Abstract
NZ2114 is a novel plectasin derivative with potent activity against gram-positive bacteria, including multiply drug-resistant strains. We used the neutropenic murine thigh infection model to characterize the time course of antimicrobial activity of NZ2114 and determine which pharmacokinetic/pharmacodynamic (PK/PD) index and magnitude best correlated with efficacy. Serum drug levels following administration of three fourfold-escalating single-dose levels of NZ2114 were measured by microbiologic assay. Single-dose time-kill studies following doses of 10, 40, and 160 mg/kg of body weight demonstrated concentration-dependent killing over the dose range (0.5 to 3.7 log10 CFU/thigh) and prolonged postantibiotic effects (3 to 15 h) against both Staphylococcus aureus and Streptococcus pneumoniae. Mice had 106.3 to 106.8 CFU/thigh of strains of S. pneumoniae or S. aureus at the start of therapy when treated for 24 h with 0.625 to 160 mg/kg/day of NZ2114 fractionated for 4-, 6-, 12-, and 24-h dosing regimens. Nonlinear regression analysis was used to determine which PK/PD index best correlated with microbiologic efficacy. Efficacies of NZ2114 were similar among the dosing intervals (P = 0.99 to 1.0), and regression with the 24-h area under the concentration-time curve (AUC)/MIC index was strong (R2, 0.90) for both S. aureus and S. pneumoniae. The maximum concentration of drug in serum/MIC index regression was also strong for S. pneumoniae (R2, 0.96). Studies to identify the PD target for NZ2114 utilized eight S. pneumoniae and six S. aureus isolates and an every-6-h regimen of drug (0.156 to 160 mg/kg/day). Treatment against S. pneumoniae required approximately twofold-less drug for efficacy in relationship to the MIC than did treatment against S. aureus. The free drug 24-h AUCs/MICs necessary to produce a stasis effect were 12.3 ± 6.7 and 28.5 ± 11.1 for S. pneumoniae and S. aureus, respectively. The 24-h AUC/MIC associated with a 1-log killing endpoint was only 1.6-fold greater than that needed for stasis. Resistance to other antimicrobial classes did not impact the magnitude of the PD target required for efficacy. The PD target in this model should be considered in the design of clinical trials with this novel antibiotic.
The epidemic of antimicrobial resistance is a growing public health threat. Unfortunately, few drug classes have been identified and brought to market in the last decade (15). One recently reported novel antibacterial compound is from the plectasin class (12). Plectasin antibiotics are defensin-like peptide antibiotics of fungal origin. These compounds exhibit broad-spectrum activity against gram-positive bacteria, including potency against multiply drug-resistant strains. The plectasins specifically bind a target molecule and interfere with bacterial biosynthesis, resulting in rapid cell death (T. Schneider et al., submitted for publication). Recent studies suggested the potential for both in vitro and in vivo efficacy with one derivative, NZ2114 (12).
Preclinical pharmacodynamic investigations have proven to be predictive of outcome in therapy of patients and thus important in the design of dosing regimens in the development of antimicrobial clinical trials (1, 2, 3, 5, 8, 10). The goals of the current experiments were to characterize the in vivo pharmacodynamic characteristics of this drug against Streptococcus pneumoniae and Staphylococcus aureus in order to identify the pharmacodynamic target for future clinical development.
MATERIALS AND METHODS
Bacteria, media, and antibiotic.
Eight strains of Streptococcus pneumoniae with variable resistance to penicillin were used. Six strains of Staphylococcus aureus (three methicillin-susceptible and three methicillin-resistant S. aureus strains) were also used for these experiments. Organisms were grown, subcultured, and quantified in Mueller-Hinton broth (Difco Laboratories, Detroit, MI) and Mueller-Hinton agar (Difco Laboratories, Detroit, MI) for all organisms except S. pneumoniae. Sheep blood agar plates (Remel, Milwaukee, WI) were utilized for S. pneumoniae. NZ2114 was supplied by Novozymes A/S.
