Abstract
Alkoxyalkyl esters of acyclic nucleoside phosphonates have previously been shown to have increased antiviral activity when they are administered orally in animal models of viral diseases, including lethal infections with vaccinia virus, cowpox virus, ectromelia virus, murine cytomegalovirus, and adenovirus. 9-(S)-(3-Hydroxy-2-phosphonomethoxypropyl)adenine [(S)-HPMPA] was previously shown to have activity against hepatitis B virus (HBV) in vitro. To assess the effect of alkoxyalkyl esterification of (S)-HPMPA, we prepared the hexadecyloxypropyl (HDP), 15-methyl-hexadecyloxypropyl (15M-HDP), and octadecyloxyethyl (ODE) esters and compared their activities with the activity of adefovir dipivoxil in vitro and in vivo. Alkoxyalkyl esters of (S)-HPMPA were 6 to 20 times more active than unmodified (S)-HPMPA on the basis of their 50% effective concentrations in 2.2.15 cells. The increased antiviral activity appeared to be due in part to the increased uptake and conversion of HDP-(S)-HPMPA to HPMPA diphosphate observed in HepG2 cells in vitro. HDP-(S)-HPMPA retained full activity against HBV mutants resistant to lamivudine (L180M, M204V), but cross-resistance to a mutant resistant to adefovir (N236T) was detected. HDP-(S)-HPMPA is orally bioavailable and provides excellent liver exposure to the drug. Oral treatment of HBV transgenic mice with HDP-(S)-HPMPA, 15M-HDP-(S)-HPMPA, and ODE-(S)-HPMPA for 14 days reduced liver HBV DNA levels by roughly 1.5 log units, a response equivalent to that of adefovir dipivoxil.
9-(S)-(3-Hydroxy-2-phosphonomethoxypropyl)adenine [(S)-HPMPA] is an acyclic nucleoside phosphonate which Holý and coworkers first reported in 1986 (7, 8, 26). (S)-HPMPA was the first acyclic nucleoside phosphonate, a growing and important class of antiviral compounds which now includes cidofovir, adefovir [9-(2-phosphonomethoxyethyl)adenine], and tenofovir [9-(2-phosphonomethoxypropyl)adenine], which are used for the treatment of cytomegalovirus (CMV), hepatitis B virus (HBV), and human immunodeficiency virus (HIV) infections, respectively (6). In this paper, we report on the synthesis and evaluation of several alkoxyalkyl ester prodrugs of (S)-HPMPA in an attempt to find oral treatments for HBV infection more effective than the currently approved antivirals.
(S)-HPMPA is a broad-spectrum antiviral which was shown to inhibit the replication of a wide variety of double-stranded DNA viruses, including orthopoxviruses, herpesviruses, adenoviruses, iridoviruses, and papovaviruses (6). (S)-HPMPA was also reported to be active in vitro against HBV replication in HB611 cells (29) and 2.2.15 cells (11) and to have 50% effective concentrations (EC50s) of 1.15 and 1.5 μM, respectively. Numerous reports have indicated that (S)-HPMPA lacks activity against HIV type 1 (HIV-1) (2, 5, 12). However, alkoxyalkyl esters of (S)-HPMPA, such as hexadecyloxypropyl-(S)-HPMPA [HDP-(S)-HPMPA] and octadecyloxyethyl-(S)-HPMPA [ODE-(S)-HPMPA], exhibit EC50s against HIV-1 in the low nanomolar range, while unmodified (S)-HPMPA is virtually inactive in vitro (13). HDP-(S)-HPMPA exhibits multiple-log increases in antiviral activity in vitro compared with the activity of unmodified (S)-HPMPA against vaccinia virus, cowpox virus, human CMV and murine CMV (3), and adenovirus (10). HDP-(S)-HPMPA is orally bioavailable and is active in vitro against lethal vaccinia virus and cowpox virus infections (24) and against lethal murine CMV infections (25). To assess the effect of alkoxyalkyl esterification of (S)-HPMPA on its in vitro and in vivo anti-HBV activity, we synthesized HDP-(S)-HPMPA, 15-methyl-HDP-(S)-HPMPA [15M-HDP-(S)-HPMPA], and ODE-(S)-HPMPA and evaluated their in vitro activities against HBV replication as well as the cellular uptake and conversion of HDP-(S)-[8-14C]HPMPA to (S)-HPMPA diphosphate (HPMPApp) in HepG2 cells. Using HDP-(S)-[8-14C]HPMPA, we also evaluated the oral pharmacokinetics and the level of drug exposure in the plasma, livers, and spleens of mice. Finally, the oral activities of the HDP, ODE, and 15M-HDP esters of (S)-HPMPA were assessed in HBV transgenic mice (15, 17, 21); and their activities were compared with the in vivo activity of adefovir dipivoxil, a compound licensed for use for the treatment of HBV infection.
MATERIALS AND METHODS
Synthesis of HDP-(S)-HPMPA, ODE-(S)-HPMPA, and 15M-HDP-(S)-HPMPA.
