trimethoprim in HIV- and CMV-seropositive patients. Br. J. Clin. Pharmacol. 47:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson, M. O., and L. B. Sheiner. 1993. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J. Pharmacokinet. Biopharm. 21:735-750. [DOI] [PubMed] [Google Scholar]
- 13.Klein, I. H. H. T., A. Abrahams, T. van Ede, R. J. Hené, H. A. Koomans, and G. Ligtenberg. 2002. Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation 73:732-736. [DOI] [PubMed] [Google Scholar]
- 14.Lee, W., and R. B. Kim. 2004. Transporters and renal drug elimination. Annu. Rev. Pharmacol. Toxicol. 44:137-166. [DOI] [PubMed] [Google Scholar]
- 15.Levey, A. S., J. Coresh, T. Greene, J. Marsh, L. A. Stevens, J. W. Kusek, and F. Van Lente, for the chronic kidney disease epidemiology collaboration. 2007. Expressing the modification of diet in renal disease study for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53:766-772. [DOI] [PubMed] [Google Scholar]
- 16.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 17.Ljubojevic, M., C. M. Herak-Kramberger, Y. Hagos, A. Bahn, H. Endou, G. Burckhardt, and I. Sabolic. 2004. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. 2004. Am. J. Physiol. Renal Physiol. 287:F124-F138. [DOI] [PubMed] [Google Scholar]
- 18.Lowance, D., H.-H. Neumayer, C. M. Legendre, J.-P. Squifflet, J. Kovarik, P. J. Brennan, D. Norman, R. Mendez, M. R. Keating, G. L. Coggon, A. Crisp, I. C. Lee, et al. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N. Engl. J. Med. 340:1462-1469. [DOI] [PubMed] [Google Scholar]
- 19.Martin, D. F., J. Sierra-Madero, S. Walmsley, R. A. Wolitz, K. Macey, P. Georgiou, C. A. Robinson, and M. J. Stempien. 2002. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N. Engl. J. Med. 346:1119-1126. [DOI] [PubMed] [Google Scholar]
- 20.Muheim, C., G. Vogel, C. Seydoux, M. Gillet, F. Mosimann, L. Von Segesser, R. Sahli, C. Estrade, G. Van Melle, and P. R. Meylan. 2002. Determinants of protracted cytomegalovirus infection in solid-organ transplant patients. Transplantation 74:226-236. [DOI] [PubMed] [Google Scholar]
- 21.Paya, C., A. Humar, E. Dominguez, K. Washburn, E. Blumberg, B. Alexander, R. Freeman, N. Heaton, M. D. Pescovitz, et al. 2004. Efficacy and safety of valganciclovir versus oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am. J. Transplant. 4:611-620. [DOI] [PubMed] [Google Scholar]
- 22.Perrottet, N., A. Beguin, P. Meylan, M. Pascual, O. Manuel, T. Buclin, J. Biollaz, and L. A. Decosterd. 2007. Determination of aciclovir and ganciclovir in human plasma by liquid chromatography-spectrofluorimetric detection and stability studies in blood samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 852:420-429. [DOI] [PubMed] [Google Scholar]
- 23.Perrottet, N., C. Robatel, P. Meylan, M. Pascual, J. P. Venetz, J. D. Aubert, M. M. Berger, L. A. Decosterd, and T. Buclin. 2008. Disposition of valganciclovir during continuous renal replacement therapy in two lung transplant recipients. J. Antimicrob. Chemother. 61:1332-1335. [DOI] [PubMed] [Google Scholar]
- 24.Pescovitz, M. D., J. Rabkin, R. M. Merion, C. V. Paya, J. Pirsch, R. B. Freeman, J. O'Grandy, C. Robinson, T. Zung, K. Wren, L. Banken, W. Buhles, and F. Brown. 2000. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob. Agents Chemother. 44:2811-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preiksaitis, J. K., D. C. Brennan, J. A. Fishmann, and U. Allen. 2005. Canadian Society of Transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am. J. Transplant. 5:218-227. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo, E., G. Fernández-Fresnedo, J. C. Ruiz, C. Piňera, M. Hera, A. L. M. de Francsico, S. Sanz de Castro, J. G. Cotorruelo, J. A. Zubimendi, and M. Arias. 2003. Assessment of glomerular filtration rate in transplant recipients with severe renal insufficiency by Nankivell, modificication of diet in renal disease (MDRD), and Cockroft-Gault equations. Transplant. Proc. 35:1671-1672. [DOI] [PubMed] [Google Scholar]
- 27.Scott, J. C., N. Partovi, and M. H. H. Ensom. 2004. Ganciclovir in solid organ transplant recipients. Is there a role for clinical pharmacokinetic monitoring? Ther. Drug Monit. 26:68-77. [DOI] [PubMed] [Google Scholar]
- 28.Sommadossi, J. P., and R. Carlisle. 1987. Toxicity of 3′-azido-3′-deoxythymidine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for normal human hematopoietic progenitor cells in vitro. Antimicrob. Agents Chemother. 31:452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda, M., S. Khamdang, S. Narikawa, H. Kimura, Y. Kobayashi, T. Yamamoto, S. H. Cha, T. Sekine, and H. Endou. 2002. Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J. Pharmacol. Exp. Therapeut. 300:918-924. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Wählby, U., A. H. Thomson, P. A. Miligan, and M. O. Karlsson. 2004. Models for time varying covariates in population pharmacokinetic-pharmacodynamic analysis. Br. J. Clin. Pharmacol. 58:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiltshire, H., S. Hirankarn, C. Farrell, C. Paya, M. D. Pescovitz, A. Humar, E. Dominguez, K. Washburn, E. Blumberg, B. Alexander, R. Freeman, N. Heaton, et al. 2005. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin. Pharmacokinet. 44:495-507. [DOI] [PubMed] [Google Scholar]
- 33.Wiltshire, H., C. Paya, M. D. Pescovitz, A. Humar, E. Dominguez, K. Washburn, E. Blumberg, B. Alexander, R. Freeman, N. Heaton, K. P. Zuideveld, et al. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477-1483. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe, E. J., V. Mathur, S. Tomlanovich, D. Jung, R. Wong, K. Griffy, and F. T. Aweeka. 1997. Pharmacokinetics of mycophenolate mofetil and intravenous ganciclovir alone and in combination in renal transplant recipients. Pharmacotherapy 17:591-598. [PubMed] [Google Scholar]