Abstract
Twice-daily 7-day regimens of tigecycline (7 mg/kg) and vancomycin (50 mg/kg) were compared in a rat tissue cage model of chronic foreign-body infection due to methicillin (meticillin)-resistant Staphylococcus aureus strain MRGR3. Subcutaneously administered tigecycline reached levels in tissue cage fluid that were nearly equivalent or slightly superior to the antibiotic MIC (0.5 μg/ml) for strain MRGR3. After 7 days, equivalent, significant reductions in bacterial counts were recorded for tigecycline-treated and vancomycin-treated rats, compared with those for untreated animals.
Antimicrobial therapy for foreign-body infections due to Staphylococcus aureus is challenging (38), in particular for multidrug-resistant hospital-associated and community-acquired isolates of methicillin (meticillin)-resistant S. aureus (MRSA) (3, 12, 15, 16). Tigecycline is a novel injectable glycylcycline broad-spectrum antibiotic that demonstrates excellent in vitro and in vivo activity against MRSA and other multiresistant organisms (9, 11, 22, 28, 32) and can overcome both major tetracycline resistance mechanisms, namely ribosomal protection (10, 23) and efflux (4, 27). Tigecycline has shown good activity in various animal models of serious MRSA infections (21, 39, 40), as well as against biofilm-embedded bacteria (14, 26).
We previously used a rat tissue cage model of S. aureus chronic foreign-body infections for evaluating a number of antimicrobial agents, namely vancomycin (17), teicoplanin (31), imipenem (30), ceftobiprole (37), daptomycin (29, 35), and several fluoroquinolones (2, 17, 36). This study reports the activity of tigecycline compared to that of the reference anti-MRSA agent vancomycin in a tissue cage model of MRSA chronic foreign-body infection.
(This study was presented in part at the 18th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, April 2008.)
MRSA strain MRGR3, whose properties were previously described (2, 5, 17, 29-31, 36, 37), was used for in vitro and in vivo studies. Strain MRGR3 is resistant to methicillin, gentamicin, erythromycin, tetracycline, and chloramphenicol (17).
MICs of freshly prepared (1, 13, 25) tigecycline (Wyeth Research, Collegeville, PA) or vancomycin (Vancocin; Teva Pharma AG, Switzerland) for MRSA strain MRGR3 or quality control S. aureus ATCC 29213 were determined by broth macrodilution in cation-adjusted Mueller-Hinton broth (CAMHB), according to Clinical and Laboratory Standards Institute guidelines (7).
The animal protocol used for evaluating the in vivo activities of tigecycline and vancomycin was previously described in detail (17, 37) and approved by the Ethics Committee of the Faculty of Medicine, University of Geneva, and the Veterinary Office of the State of Geneva. Three weeks after subcutaneous implantation of four tissue cages per animal in anesthetized Wistar rats (37), tissue cage fluids were checked for sterility (17).
Pilot pharmacokinetic studies were performed using groups of noninfected rats to find an adequate dosing regimen of tigecycline for therapy of tissue cage infections as described previously (37). Tigecycline levels in cage fluids (and blood) were estimated by a microbiological assay (21), with a detection limit of 0.25 μg/ml. To account for protein binding, all plasma or tissue cage fluid samples were diluted with 1 volume of phosphate-buffered saline and assayed in duplicate, with reference to duplicate standard concentrations (0.25 to 8 μg/ml) of tigecycline, in phosphate-buffered saline supplemented with 50% plasma or pooled tissue cage fluids, respectively.
Each tissue cage was chronically infected by inoculating 5 × 105 CFU of log-phase MRGR3 (37). Two weeks later, all rats whose cage fluids contained ≥105 CFU/ml received twice-daily doses (by the subcutaneous route for 7 days) of tigecycline (7 mg/kg), vancomycin (50 mg/kg), or no antibiotic (control group). Differences in CFU counts of cage fluid quantitative cultures, performed at day 1 (before treatment) and day 8 (12 h after the last injection of either tigecycline or vancomycin), were expressed as the change in number of log10 CFU/ml (37) and evaluated by one-way analysis of variance and post-analysis of variance pairwise comparisons between individual groups via the Tukey HSD test (http://faculty.vassar.edu/lowry/VassarStats.html), using P values of <0.05 with two-tailed significance levels.
Tigecycline resistance was screened by plating 10-fold-diluted cage fluids (100 μl) onto MH agar supplemented with 2 μg/ml tigecycline. No single colony grew on tigecycline-supplemented plates inoculated with 108 CFU of in vitro-grown cultures of strain MRGR3.