In vitro susceptibility studies.
The MICs of NZ2114, penicillin, and methicillin for the various isolates were determined by standard Clinical and Laboratory Standards Institute microdilution methods (14). All MIC assays were performed in duplicate on three occasions. The reported MIC was the mean of the replicate assays.
Murine infection model.
The neutropenic mouse thigh infection model has been used extensively for determination of pharmacokinetic/pharmacodynamic (PK/PD) index determination and prediction of antibiotic efficacy in patients (3-5). Animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care criteria (13). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital. Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, IN). Mice were rendered neutropenic (neutrophils, <100/mm3) by injecting them with cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before thigh infection. Previous studies have shown that this regimen produces neutropenia in this model for 5 days (16). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (Spectronic 88; Bausch and Lomb, Rochester, NY). After a 1:10 dilution into fresh Mueller-Hinton broth, bacterial counts of the inoculum ranged from 106.4 to 107.6 CFU/ml. Thigh infections with each of the isolates were produced by injection of 0.1 ml of inoculum into the thighs of isoflurane-anesthetized mice 2 h before therapy with NZ2114.
Drug pharmacokinetics.
Single-dose serum pharmacokinetic studies were performed in thigh-infected mice. Animals were administered subcutaneous doses (0.2 ml/dose) of NZ2114 (10, 40, and 160 mg/kg). Two groups of three mice each were included for each dose studied. Blood was removed from three mice per time point at 0.25- to 1-h intervals over 6 h. Serum was collected and processed immediately for microbiologic assay using S. aureus 6538p as the assay organism. The lower limit of detection of NZ2114 in the microbiologic assay was 0.25 μg/ml, and the lower limit of quantitation was 1.0 μg/ml. The intraday variation was 4.3% at a concentration of 4 μg/ml. Pharmacokinetic constants, including elimination half-life, area under the concentration-time curve (AUC), and peak level, were calculated using a noncompartmental model. For doses used in treatments for which actual kinetic measurements were not made, estimates were based upon linear extrapolation or interpolation from the three studied dose levels. Protein binding in the serum of neutropenic infected mice was performed using ultrafiltration methods as previously described (7, 9). The degree of binding was measured using NZ2114 concentrations of 25 and 100 μg/ml.
Treatment protocols. (i) In vivo PAE.
Two hours after infection with S. pneumoniae strain ATCC 10813 or S. aureus strain ATCC 25923, neutropenic mice were treated with single subcutaneous doses of NZ2114 (10, 40, or 160 mg/kg). Groups of two treated and untreated control mice each were sacrificed at sampling intervals ranging from 2 to 12 h. Control growth was determined at five sampling times over 24 h. The treated groups were sampled seven times over 24 h. The thighs were removed at each time point and processed immediately for CFU determination. The times for which the levels of NZ2114 (based on unbound drug concentrations) in the serum remained above the MIC for the organisms were calculated from the pharmacokinetic studies. The postantibiotic effect (PAE) was calculated by subtracting the time that it took for organisms to increase 1 log in level in the thighs of saline-treated animals from the time that it took organisms to grow the same amount in treated animals after serum levels fell below the MIC for the infecting organism (PAE = T − C, where C is the time for 1-log10 control growth and T is the time for 1-log10 treatment growth after levels have fallen below MIC) (6).
(ii) PK/PD index determination.
Neutropenic mice were infected with either penicillin-susceptible S. pneumoniae ATCC 10813 or methicillin-susceptible S. aureus ATCC 25923. Treatment with NZ2114 was initiated 2 h after infection. Groups of two mice were treated for 24 h with 20 different dosing regimens using fourfold-increasing total doses divided into one, two, four, or six doses. Total doses of NZ2114 ranged 256-fold (0.625 to 160 mg/kg/24 h). Drug doses were administered subcutaneously in 0.2-ml volumes. The mice were sacrificed after 24 h of therapy, and the thighs were removed and processed for CFU determination. Untreated control mice were sacrificed just before treatment and after 24 h.
