Abstract
Ceftobiprole is a promising new broad-spectrum cephalosporin with activity against several multidrug-resistant gram-positive and gram-negative species, including methicillin-resistant Staphylococcus aureus. In order to make efficacy predications against these resistant bacteria in soft-tissue infections, i.e., skin and skin structure infections, ceftobiprole's ability to reach the site of action should be explored. Therefore, a microdialysis study was conducted in 12 healthy volunteers to determine the penetration of ceftobiprole into skeletal muscle and subcutaneous (s.c.) adipose tissue after a single intravenous dose of 500 mg. Plasma and tissue interstitial space fluid (ISF) drug concentrations were measured for 24 h from the start of the 2-h intravenous infusion. Pharmacokinetic parameters were determined using noncompartmental analysis. The penetration of ceftobiprole into the ISF of tissues was assessed by comparing the ratios between tissue and plasma of the free drug area under the concentration-time curve (fAUC). It was found that ceftobiprole distributes into the muscle (fAUCmuscle/fAUCplasma of 0.69 ± 0.13) and s.c. adipose tissue (fAUCs.c.adipose/fAUCplasma of 0.49 ± 0.28). The concentrations in both skeletal muscle and s.c. adipose tissue met the efficacy breakpoint (percentage of the time that free drug concentrations remained above the MIC) for at least 40% of the 8-h dosing interval for organisms with a MIC of 2 mg/liter. Therefore, ceftobiprole qualifies as a potential agent with drug penetration capabilities to treat complicated skin and skin structure infections due to both gram-negative and gram-positive pathogens with MICs equal to or below 2 mg/liter.
Ceftobiprole, the active compound in ceftobiprole medocaril, is a promising new cephalosporin with activity against both gram-negative and gram-positive bacteria. This includes good activity against methicillin-resistant Staphylococcus aureus with reported MIC90 values of 2 mg/liter (12) and 4 mg/liter (9) and against penicillin-resistant Streptococcus pneumoniae with a MIC90 of 2 mg/liter (9). Currently, ceftobiprole is under regulatory review and is approved only in Canada and Switzerland. Two dosing regimens are recommended, 500 mg as a 2-h intravenous (i.v.) infusion every 8 h in cases of gram-positive and/or gram-negative infections, including diabetic foot infections, and 500 mg as a 1-h i.v. infusion every 12 h in cases of documented gram-positive infections excluding diabetic foot infections (11). This study focuses on the more general dosing regimen, i.e., the 2-h 500-mg infusion every 8 h, and references to the dosing interval refer to 8 h. This prolonged infusion time, 2 h, was chosen in an attempt to prolong the percentage of the time that the concentration remains above the MIC (T>MIC).
Traditionally, plasma samples have been taken to determine the pharmacokinetic (PK) properties of a compound and make efficacy predications based on PK/pharmacodynamic relationships. However, these drug concentrations are sometimes presented as total drug concentrations while only the free drug is pharmacologically active (18, 25). Also, free drug concentrations at the site of action/infection are much more relevant in determining efficacy (15, 24), and exploring the concentration at the site of action has been recommended by regulatory agencies (1, 2, 8). One technique that has proven useful for the measurement of free-drug concentrations in subcutaneous (s.c.) adipose tissue and skeletal muscle is microdialysis (6, 7, 10, 14, 16, 21).
The aim of this study is to examine the penetration of ceftobiprole from plasma into s.c. adipose tissue and skeletal muscle using microdialysis and to determine if efficacy breakpoints of relevant pathogens are met.
(This work was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008.)
MATERIALS AND METHODS
This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Prior to study initiation, approval was received from the local ethics committee, the Institutional Review Board at the University of Florida/Shands Hospital. All volunteers consented orally and in writing prior to participation in the study, and written consent was obtained.
Healthy volunteers.