The MIC of tigecycline in CAMHB for MRSA strain MRGR3 was 0.5 μg/ml, namely at the upper limit of susceptibility breakpoints (7), and was unaffected by supplementation of CAMHB with 50% tissue cage fluid (data not shown). Since tigecycline did not produce a 3-log10 reduction in the number of MRGR3 CFU/ml, it was not considered bactericidal. Nevertheless, supra-MIC levels (1, 2, and 4 μg/ml) of tigecycline produced a 2- to 3-log10 decrease in the number of MRGR3 CFU/ml at 24 h. The vancomycin MIC and minimal bactericidal concentration for strain MRGR3 were 1 and 2 μg/ml, respectively (17).
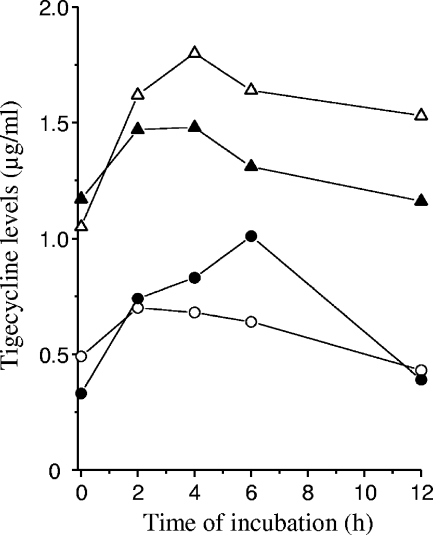
Average tigecycline levels, scored for tissue cage fluids (n = 6) from 0 to 12 h after subcutaneous administration, remained quite constant over time, showing ≤3-fold variations between results at different time points and moderate animal-to-animal differences (Fig. 1). A 7-mg/kg twice-daily regimen yielded cage fluid levels of 0.39 to 0.70 μg/ml tigecycline at day 4 and 0.33 to 1.01 μg/ml at day 7, such results thus being nearly equivalent or slightly superior to the antibiotic MIC for MRGR3. Tigecycline plasma levels at 2 h on day 4 were 1.87 ± 0.66 μg/ml, in agreement with other reports (8, 21). A 14-mg/kg twice-daily regimen led to plasma and tissue cage fluid tigecycline levels ca. twofold higher than the 7-mg/kg regimen (Fig. 1). Average peak and trough cage fluid levels of vancomycin were previously determined (17) as 12 and 2 μg/ml at 4 and 12 h, respectively.
FIG. 1.
Pharmacokinetic levels of tigecycline in tissue cage fluids of rats on day 4 (open symbols) or day 7 (closed symbols) of therapy every 12 h with 7 mg/kg (○) or 14 mg/kg (▵) of tigecycline. Each value is the mean result of six determinations.
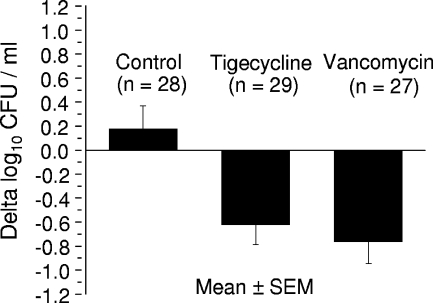
At day 1, mean bacterial counts for MRGR3-infected cages were not significantly different (P = 0.65) in controls (6.85 ± 0.19 log10 CFU/ml; n = 28), tigecycline-treated rats (6.92 ± 0.13 log10 CFU/ml; n = 29), or vancomycin-treated rats (6.70 ± 0.18 log10 CFU/ml; n = 27). At day 8, significant (P < 0.01 versus controls) reductions were recorded in bacterial counts in cage fluids of both tigecycline-treated (−0.62 ± 0.17 CFU/ml; n = 29) and vancomycin-treated (−0.76 ± 0.18 log10 CFU/ml; n = 27) rats, whereas the bacterial counts for controls slightly increased (+0.18 ± 0.19 log10 CFU/ml; n = 28) (Fig. 2). The reductions in CFU counts for vancomycin-treated and tigecycline-treated rats were not significantly different. Finally, no MRGR3 isolate showing increased tigecycline MIC was observed in any posttherapy cage fluid sample (n = 29). The lack of emergence of MRGR3 derivates with diminished susceptibility to tigecycline is consistent with the difficulty in selecting laboratory-derived, tigecycline-resistant mutants of S. aureus (18), and it contrasts with the emergence of resistant subpopulations during low-dose daptomycin therapy of S. aureus-infected tissue cages (35).
FIG. 2.
Decrease in viable counts of MRSA MRGR3 in tissue cage fluids of rats treated for 7 days with tigecycline or vancomycin.