(iii) PK/PD index magnitude studies.
Similar dosing studies using five or six fourfold-increasing NZ2114 doses administered every 6 h were utilized to treat thigh-infected neutropenic animals with eight strains of S. pneumoniae (two penicillin-susceptible, three penicillin-intermediate, and three penicillin-resistant strains) and six strains of S. aureus (three methicillin-susceptible and three methicillin-resistant strains). The total daily dose of NZ2114 used in these studies varied from 0.156 to 160 mg/kg/day.
Data analysis.
The results of these studies were analyzed using the sigmoid dose-effect model. The model, as follows, is derived from the Hill equation: E = (Emax × DN)/(ED50N − DN), where E is the effect or, in this case, the log change in CFU per thigh between treated mice and untreated controls after the 24-h period of study, Emax is the maximum effect, D is the 24-h total dose, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect curve (4, 11). The indices Emax, ED50, and N were calculated using nonlinear least-squares regression. The correlation between efficacy and each of the three PK/PD indices (T > MIC, AUC/MIC, and peak/MIC) studied was determined by nonlinear regression (Sigma Stat; SPSS. Inc., San Rafael, CA). The coefficient of determination, or R2, was used to estimate the variance that could be due to regression with each of the PK/PD indices.
We utilized the 24-h static dose as well as the doses necessary to achieve a 1-log10 reduction in colony counts compared to numbers at the start of therapy to compare the impacts of the dosing intervals on treatment efficacy. If these dose values remained similar among each of the dosing intervals, this would support the 24-h AUC/MIC as the predictive index. If the dose values increased as the dosing interval was lengthened, this would suggest that T > MIC is the predictive index. Lastly, if the dose values decreased as the dosing interval was increased, this would support peak/MIC as the pharmacodynamically important index. The static dose and 1-log kill values for each of the dosing intervals were compared statistically using analysis of variance.
To allow a comparison of the potencies of NZ2114 against a variety of organisms, we utilized the 24-h static dose. The magnitude of the PK/PD index associated with each endpoint dose was calculated from the equation log10 D = log10 (E/Emax − E)/N + log10 ED50, where D is the drug dose, E is the control growth for dose (D), E is the control growth + 1 log for a D of 1-log kill, Emax is the maximal effect, N is the slope of the dose-response relationship, and ED50 is the dose needed to achieve 50% of the maximal effect. The significance of differences among the various dosing endpoints was determined by using analysis of variance on ranks.
RESULTS
In vitro susceptibility testing.
The MICs of NZ2114 for the 14 study strains are shown in Table 1. NZ2114 MICs varied more than 30-fold (range, 0.06 to 2.0 μg/ml).
TABLE 1.
In vitro and in vivo activities of NZ2114 against select strains of S. pneumoniae and S. aureus
| Strain | MIC (μg/ml) | Static dosea
|
1 Log killb
|
||||
|---|---|---|---|---|---|---|---|
| mg/kg/24 h | 24-h AUC/MICc | Mean ± SD | mg/kg/24 h | 24-h AUC/MICc | Mean ± SD | ||
| S. pneumoniae | 12.3 ± 6.7 | 20.1 ± 12.2 | |||||
| 10813 | 0.06 | 1.1 | 6.2 | 2.0 | 11.6 | ||
| 43916d | 0.125 | 6.0 | 16.4 | 10.2 | 28.0 | ||
| 140 | 1.0 | 20 | 6.8 | 17.2 | 5.8 | ||
| 1293e | 0.06 | 2.7 | 15.2 | 5.6 | 31.5 | ||
| 6301d | 0.5 | 13.7 | 9.2 | 19.5 | 13.2 | ||
| 6303d | 0.125 | 1.24 | 3.4 | 2.12 | 5.8 | ||
| 1325e | 0.25 | 15.5 | 21.2 | 26.5 | 36.0 | ||
| 1020e | 0.125 | 7.4 | 20.2 | 10.6 | 28.8 | ||
| S. aureus | 28.5 ± 11.1 | 47.6 ± 18.1 | |||||
| 25923 | 1.0 | 68.8 | 25.6 | 100 | 38.4 | ||
| 33591f | 2.0 | 167 | 32.4 | 408 | 52.0 | ||
| Smith | 2.0 | 158 | 32.0 | 448 | 61.6 | ||
| 31005 | 2.0 | 218 | 37.7 | 516 | 68.8 | ||
| 307109f | 2.0 | 199 | 35.6 | 308 | 47.2 | ||
| WIS1f | 2.0 | 73.6 | 7.4 | 93 | 17.7 | ||
Over 24 h.
