Abstract
Sitafloxacin showed MICs of less than or equal to 0.5 μg/ml against 105 isolates of Helicobacter pylori, including 44 isolates with mutations in the gyrA gene. The highest MICs for garenoxacin and levofloxacin were 8 and 64 times, respectively, higher than the highest MICs observed for sitafloxacin.
The guidelines for the management and treatment of Helicobacter pylori infections established by the European Helicobacter Study Group Third Masstricht Consensus Report recommend an eradication antimicrobial chemotherapy consisting of amoxicillin, clarithromycin, and a proton pump inhibitor alone or in combination with metronidazole and clarithromycin (10). On the other hand, a trend toward increased clarithromycin resistance in Japan has been reported (8); furthermore, high metronidazole resistance rates associated with H. pylori eradication failure have been seen in the United States, Europe, and Asia with the exception of Japan (11). In the search for alternative eradication treatment regimens, it has been recently reported in the United States and Europe that levofloxacin may be efficacious in H. pylori eradication therapy (7, 13). At the same time, the increased use of levofloxacin-based eradication regimens has led to increasing resistance to levofloxacin in H. pylori as a result of mutations in the quinolone resistance-determining region (QRDR) of the gyrA gene correlating with the decreased effectiveness of levofloxacin in eradication regimens (16). Sitafloxacin is a recently developed fluoroquinolone with wide-spectrum activity, ranging from gram-positive cocci to gram-negative bacilli (1, 15). We studied the effect of mutations in the gyrA gene and its impact on the antimicrobial activity of sitafloxacin in H. pylori.
(This study was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 25 to 28 October 2008.)
A total of 105 H. pylori isolates were recovered from the gastric mucosa of patients presenting with gastroduodenal diseases in health care facilities in Japan between 2004 and 2005. Of the 105 patients, 57 (54.3%) were males and 48 (45.7%) were females, and the average age was 57.9 years (range in age from 21 to 84). None of the patients had previously undergone eradication therapy. The spectrum of peptic ulcers included 39 (37.1%) cases of chronic gastritis, 21 (20.0%) gastric ulcers, 18 (17.1%) duodenal ulcers, 9 (8.6%) gastric cancers, 8 (7.6%) gastroduodenal ulcers, and 10 (9.5%) cases with other causes or an unspecified diagnosis. Only one isolate per patient was included among the 105 isolates.
Susceptibilities to sitafloxacin (Daiichi Sankyo, Japan), garenoxacin [a novel des-fluoro(6)quinolone (Astellas, Japan) (6)], and levofloxacin (Daiichi Sankyo, Japan) were determined by agar dilution method according to CLSI guidelines by using drugs with known potency (4, 5). The agar dilution method was performed by serial twofold dilution on Mueller-Hinton agar (Becton Dickinson, MD) with 5% sheep blood using 1 to 3 μl of a McFarland 2.0-adjusted inoculum and incubation at 35 ± 2°C for 72 h under microaerophilic conditions. For quality control, H. pylori ATCC 43504 was tested with each run.
PCR amplification and sequence analysis of the QRDR of gyrA was performed as previously described by Nishizawa et al. (12). The specific primers used for amplification were GYRA-F (5′-TTTRGCTTATTCMATGAGCGT-3′) and GYRA-R (5′-GCAGACGGCTTGGTARAATA-3′). For sequencing, the ABI Prism 3130xl genetic analyzer (Perkin-Elmer, ABI, CA) was used. Sequences were compared to that of the H. pylori wild-type strain (GenBank accession no. L29481).
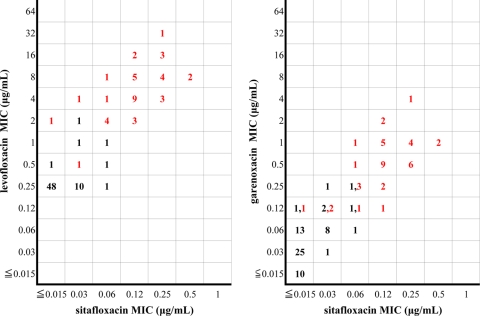
Forty-four of the 105 H. pylori isolates exhibited mutations in the gyrA gene (Table 1). Mutations at Asn87 were observed in 14 isolates, while mutations were seen at Asp91 in 25 isolates. The remaining five isolates had mutations in other regions. Table 2 shows the effect of changes in the gyrA gene in the QRDR and its effect on the MICs of sitafloxacin, garenoxacin, and levofloxacin. Mutations involving Asn87 resulted in a shift to higher MIC levels of the drugs than mutations in other regions. Sitafloxacin demonstrated the narrowest MIC distribution with a MIC of ≤0.5 μg/ml against all isolates. In contrast, the highest MICs for garenoxacin and levofloxacin were 8 and 64 times, respectively, higher than the highest MIC observed for sitafloxacin. A scattergram depicting sitafloxacin MICs versus levofloxacin or garenoxacin MICs for 105 isolates with or without gyrA mutations is shown in Fig. 1. From the scattergram, the increase in garenoxacin and levofloxacin MICs relative to the sitafloxacin MICs is observed. With respect to levofloxacin-resistant isolates with MICs ranging from 2 to 32 μg/ml, garenoxacin and sitafloxacin demonstrated MICs ranging from 0.125 to 4 μg/ml and ≥0.015 to 0.5 μg/ml, respectively.