Several studies performed with the rat tissue cage model demonstrated the low initial in vivo response of foreign-body-associated chronic MRSA infections (2, 5, 6, 17, 20, 29-31, 35-37). A much greater reduction of viable MRSA counts in cage fluids requires longer periods of antibiotic therapy (5), as found in clinical situations with foreign-body infections (38). Major pharmacokinetic properties of tigecycline, observed in human and animal studies, are very low plasma levels, long half-lives, and high volumes of distribution indicating extensive tigecycline distribution into the tissues (8, 11, 19, 28, 32, 40). In line with previous observations that showed a requirement for active, preferentially bactericidal, antibiotic levels for obtaining significant reductions of CFU counts in MRSA-infected cage fluids (29, 37), we selected for therapy a twice-daily 7-mg/kg regimen yielding cage fluid tigecycline levels above the MIC for strain MRGR3 for >50% of the dosing interval (32, 33), while minimizing the occurrence of side effects previously observed with higher-dose regimens (39). Our regimen is similar to those required for activity in other animal models of hard-to-treat S. aureus infections, such as endocarditis or osteomyelitis (21, 39), although its relevance to human therapy is not fully defined (32). In addition, the incomplete in vitro killing activity of tigecycline, namely a <3-log10 reduction in number of MRGR3 CFU at 24 h, prevents a pharmacodynamic analysis of tigecycline in vivo activity more detailed than those of previously evaluated bactericidal antibiotics in MRSA-infected cages (29, 37). We can also speculate that other properties of tigecycline, namely its in vivo activity against intracellular, slowly growing, or biofilm-forming bacteria, might significantly contribute to tigecycline activity in MRSA-infected cages (34). Indeed, high intracellular levels of tigecycline were shown to accumulate in human polymorphonuclear neutrophils and prevent growth of phagocytized bacteria (24). Further studies are needed to elucidate the mechanisms of tigecycline activity against hard-to-treat MRSA infections.
Acknowledgments
This study was supported in part by research grants from Wyeth Pharmaceuticals (Collegeville, PA) and grants 320000-116518 (to P.V.) and 310030-125109 (to D.P.L.) from the Swiss National Science Foundation.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Bradford, P. A., P. J. Petersen, M. Young, C. H. Jones, M. Tischler, and J. O'Connell. 2005. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob. Agents Chemother. 49:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagni, A., C. Chuard, P. E. Vaudaux, J. Schrenzel, and D. P. Lew. 1995. Comparison of sparfloxacin, temafloxacin, and ciprofloxacin for prophylaxis and treatment of experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 39:1655-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 4.Chopra, I. 2002. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist. Updates 5:119-125. [DOI] [PubMed] [Google Scholar]
- 5.Chuard, C., M. Herrmann, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1991. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob. Agents Chemother. 35:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuard, C., J. C. Lucet, P. Rohner, M. Herrmann, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1991. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J. Infect. Dis. 163:1369-1373. [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Crandon, J. L., M. A. Banevicius, and D. P. Nicolau. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob. Agents Chemother. 53:1165-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draghi, D. C., S. Tench, M. J. Dowzicky, and D. F. Sahm. 2008. Baseline in vitro activity of tigecycline among key bacterial pathogens exhibiting multidrug resistance. Chemotherapy 54:91-100. [DOI] [PubMed] [Google Scholar]
- 10.Fluit, A. C., A. Florijn, J. Verhoef, and D. Milatovic. 2005. Presence of tetracycline resistance determinants and susceptibility to tigecycline and minocycline. Antimicrob. Agents Chemother. 49:1636-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrison, M. W., J. J. Neumiller, and S. M. Setter. 2005. Tigecycline: an investigational glycylcycline antimicrobial with activity against resistant gram-positive organisms. Clin. Ther. 27:12-22. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K. 2001. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 1:147-155. [DOI] [PubMed] [Google Scholar]
- 13.Hope, R., M. Warner, S. Mushtaq, M. E. Ward, T. Parsons, and D. M. Livermore. 2005. Effect of medium type, age and aeration on the MICs of tigecycline and classical tetracyclines. J. Antimicrob. Chemother. 56:1042-1046. [DOI] [PubMed] [Google Scholar]