Dose over 24 h associated with 1-log reduction compared to start of therapy.
AUC/MICs are based on free drug.
Penicillin intermediate.
Penicillin resistant.
Methicillin resistant.
Pharmacokinetics.
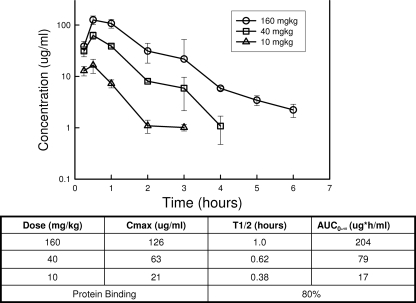
The time course of serum levels of NZ2114 in infected neutropenic mice following subcutaneous doses of 10, 40, and 160 mg/kg is shown in Fig. 1. Peak levels were observed by 30 min. The elimination half-life in the mice ranged from 0.38 to 1.0 h. The AUC and peak values for the escalating single doses ranged from 17 to 204 and 21 to 126, respectively. The protein binding of NZ2114 in mouse serum, as determined by ultrafiltration, was 80%. This is similar to the degree of binding in other animal species and in human serum (D. Sandvang, personal communication).
FIG. 1.
Serum pharmacokinetics of plectasin derivative NZ2114 following single subcutaneous doses. Each symbol represents the mean concentration from three mice. The error bars represent the standard deviations. Three dose levels (fourfold escalating) of NZ2114 were studied. The measured Cmax and calculated elimination half-life and AUC (zero to infinity) are shown in the table. Protein binding was determined by ultrafiltration using concentrations of 25 and 100 μg/ml. A microbiologic assay using S. aureus 6538p was used for determination of all NZ2114 concentrations.
In vivo PAE.
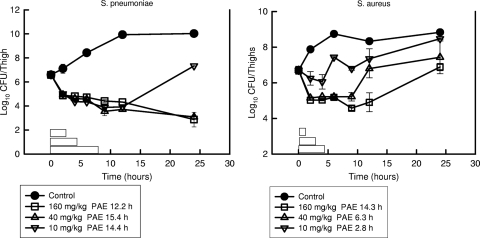
At the start of therapy, mice had 106.6 to 106.7 CFU/thigh of S. pneumoniae and S. aureus, respectively. Growth of 1 log10 CFU/thigh in saline-treated animals occurred in 4.1 and 2.0 h in S. pneumoniae- and S. aureus-infected animals, respectively. Based upon the serum pharmacokinetic determinations, serum NZ2114 levels following the single doses of 10, 40, and 160 mg/kg remained above the MIC for S. pneumoniae strain ATCC 10813 (MIC, 0.06 μg/ml) for 2.3, 4.5, and 7.7 h based on free drug levels, respectively. Rapid, dose-dependent killing of organisms occurred following each of the dose levels for both strains. Maximal killing compared to organism burden at the start of therapy ranged from 2.7 to 3.7 log10 CFU/thigh over the dose range for S. pneumoniae. Over a similar dose range, maximal killing of S. aureus ranged from 0.5 to 1.7 log10 CFU/thigh. Organism regrowth with S. pneumoniae did not occur for more than 12 h. For the lowest dose in the S. aureus study, regrowth began 4 h after dosing. The times above the MIC for these doses against S. aureus strain ATCC 25923 (MIC, 1.0 μg/ml) were 1.2, 2.6, and 4.6 h based on free drug levels. The time-kill curves for both of the studies are shown in Fig. 2. Against S. pneumoniae, escalating doses produced free drug PAEs ranging from 12.2 to 15.4 h. The study with S. aureus produced free drug PAEs ranging from 2.8 to 14.3 h. No detectable drug carryover was observed in any of the treatment groups.