This study included 15 healthy volunteers (nine males and six females) between the ages of 20 and 34. Health was determined based on physical examination, medical history, vital signs, 12-lead electrocardiogram, clinical laboratory tests (serum chemistry, hematology, and urinalysis), body mass index, negative hepatitis B surface antigen, negative hepatitis C antibodies, negative human immunodeficiency virus antibodies, and normal renal function based on serum creatinine and the Cockcroft-Gault equation. Subjects also had a negative urine drug test and alcohol breath test at screening and admission and were nonsmokers. Females in the study had a negative pregnancy test at screening and admission and were postmenopausal, sterile, abstinent, or practicing an effective measure of birth control. Additionally, subjects did not use any other medication for 1 week prior to the study drug administration until after the last blood draw with the exceptions of acetaminophen for pain, hormonal contraceptive medications, or hormonal replacement drugs.
Microdialysis.
Microdialysis is a sampling technique that is based on simple diffusion of free analyte through the semipermeable membrane at the tip of a microdialysis probe. This technique has been explained in detail previously (22). Once in the interstitial space fluid (ISF) of the tissue of interest, the flexible probe is continuously perfused with a physiological solution at a low flow rate by use of a syringe pump. The equilibrium between the probe and the tissue is incomplete, and therefore, the probes must be calibrated once in place, typically by retrodialysis (23). In this technique, a low concentration of the analyte is perfused through the microdialysis probe, and the disappearance into the tissue is measured from the dialysate. This recovery value is later used to calculate the true tissue ISF concentration. The recovery value is calculated as follows: recovery value = 100 − (Cdialysate/Cperfusate × 100), where Cperfusate is the analyte concentration flowing into the probe and Cdialysate is the concentration of the analyte leaving the probe.
Study design. (i) Pilot study.
Pilot study subjects were admitted to the General Clinical Research Center at Shands Hospital the morning of the study and underwent only the calibration procedures. After the site of probe insertion was cleaned and disinfected, two microdialysis probes (CMA 60; CMA Microdialysis AB, Solna, Sweden) were implanted without anesthesia into the same thigh by a study physician, one into the skeletal muscle and one into the s.c. adipose tissue. The probes were perfused with lactated Ringer's solution for 30 min at a flow rate of 1.5 μl/min. The pilot study subjects then received ceftobiprole via the microdialysis probes at a concentration of 200 mg/liter and a flow rate of 1.5 μl/min for 60 min. A sample was collected during the last 30 min to determine the individual percent recovery values. The probes were then flushed with blank lactated Ringer's solution for 3 h, and samples were collected every 30 min.
(ii) Main study.
Subjects in the main study (six male and six female) were admitted to the General Clinical Research Center the evening before dosing. On the morning of dosing, two microdialysis probes were implanted, and each was calibrated via retrodialysis as described above. After a 4-h washout period, subjects received a single 666.7-mg dose of ceftobiprole medocaril (Johnson & Johnson Pharmaceutical Research and Development, Raritan, NJ), corresponding to 500 mg of ceftobiprole, as a 2-h i.v. infusion. The microdialysis probes were perfused with lactated Ringer's solution at a flow rate of 1.5 μl/min by a syringe pump from the start of the washout phase until after the 24-h sample was collected. Dialysate samples were collected in 20-min intervals from predose through 12 h after the start of the infusion and at 16 and 24 h. Blood samples for PK determinations were collected at predose, 40 min, 1 h, 1 h 40 min, 2 h, 2 h 20 min, 3 h, 4 h, 6 h, 8 h, 12 h, 16 h, and 24 h. Blood samples for protein binding determination were collected at 2 h and 12 h. Blood sampling occurred from the opposite arm that the drug was administered, and K2EDTA tubes were used for collection.
Analysis methods. (i) Sample analysis: microdialysis samples.
The dialysate samples were stored at −80°C until analysis at the University of Florida, Department of Pharmaceutics (Gainesville, FL), using a validated high-performance liquid chromatography-UV method. The limit of quantification for this method was 0.1 μg/ml. An Agilent 1100 series high-performance liquid chromatograph with a UV detector was used with a reverse-phase column (Supelco Discovery C18). The mobile phase consisted of water and acetonitrile (95:5). The flow rate was 1 ml/min. The injection volume was 20 μl, and the detection wavelength was 300 nm. The true tissue ISF drug concentrations were calculated from the dialysate concentrations and adjusted with the recovery value. The calculation was as follows: CISFtissue = 100 × Cdialysate/recovery value.
(ii) Sample analysis: plasma samples.