- 14.Labthavikul, P., P. J. Petersen, and P. A. Bradford. 2003. In vitro activity of tigecycline against Staphylococcus epidermidis growing in an adherent-cell biofilm model. Antimicrob. Agents Chemother. 47:3967-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 47:3040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy, F. D. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Investig. 111:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAleese, F., P. Petersen, A. Ruzin, P. M. Dunman, E. Murphy, S. J. Projan, and P. A. Bradford. 2005. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob. Agents Chemother. 49:1865-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murillo, O., A. Domenech, G. Euba, R. Verdaguer, F. Tubau, J. Cabo, C. Cabellos, F. Gudiol, and J. Ariza. 2008. Efficacy of linezolid alone and in combination with rifampin in staphylococcal experimental foreign-body infection. J. Infect. 57:229-235. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, T. M., J. M. Deitz, P. J. Petersen, S. M. Mikels, and W. J. Weiss. 2000. Therapeutic efficacy of GAR-936, a novel glycylcycline, in a rat model of experimental endocarditis. Antimicrob. Agents Chemother. 44:3022-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noskin, G. A. 2005. Tigecycline: a new glycylcycline for treatment of serious infections. Clin. Infect. Dis. 41(Suppl. 5):S303-S314. [DOI] [PubMed] [Google Scholar]
- 23.Olson, M. W., A. Ruzin, E. Feyfant, T. S. Rush III, J. O'Connell, and P. A. Bradford. 2006. Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob. Agents Chemother. 50:2156-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong, C. T., C. P. Babalola, C. H. Nightingale, and D. P. Nicolau. 2005. Penetration, efflux and intracellular activity of tigecycline in human polymorphonuclear neutrophils (PMNs). J. Antimicrob. Chemother. 56:498-501. [DOI] [PubMed] [Google Scholar]
- 25.Petersen, P. J., and P. A. Bradford. 2005. Effect of medium age and supplementation with the biocatalytic oxygen-reducing reagent oxyrase on in vitro activities of tigecycline against recent clinical isolates. Antimicrob. Agents Chemother. 49:3910-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose, W. E., and M. J. Rybak. 2006. Tigecycline: first of a new class of antimicrobial agents. Pharmacotherapy 26:1099-1110. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein, E., and D. Vaughan. 2005. Tigecycline: a novel glycylcycline. Drugs 65:1317-1336. [DOI] [PubMed] [Google Scholar]
- 29.Schaad, H. J., M. Bento, D. P. Lew, and P. Vaudaux. 2006. Evaluation of high-dose daptomycin for therapy of experimental Staphylococcus aureus foreign body infection. BMC Infect. Dis. 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaad, H. J., C. Chuard, P. Vaudaux, P. Rohner, F. A. Waldvogel, and D. P. Lew. 1994. Comparative efficacies of imipenem, oxacillin and vancomycin for therapy of chronic foreign body infection due to methicillin-susceptible and -resistant Staphylococcus aureus. J. Antimicrob. Chemother. 33:1191-1200. [DOI] [PubMed] [Google Scholar]
- 31.Schaad, H. J., C. Chuard, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1994. Teicoplanin alone or combined with rifampin compared with vancomycin for prophylaxis and treatment of experimental foreign body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 38:1703-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein, G. E., and W. A. Craig. 2006. Tigecycline: a critical analysis. Clin. Infect. Dis. 43:518-524. [DOI] [PubMed] [Google Scholar]
- 33.van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaudaux, P. 1998. Phenotypic antibiotic tolerance of Staphylococcus aureus in implant-related infections: relationship with in vitro colonization of artificial surfaces. Drug Resist. Updates 1:352-357. [DOI] [PubMed] [Google Scholar]
- 35.Vaudaux, P., P. Francois, C. Bisognano, D. Li, D. P. Lew, and J. Schrenzel. 2003. Comparative efficacy of daptomycin and vancomycin in the therapy of experimental foreign body infection due to Staphylococcus aureus. J. Antimicrob. Chemother. 52:89-95. [DOI] [PubMed] [Google Scholar]
- 36.Vaudaux, P., P. Francois, C. Bisognano, J. Schrenzel, and D. P. Lew. 2002. Comparison of levofloxacin, alatrofloxacin, and vancomycin for prophylaxis and treatment of experimental foreign-body-associated infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:1503-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaudaux, P., A. Gjinovci, M. Bento, D. Li, J. Schrenzel, and D. P. Lew. 2005. Intensive therapy with ceftobiprole medocaril of experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3789-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldvogel, F. A., and A. L. Bisno. 2000. Infections associated with indwelling medical devices, p.1-436. American Society for Microbiology, Washington, DC.
- 39.Yin, L. Y., L. Lazzarini, F. Li, C. M. Stevens, and J. H. Calhoun. 2005. Comparative evaluation of tigecycline and vancomycin, with and without rifampicin, in the treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis in a rabbit model. J. Antimicrob. Chemother. 55:995-1002. [DOI] [PubMed] [Google Scholar]
- 40.Zhanel, G. G., K. Homenuik, K. Nichol, A. Noreddin, L. Vercaigne, J. Embil, A. Gin, J. A. Karlowsky, and D. J. Hoban. 2004. The glycylcyclines: a comparative review with the tetracyclines. Drugs 64:63-88. [DOI] [PubMed] [Google Scholar]