FIG. 2.
Impact of plectasin derivative NZ2114 dose escalation on burden of either S. pneumoniae or S. aureus in the thighs of neutropenic mice over time. Each symbol represents the mean CFU/thigh from two mice (four thighs). The error bars represent the standard deviations. Solid symbols represent growth of organisms in control mice over time. Open symbols represent the burden of organisms in NZ2114-treated mice. The horizontal boxes represent the duration of time that free drug NZ2114 serum concentrations remained above the MIC of the infecting organism. The PAE is expressed in hours.
PK/PD index determination.
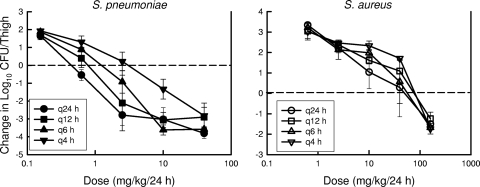
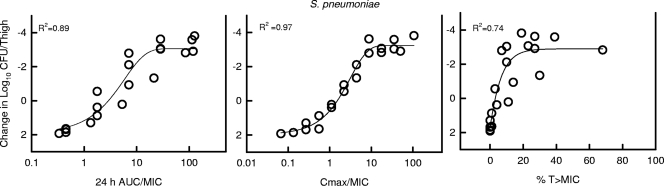
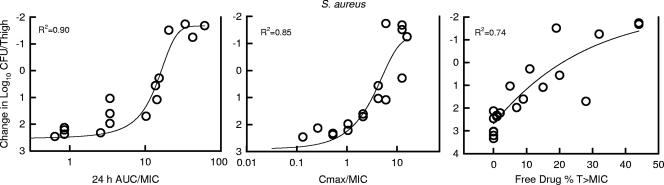
At the start of therapy, mice had 6.8 ± 0.07 and 6.3 ± 0.32 log10 CFU/thigh of S. pneumoniae strain ATCC 10813 and S. aureus strain ATCC 29213, respectively. The organisms grew 2.1 ± 0.2 and 3.4 ± 0.03 log10 CFU/thigh after 24 h in untreated control mice, respectively. Escalating doses of NZ2114 resulted in the concentration-dependent killing of both strains. The highest doses studied reduced organism burden by 4.8 ± 0.26 and 1.7 ± 0.01 log10 CFU/thigh compared to numbers at the start of therapy for S. pneumoniae and S. aureus, respectively. The dose-response relationships for the four dosing intervals against S. pneumoniae and S. aureus are shown in Fig. 3. The curves were similar among each of the dosing intervals against S. aureus. In the study with S. pneumoniae, there was a slight shift in the dose-response curve to the left (indicating enhanced effect) as the dosing interval was lengthened. The dose levels required to produce stasis and a 1-log reduction were calculated for each dosing interval (data not shown). At each of these treatment endpoints, we did not observe a significant difference, as the dosing interval was lengthened from every 4 h to every 24 h (data not shown). These analyses suggest that treatment efficacy was dependent upon dose level and independent of the dosing intervals studied. The relationships between microbiologic effect and each of the pharmacodynamic indices, 24-h AUC/MIC, percent time above the MIC, and peak/MIC against S. pneumoniae strain ATCC 10813, are shown in Fig. 4. Therapeutic outcome correlated well with both the 24-h AUC/MIC and maximum concentration of drug in serum (Cmax)/MIC indices. For S. aureus the relationship was strongest for the 24-h AUC/MIC index (R2 = 0.90 for 24-h AUC/MIC; R2 = 0.85 for Cmax/MIC), and for S. pneumoniae the Cmax/MIC index correlated somewhat better (R2 = 0.89 for 24-h AUC/MIC; R2 = 0.97 for Cmax/MIC) (Fig. 5). Regression with the percent T > MIC index resulted in a less strong relationship (R2 = 0.74 for each organism). The reasonable fit of the data with each of the PK/PD indices is due to the interrelationships among each of the indices (5). Consideration of total or unbound drug levels did not appreciably impact the relationship between efficacy and percent T > MIC (data not shown).