TABLE 1.
Genetic characteristics of Helicobacter pylori isolates in this study
| Strain and no. of isolates | Amino acid substitution at position:
|
||
|---|---|---|---|
| 87 | 91 | Other | |
| Strains with wild-type gyrA | |||
| 61 | Asn | Asp | |
| Strains with gyrA mutations at Asn87 (n = 14) | |||
| 11 | Lys | Asp | |
| 2 | Lys | Asp | Asp143Glu |
| 1 | Ile | Asp | |
| Strains with gyrA mutations at Asp91 (n = 25) | |||
| 9 | Asn | Gly | |
| 1 | Asn | Gly | Asp145Gly |
| 5 | Asn | Asn | |
| 2 | Asn | Asn | Asp143Glu |
| 6 | Asn | Tyr | |
| 1 | Asn | Tyr | Ala97Val |
| 1 | Asn | Tyr | Ala129Val |
| Strains with gyrA mutations in other regions (n = 5) | |||
| 1 | Asn | Asp | Thr62Ile |
| 1 | Asn | Asp | Asp99Val |
| 1 | Asn | Asp | Arg130Lys |
| 1 | Asn | Asp | Asp143Glu |
| 1 | Asn | Asp | Lys158Arg |
TABLE 2.
Correlation of quinolone MICs with gyrA mutations for 105 Helicobacter pylori isolates
| Agent | gyrA mutation | No. of isolates | MIC range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| Sitafloxacin | Asn87 | 14 | 0.03-0.5 | 0.25 | 0.5 |
| Asp91 | 25 | ≤0.015-0.25 | 0.12 | 0.25 | |
| Other | 5 | ≤0.015-0.12 | |||
| None | 61 | ≤0.015-0.06 | 0.03 | 0.03 | |
| Garenoxacin | Asn87 | 14 | 0.12-4 | 0.5 | 1 |
| Asp91 | 25 | 0.12-2 | 0.5 | 1 | |
| Other | 5 | ≤0.015-0.5 | |||
| None | 61 | ≤0.015-0.25 | 0.03 | 0.06 | |
| Levofloxacin | Asn87 | 14 | 4-32 | 8 | 16 |
| Asp91 | 25 | 2-8 | 4 | 8 | |
| Other | 5 | 0.12-4 | |||
| None | 61 | 0.12-2 | 0.25 | 0.25 |
FIG. 1.
Scattergram of sitafloxacin MICs versus levofloxacin or garenoxacin MICs for 105 Helicobacter pylori isolates. The red and black numbers indicate the frequency of isolates with and without gyrA mutations, respectively.
In recent years, the increased resistance to clarithromycin and metronidazole of H. pylori has led to the inclusion of fluoroquinolones in eradication therapy in light of their minimal side effects and improved eradication rates (7, 13). However, resistance to a fluoroquinolone as a result of mutations in gyrA has been reported (12). Furthermore, a significant reduction in eradication effectiveness was observed when levofloxacin was used against H. pylori isolates exhibiting gyrA mutations compared to H. pylori isolates with no mutations in gyrA (16). Nishizawa et al. found elevated MICs to gatifloxacin along with mutations in gyrA in 47.9% of the isolates recovered from patients who had failed H. pylori eradication therapy (12).
We observed that 41 (39%) of the H. pylori isolates recovered from patients prior to undergoing their first eradication regimen already showed resistance to levofloxacin based on a breakpoint of >1 μg/ml (2). The rate of resistance to levofloxacin we observed was considerably higher than the figure found in the American College of Gastroenterology Guideline (3). The prevalence of levofloxacin-resistant H. pylori may be associated with the increasing use of fluoroquinolones in clinical practice for many indications in Japan since the 1980s. There was little difference observed in the rate of resistance to clarithromycin between levofloxacin-resistant (14/41 [34%]) and -susceptible isolates (20/64 [31%]) in this study (data not shown). Compared to data generated in southeast Asia and Europe (9, 14), the prevalence of metronidazole-resistant isolates observed in our study was lower (5/105 [4.8%]). Two of the five isolates were resistant to levofloxacin.