Plasma samples were stored at −80°C until shipment with dry ice to SFBC Analytical Laboratories, North Wales, PA. They were analyzed there using a validated liquid chromatography-tandem mass spectrometry method for ceftobiprole with K2EDTA and citric acid. The limit of quantification was 0.050 μg/ml. A Perkin-Elmer 200 HPLC autosampler (4°C) and pump were used. A gradient elution with two mobile phases (phase A consisting of formic acid-methanol-water at 1:5:95; phase B consisting of formic acid-methanol-water at 1:50:50) on a reverse-phase column (Synergi 4μ Polar-RP; 50 by 2 mm) was used. A tandem mass spectrometer (PE Sciex API 4000 Series) with a turbo ionspray interface was set to monitor ceftobiprole at m/z 535 to 308 and m/z 539 to 312 for the internal standard (ceftobiprole-d4). Plasma samples were prepared by adding 50 μl of sample to 50 μl of internal standard, mixing, and then adding 200 μl of 0.1% formic acid in acetonitrile and 300 μl of 10% perchloric acid. The samples were then vortexed for 1 min and centrifuged at 3,000 rpm for 15 min. Protein binding was determined by ultrafiltration.
Data analysis.
Total plasma drug concentrations were adjusted based on individual protein binding prior to PK analysis to determine the concentration of free drug in plasma. PK analysis was performed using commercially available software (WinNonlin, version 5.2; Pharsight Corp., Mountain View, CA) by noncompartmental analysis. The area under the concentration-time curve from the first to the last sampling (AUC0-last) was calculated using the linear trapezoidal rule. The AUC0-∞ was calculated as AUC0-last plus Clast/λz. The elimination rate constant, λz, was calculated using linear regression of the concentration-time data. The points chosen for calculation of λz were based on the best fit of the terminal phase and visual inspection. The AUCs were compared using Wilcoxon's matched paired tests. A P value of <0.05 in a two-sided test was considered significant.
RESULTS
The aim of this study was to assess the tissue penetration of ceftobiprole into skeletal muscle and s.c. adipose tissue. A pilot study was first conducted to test the feasibility of using microdialysis with ceftobiprole and to determine the washout period needed to ensure that no drug was remaining in the tissues and the microdialysis system at the time of dosing.
The mean recovery values (± standard deviations [SD]) in the pilot study were 64.1% ± 12.6% and 47.5% ± 3.3% for s.c. adipose and muscle tissue, respectively. In the pilot study one probe malfunctioned after insertion, and therefore the mean recovery value for the muscle is calculated from two samples. From the pilot study it was determined that the recovery was high enough to continue to the main study and that a 4-h washout period should be allotted after calibration and prior to dosing.
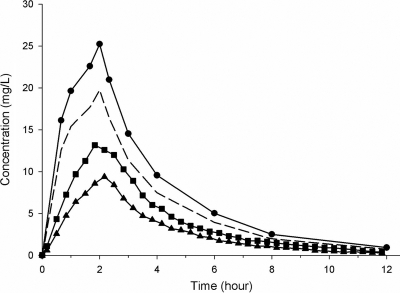
The mean protein binding between all subjects was 21.7% ± 6.6%. The mean recovery values in muscle and s.c. adipose tissue for the main study were 58.3% ± 5.1% and 59.4% ± 5.4%, respectively, and the measured concentrations were adjusted accordingly. The mean concentration-time profiles of drug in plasma, muscle, and s.c. adipose tissue and of free drug in plasma are presented in Fig. 1. The PK parameters are summarized in Table 1. The mean fAUC0-∞ (± SD; f indicates free drug) ratios of tissue ISF compared to plasma were 0.69 ± 0.13 and 0.49 ± 0.28 for skeletal muscle and s.c. adipose tissue, respectively. The results show that there is a significant difference between the fAUC in plasma and the AUCs of both soft tissues. Additionally, the AUC of skeletal muscle is significantly higher than the AUC of s.c. adipose tissue.
FIG. 1.
Mean ceftobiprole concentration in plasma (circles), skeletal muscle (squares), and s.c. adipose tissue (triangles) over 12 h. The concentration of free drug in plasma (dashed line) was calculated based on the plasma protein binding of each individual patient.
TABLE 1.