FIG. 3.
Impact of plectasin derivative NZ2114 dosing interval on efficacy against a strain of S. aureus and a strain of S. pneumoniae in the neutropenic murine thigh infection model. Five total (mg/kg/24-h) doses were fractionated into one, two, four, or six doses over the 24-h treatment period (q24 h, q12 h, q6 h, and q4 h, respectively). Total escalating doses varied fourfold. Efficacy is expressed as change in CFU/thigh compared to organism burden at the start of therapy. Each symbol represents the mean CFU/thigh from two mice (four thighs). The error bars represent the standard deviations. The horizontal dashed line represents the burden of organisms at the start of therapy.
FIG. 4.
Relationship between the NZ2114 pharmacodynamic index (24-h AUC/MIC, Cmax/MIC, and percent time above MIC) and efficacy over 24 h against S. pneumoniae 10813 in a neutropenic murine thigh infection model. Unbound (free drug) concentrations were used for index calculations. Efficacy is expressed as change in CFU/thigh compared to organism burden at the start of therapy. Each symbol represents the mean CFU/thigh from two mice (four thighs). The sigmoid line represents the best fit using the sigmoid Emax model. R2 is the coefficient of determination.
FIG. 5.
Relationship between the NZ2114 pharmacodynamic index (24-h AUC/MIC, Cmax/MIC, and percent time above MIC) and efficacy over 24 h against S. aureus 25923 in a neutropenic murine thigh infection model. Unbound (free drug) concentrations were used for index calculations. Efficacy is expressed as change in CFU/thigh compared to organism burden at the start of therapy. Each symbol represents the mean CFU/thigh from two mice (four thighs). The sigmoid line represents the best fit using the sigmoid Emax model. R2 is the coefficient of determination.
PK/PD magnitude determination.
Calculation of the doses necessary to achieve a static effect and a 1-log10 kill against multiple organisms is shown in Table 1. The growth curves of the eight pneumococcal and six staphylococcal strains in the thighs of control animals were relatively similar. At the start of therapy, mice had 7.0 ± 0.44 (range, 5.6 to 7.6) log10 CFU of pneumococci or S. aureus/thigh. The organisms grew to 2.4 ± 0.54 log10 CFU/thigh (range, 1.5 to 3.4) in untreated control mice. The maximal reduction in S. pneumoniae with NZ2114-treated mice compared to untreated controls ranged from 2.8 ± 0.3 to 4.6 ± 0.2 log10 CFU/thigh (mean, 3.4 ± 0.60). Less killing was observed against the S. aureus strains (mean, 1.9 ± 0.42 log10 CFU/thigh).