Bogaerts et al. has previously reported that two amino acid substitutions due to mutations in gyrA elevated MICs to levofloxacin, ciprofloxacin, and moxifloxacin (2). While we did not observe H. pylori isolates with mutations in both Asn87 and Asp91, there is a need for continued surveillance for the emergence of high resistance in Japan in light of the high use of fluoroquinolones.
In this study, we observed the superior antibacterial activity of sitafloxacin against H. pylori compared to that of levofloxacin and garenoxacin even in the presence of mutations in gyrA. With respect to other organisms, sitafloxacin has been reported to have high affinity to DNA gyrase and topoisomerase IV along with superior antibacterial activity (1). As H. pylori lacks the topoisomerase IV enzyme, the high affinity of sitafloxacin to DNA gyrase may account for its lower MIC against H. pylori. Our report is the first to demonstrate the antibacterial activity of sitafloxacin against H. pylori isolates with gyrA mutations. Based on sitafloxacin's superior antibacterial activity, clinical trials of second- or third-line eradication therapy including sitafloxacin are warranted.
Footnotes
Published ahead of print on 20 April 2009.
REFERENCES
- 1.Akasaka, T., S. Kurosaka, Y. Uchida, M. Tanaka, K. Sato, and I. Hayakawa. 1998. Antibacterial activities and inhibitory effects of sitafloxacin (DU-6859a) and its optical isomers against type II topoisomerases. Antimicrob. Agents Chemother. 42:1284-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaerts, P., C. Berhin, H. Nizet, and Y. Glupczynski. 2006. Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter 11:441-445. [DOI] [PubMed] [Google Scholar]
- 3.Chey, W. D., B. C. Wong, and the Practice Parameters Committee of the American College of Gastroenterology. 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 102:1808-1825. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huczko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisbert, J. P., and F. Morena. 2006. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment. Pharmacol. Ther. 23:35-44. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi, I., K. Murakami, M. Kato, S. Kato, T. Azuma, S. Takahashi, N. Uemura, T. Katsuyama, Y. Fukuda, K. Haruma, M. Nasu, and T. Fujioka. 2007. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J. Clin. Microbiol. 45:4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulsuntiwong, P., C. Chomvarin, K. Chaicumpar, W. Namwat, W. Kaewkes, P. Mairlang, and A. Sangchan. 2008. Antimicrobial susceptibility of Helicobacter pylori isolated from gastric biopsies in dyspeptic patients. Southeast Asian J. Trop. Med. Public Health 39:1102-1109. [PubMed] [Google Scholar]
- 10.Malfertheiner, P., F. Megraud, C. O'Morain, F. Bazzoli, E. El-Omar, D. Graham, R. Hunt, T. Rokkas, N. Vakil, and E. J. Kuipers. 2007. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mégraud, F. 2004. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishizawa, T., H. Suzuki, K. Kurabayashi, T. Masaoka, H. Muraoka, M. Mori, E. Iwasaki, I. Kobayashi, and T. Hibi. 2006. Gatifloxacin resistance and mutations in gyrA after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob. Agents Chemother. 50:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nista, E. C., M. Candelli, M. A. Zocco, F. Cremonini, V. Ojetti, R. Finizio, C. Spada, G. Cammarota, G. Gasbarrini, and A. Gasbarrini. 2006. Levofloxacin-based triple therapy in first-line treatment for Helicobacter pylori eradication. Am. J. Gastroenterol. 101:1985-1990. [DOI] [PubMed] [Google Scholar]
- 14.Romano, M., M. R. Iovene, M. I. Russo, A. Rocco, R. Salerno, D. Cozzolino, A. P. Pilloni, M. A. Tufana, and G. Nardone. 2008. Failure of first-line eradication treatment significantly increases prevalence of antimicrobial-resistant Helicobacter pylori clinical isolates. J. Clin. Pathol. 61:1112-1115. [DOI] [PubMed] [Google Scholar]
- 15.Sato, K., K. Hoshino, M. Tanaka, I. Hayakawa, and Y. Osada. 1992. Antimicrobial activity of DU-6859, a new potent fluoroquinolone, against clinical isolates. Antimicrob. Agents Chemother. 36:1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirasaka, D., N. Aoyama, I. Miki, Y. Matsumoto, H. Miyaji, M. Toyoda, T. Mitani, Y. Morita, T. Tamura, and T. Azuma. 2006. The efficiency and safety of second-eradication therapy for Helicobacter pylori with metronidazole and levofloxacin. J. Gastroenterol. 103S:782. (In Japanese.) [Google Scholar]