Noncompartmental pharmacokinetic analysis
| PK parametera | Value for the parameter (mean ± SD) in:b
|
|||
|---|---|---|---|---|
| Plasma (total drug) | Plasma (free drug) | s.c. adipose tissue | Muscle | |
| Cmax (mg/liter) | 25.8 ± 2.96 | 20.2 ± 2.63 | 9.61 ± 4.74 | 14.0 ± 3.22 |
| Tmax (mg/liter) | 1.92 ± 0.15 | ND | 2.25 ± 0.21 | 2.25 ± 0.14 |
| t1/2 (h) | 2.61 ± 0.33 | ND | 2.56 ± 0.39 | 2.61 ± 0.52 |
| AUC0-last (h·mg/liter) | 97.1 ± 10.3 | 76.0 ± 8.81 | 34.3 ± 19.0 | 50.6 ± 10.9 |
| AUC0-∞ (h·mg/liter) | 98.0 ± 10.5 | 76.9 ± 8.94 | 36.5 ± 19.4 | 53.2 ± 11.5 |
| CL (liter/h) | 5.15 ± 0.53 | ND | ND | ND |
| Vz (liters) | 19.4 ± 3.61 | ND | ND | ND |
| Vss (liters) | 14.6 ± 2.17 | ND | ND | ND |
| AUCISF/fAUCplasma | 0.49 ± 0.28 | 0.69 ± 0.13 | ||
t1/2, half-life; CL, clearance; Vz, apparent volume of distribution.
ND, not determined.
DISCUSSION
This study shows that ceftobiprole distributes into the ISF of s.c. adipose tissue (fAUCs.c.adipose/fAUCplasma, 0.49 ± 0.28) and skeletal muscle, (fAUCmuscle/fAUCplasma, 0.69 ± 0.13). The degree of tissue penetration with ceftobiprole correlates well with other cephalosporins such as cefpodoxime (14), cefixime (14), cefpirome (10, 21), and cefaclor (7) (Table 2). There was a significant difference between the fAUC of plasma and the AUC of both tissues and between the AUC of muscle and the AUC of s.c. adipose tissue. The differences in penetration ratios can be due to several factors including the perfusion of the particular tissue, local capillary density (7), the degree of tissue binding, the possibility of active transporters (6), loss of drug from the peripheral compartments (13), and physiochemical properties of the compound (6), such as lipophilicity. Therefore, it is important to measure the free, active drug in each tissue and not make the assumption that free-drug levels in plasma equal free-drug levels in tissue, even in well-perfused tissues.
TABLE 2.
Soft-tissue penetration of four cephalosporins determined by microdialysis
| Parameter | Mean AUC (mg·h/liter) for the indicated drug (dosing regimen)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cefpodoxime (400 mg orally)a | Cefixime (400 mg orally)a | Cefpirome (2 g i.v. in 15 min)b | Cefpirome (2 g i.v. in 10 min)c | Cefpirome (2 g i.v. in 8 h)d | Cefaclor (500 mg of IR orally)e | Cefaclor (500 mg of MR orally)f | Cefaclor (750 mg of MR orally)f | |
| AUCplasma | 22.4 | 25.6 | 17.6 | 13.7 | 22.1 | |||
| fAUCplasma | 17.7 | 9.0 | 275.0 | 230.7 | 175.0 | 13.2 | 10.3 | 16.6 |
| fAUCmuscle | 15.4 | 7.3 | 130.0 | 80.0 | 9.49 | 7.02 | 11.53 | |
| fAUCs.c.adipose | 218.5 | 117.0 | 87.2 | |||||
| fAUCmuscle/fAUCplasma | 0.89 | 0.84 | 0.56 | 0.46 | 0.73 | 0.67 | 0.70 | |
| fAUCs.c.adipose/fAUCplasma | 0.79 | 0.51 | 0.50 | |||||
AUC0-∞ is presented. fAUC was calculated based on protein binding (14).
AUC0-4 is presented. Data are from healthy volunteers only (21).
AUC0-8 is presented (10).
Only 8 h was observed. AUC0-8 is presented (10).
AUC0-∞ is presented. fAUC was calculated based on protein binding (7). IR, immediate release.
AUC0-∞ is presented. fAUC was calculated based on protein binding (7). MR, maximum release.