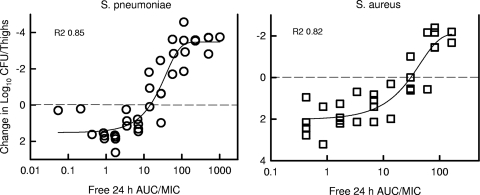
The 24-h AUC/MIC index was used for determination of the pharmacodynamic magnitude of exposure associated with efficacy. Table 1 shows the 24-h dose and free drug 24-h AUC/MIC ratios necessary to achieve a net static effect and 1-log10 reduction in organism burden. The static doses varied from 1.1 mg/kg every 24 h to 218 mg/kg every 24 h against the strains of S. pneumoniae and S. aureus. The corresponding free drug 24-h AUC/MICs varied from 3.4 to 37.7. The mean 24-h AUC/MICs associated with a static effect were slightly lower for S. pneumoniae than for S. aureus (12.3 ± 6.7 versus 28.5 ± 11.1, respectively) (P = 0.006). The exposure associated with bactericidal activity or a 1-log10 reduction in organism burden compared to the start of therapy was less than twofold larger than that associated with a stasis endpoint. The presence of antimicrobial resistance in both bacterial species did not alter the 24-h AUC/MIC required to produce efficacy. The relationship between the 24-h free drug AUC/MIC and efficacy against the two organism groups is demonstrated graphically in Fig. 6. The exposure-response relationships were relatively strong, with R2 values of 0.85 and 0.82 for S. pneumoniae and S. aureus, respectively.
FIG. 6.
Relationship between NZ2114 free drug 24-h AUC/MIC and efficacy against eight S. pneumoniae (left) and six S. aureus (right) strains. Each symbol represents the mean CFU/thigh from two mice (four thighs). Efficacy on the y axis is expressed as the change in CFU/thigh compared to the burden of organisms at the start of therapy. The dashed horizontal line represents the burden of organisms in thighs at the start of therapy. The sigmoid line represents the best-fit curve using the sigmoid Emax model. R2 is the coefficient of determination.
DISCUSSION
The number of infections due to resistant gram-positive bacteria, including penicillin-resistant Streptococcus pneumoniae and methicillin-resistant Staphylococcus aureus, continues to increase (15). The development of effective antimicrobial agents to treat these infections is an area of intense research. Peptide antimicrobial agents represent a promising new class of compounds which collectively act at a number of different bacterial targets and have demonstrated potency against these emerging pathogens (12).
Previous in vivo studies in two pneumococcal murine infection models showed both enhanced animal survival and a rapid decline in organism burden following single doses of the native plectasin molecule (12). A logical next step in the evaluation of these molecules is to use animal infection molecules to understand the pharmacodynamic activity of this drug class. Antimicrobial pharmacodynamic studies characterize the time course of antibiotic effect. The time course of antimicrobial activity can be determined by two characteristics: (i) the effect of increasing drug concentrations on the extent of organism killing and (ii) the presence or absence of antimicrobial effects that persist after the levels in serum have fallen below the MIC (5). The use of animal infection models to understand the pharmacodynamic activity of antimicrobial agents is one approach that has proven helpful for (i) the design of effective dosing regimens in humans and (ii) the development of appropriate in vitro susceptibility breakpoints (3).
In these studies, escalating doses of NZ2114 produced a rapid reduction in viable counts. Each of the doses examined reduced the burden of organisms more than 2 log in the first 6 h after a single dose of NZ2114, and nearly 2 additional logs of killing was observed while free drug concentrations exceeded the MIC of the infecting S. pneumoniae strain. After drug concentrations fell below the MIC, we observed prolonged growth suppression. A similar time course pattern was observed in a study with a strain of S. aureus. However, the degree of organism killing was somewhat lower against the S. aureus strain. Prolonged PAEs were also seen against S. aureus, and the duration of the PAE was dose dependent. The efficacy of antibiotics characterized by this pattern of activity is best correlated with either the 24-h AUC/MIC or the Cmax/MIC index.
Results from analysis of the multiple-dosing regimen studies indicate that the 24-h AUC/MIC is the best index for predicting the efficacy of NZ2114. The dose-response relationship against S. aureus was not impacted by a change in dosing interval, and pharmacodynamic regression was clearly strongest with the 24-h AUC/MIC index. In the pneumococcal investigation, the dosing interval did appear to have some impact on the dose-response relationship. As the dosing strategy provided larger doses more infrequently, the dose-response curves shifted slightly to the left. This visual pharmacodynamic relationship would suggest that the Cmax/MIC is important (5). However, statistical comparison of dosing endpoints (static dose and 1-log kill) among the dosing intervals did not show significance. The regression analysis results based upon the coefficient of determination (R2) suggested that both the Cmax/MIC and 24-h AUC/MIC indices predict efficacy with NZ2114.
Next, studies were undertaken to identify the magnitude of the pharmacodynamic index needed for efficacy of NZ2114. We considered the 24-h AUC/MIC index for these analyses. Experiments included 14 organisms with a wide range of NZ2114 MICs (more than 30-fold). The strains were chosen to include those resistant to beta-lactams. In vitro studies with NZ2114 demonstrated antimicrobial activity against S. pneumoniae and S. aureus including strains resistant to other antimicrobial classes including beta-lactams, macrolides, quinolones, and vancomycin (14a). Similarly, in the current in vivo experiments penicillin resistance in S. pneumoniae and methicillin resistance in S. aureus had no impact upon the in vivo pharmacodynamic target of NZ2114.
The NZ2114 dose-response relationships for each species were very similar. However, the dose-response curves for S. pneumoniae were shifted somewhat to the left compared to those for S. aureus. This slight difference in activity is similar to that observed in the time course studies. The free (unbound) NZ2114 24-h AUC/MIC associated with a net static effect against S. pneumoniae was roughly twofold lower than that for S. aureus. The dose-response curves were relatively steep against both bacterial species, and the pharmacodynamic exposure associated with a killing endpoint (1-log kill) was less than twofold greater than that needed for a stasis effect.
The microbiologic outcomes in this infection model have correlated with clinical and microbiologic efficacy of several antimicrobial classes in humans (1, 2, 3, 4, 5). The results of these studies should be utilized to examine the pharmacokinetics of NZ2114 in humans in the context of MIC distribution of target organisms to help guide appropriate dosing regimen design and to determine preliminary susceptibility breakpoints.
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., J. B. Anon, S. M. Bhavnani, O. O. Okusanya, R. N. Jones, M. R. Paglia, J. Kahn, and G. L. Drusano. 2008. Use of pharmacodynamic endpoints for the evaluation of levofloxacin for the treatment of acute maxillary sinusitis. Diagn. Microbiol. Infect. Dis. 61:13-20. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 4.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 7.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams and Wilkins Co., Baltimore, MD.
- 8.Craig, W. A., and D. R. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 9.Craig, W. A., and P. G. Welling. 1977. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin. Pharmacokinet. 2:252-268. [DOI] [PubMed] [Google Scholar]
- 10.Drusano, G. L., S. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II-III dose for everninomycin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calamae, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 12.Mygind, P. H., R. L. Fischer, K. M. Schnorr, M. T. Hansen, C. P. Sönksen, S. Ludvigsen, D. Raventós, S. Buskov, B. Christensen, L. De Maria, O. Taboureau, D. Yaver, S. G. Elvig-Jørgensen, M. V. Sørensen, B. E. Christensen, S. Kjaerulff, N. Frimodt-Moller, R. I. Lehrer, M. Zasloff, and H. H. Kristensen. 2005. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437:975-980. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 14.NCCLS. 2000. Performance standards for antimicrobial disk 1 susceptibility tests. Approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14a.Sandvang, D., P. H. Mygind, M. E. Jones, D. F. Sahm, and H. Kristensen. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-1663, p. 243.
- 15.Talbot, G. H., J. Bradley, J. E. Edwards Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 16.Zuluaga, A. F., B. E. Salazar, C. A. Rodriguez, A. X. Zapata, M. Agudelo, and O. Vesga. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]