The PKs determined in this study are in good agreement with previously summarized results (17). The half-life, clearance, maximum concentration [Cmax], and AUC of 2.61 h, 5.15 liter/h, 25.8 mg/liter, and 98.0 mg·h/liter, respectively, were all within 1 standard deviation of the previously reported parameters with the same dosing regimen (17; also B. Murthy, D. Skee, D. Wexler, D. Balis, I. Chang, D. Desai-Kreiger, and G. Noel, presented at the 17th European Conference of Clinical Microbiology and Infectious Disease, Munich, Germany, 31 March to 3 April 2007). The volume of distribution at steady state (Vss) of 14.6 liters is lower in this study than the reported value of 21.7 liters for a single dose. However, it is in agreement with the multiple-dose Vss of 15.5 liters. The value of V suggests that this compound distributes to the ISF, which is common with this antibiotic class. This property is advantageous because the ISF is often the location of infectious pathogens.
The major advantage to the microdialysis technique is the ability to measure the free drug at the site of action, usually the ISF of soft tissues in regard to skin and skin structure infections. It is this concentration that should be used to measure if efficacy breakpoints are met. For β-lactams the percentage of the time the free drug concentration remains above the MIC (fT>MIC) is thought to predict efficacy. It has been shown in neutropenic animals that efficacy is established if the concentration remains above the MIC for at least 40 to 50% of the dosing interval (3-5), i.e., 3.2 to 4 h of an 8-h dosing interval. The MIC90 for ceftobiprole against methicillin-resistant S. aureus (12) and penicillin-resistant S. pneumoniae (9) has been reported as 2 mg/liter. The drug concentrations in the ISF of both skeletal muscle and s.c. adipose tissue remained above 2 mg/liter for at least 50% of the dosing interval, and, therefore, this dosing regimen should be efficacious with these subcutaneous soft tissue pathogens. Also, sufficient concentrations are achieved to meet the efficacy breakpoint in organisms with an MIC90 of 4 mg/liter in skeletal muscle, i.e., 53.6% ± 9.62% fT>MIC. In s.c. adipose tissue the fT>MIC of 35.1% ± 22.2% is close to 40% of an 8-h dosing interval, and it has been suggested that the fT>MIC is less than 40% for S. aureus and S. pneumoniae (5). It is important to remember that further studies should be conducted in patients to see the relationship between the host response, free ceftobiprole concentration at the site of action, and clinical outcome. Additionally, ceftobiprole has demonstrated clinical cure rates similar to those of vancomycin in gram-positive complicated skin and skin structure infections and to vancomycin plus ceftazidime in both gram-positive and gram-negative complicated skin and skin structure infections in large-scale pivotal studies (19, 20). This study demonstrates that ceftobiprole distributes into the ISF of soft tissues in healthy volunteers. This finding and ceftobiprole's wide range of activity make it a promising new single agent for the treatment of complicated skin and skin structure infections.
Acknowledgments
We thank the General Clinical Research Center at the University of Florida/Shands Hospital, without whose support this project would not have been possible. We thank the GLP laboratory of the Department of Pharmaceutics at the University of Florida.
We also thank Johnson & Johnson Pharmaceutical Research and Development for funding this study. This research was also supported in part by the University of Florida General Clinical Research Center, grant M01 RR000082 NCRR/NIH.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Center for Drug Evaluation and Research. 2003. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. U.S. Department of Health and Human Services, Food and Drug Administration, Silver Spring, MD.
- 2.Chaurasia, C. S., M. Muller, E. D. Bashaw, E. Benfeldt, J. Bolinder, R. Bullock, P. M. Bungay, E. C. DeLange, H. Derendorf, W. F. Elmquist, M. Hammarlund-Udenaes, C. Joukhadar, D. L. Kellogg, Jr., C. E. Lunte, C. H. Nordstrom, H. Rollema, R. J. Sawchuk, B. W. Cheung, V. P. Shah, L. Stahle, U. Ungerstedt, D. F. Welty, and H. Yeo. 2007. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 24:1014-1025. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):S233-S237. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 2002. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-22. In D. H. Nightingale, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and clinical practice. Informa Healthcare, Inc., New York, NY.
- 5.Craig, W. A., and D. R. Andes. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehghanyar, P., C. Burger, M. Zeitlinger, F. Islinger, F. Kovar, M. Muller, C. Kloft, and C. Joukhadar. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de La Pena, A., M. Brunner, H. G. Eichler, E. Rehak, J. Gross, U. Thyroff-Friesinger, M. Muller, and H. Derendorf. 2002. Comparative target site pharmacokinetics of immediate- and modified-release formulations of cefaclor in humans. J. Clin. Pharmacol. 42:403-411. [DOI] [PubMed] [Google Scholar]
- 8.European Agency for the Evaluation of Medicinal Products/Committee for Proprietary Medicinal Products. 27 July 2000, posting date. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. European Agency for the Evaluation of Medicinal Products London, United Kingdom. http://www.emea.europa.eu/pdfs/human/ewp/265599en.pdf.
- 9.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollenstein, U., M. Brunner, B. X. Mayer, S. Delacher, B. Erovic, H. G. Eichler, and M. Muller. 2000. Target site concentrations after continuous infusion and bolus injection of cefpirome to healthy volunteers. Clin. Pharmacol. Ther. 67:229-236. [DOI] [PubMed] [Google Scholar]
- 11.Janssen-Ortho, Inc. 2008. Zeftera product monograph. Janssen-Ortho, Inc., Toronto, Ontario, Canada. http://www.janssen-ortho.com/JOI/pdf_files/Zeftera_E.pdf.
- 12.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 13.Karjagin, J., S. Lefeuvre, K. Oselin, K. Kipper, S. Marchand, A. Tikkerberi, J. Starkopf, W. Couet, and R. J. Sawchuk. 2008. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin. Pharmacol. Ther. 83:452-459. [DOI] [PubMed] [Google Scholar]
- 14.Liu, P., M. Muller, M. Grant, B. Obermann, and H. Derendorf. 2005. Tissue penetration of cefpodoxime and cefixime in healthy subjects. J. Clin. Pharmacol. 45:564-569. [DOI] [PubMed] [Google Scholar]
- 15.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 16.Muller, M. 2000. Microdialysis in clinical drug delivery studies. Adv. Drug Deliv. Rev. 45:255-269. [DOI] [PubMed] [Google Scholar]
- 17.Murthy, B., and A. Schmitt-Hoffmann. 2008. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 47:21-33. [DOI] [PubMed] [Google Scholar]
- 18.Nix, D. E., K. R. Matthias, and E. C. Ferguson. 2004. Effect of ertapenem protein binding on killing of bacteria. Antimicrob. Agents Chemother. 48:3419-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel, G. J., K. Bush, P. Bagchi, J. Ianus, and R. S. Strauss. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 46:647-655. [DOI] [PubMed] [Google Scholar]
- 20.Noel, G. J., R. S. Strauss, K. Amsler, M. Heep, R. Pypstra, and J. S. Solomkin. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 52:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauermann, R., G. Delle-Karth, C. Marsik, I. Steiner, M. Zeitlinger, B. X. Mayer-Helm, A. Georgopoulos, M. Muller, and C. Joukhadar. 2005. Pharmacokinetics and pharmacodynamics of cefpirome in subcutaneous adipose tissue of septic patients. Antimicrob. Agents Chemother. 49:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahl, M., R. Bouw, A. Jackson, and V. Pay. 2002. Human microdialysis. Curr. Pharm. Biotechnol. 3:165-178. [DOI] [PubMed] [Google Scholar]
- 23.Stahle, L., P. Arner, and U. Ungerstedt. 1991. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 49:1853-1858. [DOI] [PubMed] [Google Scholar]
- 24.Theuretzbacher, U. 2007. Tissue penetration of antibacterial agents: how should this be incorporated into pharmacodynamic analyses? Curr. Opin. Pharmacol. 7:498-504. [DOI] [PubMed] [Google Scholar]
- 25.Zeitlinger, M., R. Sauermann, M. Fille, J. Hausdorfer, I. Leitner, and M. Muller. 2008. Plasma protein binding of fluoroquinolones affects antimicrobial activity. J. Antimicrob. Chemother. 61:561-567. [DOI] [PubMed] [Google Scholar]