Briefly, bis-trityl-(S)-2,3-dihydroxypropyl-adenine was alkylated with long-chain octadecyloxypropyl- and hexadecyloxypropyl-toluenesulfonyloxy-methyl-phosphonates and deprotected to provide the hexadecyloxpropyl and octadecyloxyethyl esters of (S)-HPMPA, HDP-(S)-HPMPA and ODE-(S)-HPMPA, respectively, as reported previously (3). The identity and the purity (≥98%) of the compounds were confirmed by 1H and 31P nuclear magnetic resonance (NMR), mass spectroscopy, and elemental combustion analysis (3). HDP-(S)-[8-14C]HPMPA (50 mCi/mmol) was prepared by custom synthesis at Moravek Biochemicals (Brea, CA). To make the penultimate branched methyl analog of HDP-(S)-HPMPA, the Grignard coupling reaction between isoamylmagnesium bromide and 12-bromo-1-dodecanol was carried out as described by Yuasa and Tsuruta (30), giving 15-methylhexadecanol, which was converted to the methanesulfonate and treated with 1,3-propanediol (NaH/N,N-dimethylformamide) to give 3-(15-methylhexadecyloxy)propan-1-ol, as previously described by Kini et al. (18). Cyclic (S)-HPMPA (cHPMPA) was prepared by a previously described method (14, 26).
The penultimate branched alkyl analog, 15-methyl-hexadecyloxypropyl-(S)-HPMPA, was then synthesized as follows: diethylazodicarboxylate (200 mg, 1.0 mmol) was added to a mixture of cHPMPA (200 mg, 0.7 mmol), 3-(15-methylhexadecyloxy)propan-1-ol (220 mg, 0.7 mmol), triphenylphosphine (265 mg, 1.0 mmol), and dry N,N-dimethylformamide (10 ml); and the mixture was stirred overnight at room temperature. After evaporation of the solvent in vacuo, the residue was adsorbed on silica gel and purified by flash column chromatography. The product, 15M-HDP-cyclic-(S)-HPMPA was eluted with 15% ethanol-dichloromethane and recrystallized in p-dioxane to give 187 mg 15M-HDP-cHPMPA (46% yield). 1H NMR (CD3OD) δ 8.24 (d, 1H), 8.04 (d, 1H), 4.59-4.15 (m, 9H), 3.51 (t, 2H), 3.41 (t, 2H), 1.94 (quintet, 2H), 1.54 (m, 2H), 1.27 (broad s, 26 H), 1.16 (m, 1H), 0.87 (d, 6H). The 15M-HDP-cyclic-(S)-HPMPA (150 mg, 0.26 mmol) was suspended in 1 N NaOH (10 ml) and heated to 60°C for 1 h, during which time the solution became clear. The solution was cooled to 25°C and acidified with acetic acid to a pH of approximately 5. The precipitate was collected by vacuum filtration and purified by flash column chromatography. The product eluted with 20% methanol-dichloromethane to give 15M-HDP-(S)-HPMPA (110 mg, 72% yield). The following data were used to confirm the structure and purity (≥98%) of the compound: 1H NMR (CD3OD) δ 0.88 (d, 6H), 1.16 (m,1H), 1.27 (br s, 26 H), 1.52 (m, 2H), 1.82 (quintet, 2H), 3.38 (t, 2H), 3.49 (t, 2H), 3.51-3.80 (m, 3H), 3.85-3.91 (m, 2H), 4.35-4.40 (m, 2H) 8.21 (s, 1H), 8.22 (s, 1H); 31P NMR δ 16.86; mass spectrometry (electrospray) m/z 600.32 [M + H]+.
Analysis of anti-HBV activity.
Confluent cultures of 2.2.15 cells were maintained on 96-well flat-bottom tissue culture plates in RPMI 1640 medium with 2% fetal bovine serum, as described previously (19). Cultures were treated with nine consecutive daily doses of the test compounds (six for each test concentration on two replicate plates). The culture medium was changed every day with medium containing the indicated concentration of the test compounds. Extracellular HBV DNA levels were assessed by quantitative blot hybridization 24 h after the last treatment. No specific isolation of HBV particles was utilized. Prior to analysis, cellular material was pelleted out of the medium, and the culture medium was specifically not frozen and thawed (stored at 4°C) to minimize any potential for the release of viral DNA from residual cellular material that may have been released into the medium (19). Cytotoxicity was assessed by measurement of the uptake of neutral red dye and quantitative analysis of the absorbance of the internalized dye at 510 nM 24 h following the last treatment (three cultures per test concentration). The activities of the compounds against lamivudine-resistant and adefovir-resistant HBV mutants were determined in a 5-day assay by use of a transient transfection method with Huh7 cells, as described previously (16).
Cell uptake and metabolism of HDP-(S)-[8-14C]HPMPA.
Radioactively labeled drugs were added at a final concentration of 10 μM (specific activity, 50 mCi/mmol) to 25-cm2 flasks of nearly confluent HepG2 cells, and the flasks were incubated for 24 h. The medium was removed and the monolayer was washed twice with cold phosphate-buffered saline, followed by addition of 0.6 ml of distilled water. The flasks were twice frozen and thawed and sonicated for 5 min in a cold sonicator bath, and the contents of the flasks were scraped into a glass tube. Cold trichloroacetic acid was added to a final concentration of 8%, and the contents were vortexed and centrifuged for 10 min at 4°C. The supernatant was removed, an aliquot was counted, and another aliquot was immediately subjected to high-pressure liquid chromatography analysis by Partisil SAX ion exchange, as described previously (1). The retention times of labeled HPMPA, (S)-HPMPA monophosphate (HPMPAp), and HPMPApp (32 to 33 min) were identical to those of the chemically pure reference standards.
Pharmacokinetics of orally administered drug in mice.
Female Swiss-Webster mice (weight, approximately 25 g) received a single dose of 10 mg/kg of body weight HDP-(S)-[8-14C]HPMPA (specific activity, 50 mCi/mmol) in 0.7% saline by oral gavage or intraperitoneal injection, as reported previously (4). Blood was collected at 1, 3, 6, 12, and 24 h after treatment and placed in heparinized Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ). The mice were killed; and the liver, spleen, kidney, lung, heart, and brain tissues were removed, diced, washed in cold phosphate-buffered saline, blotted dry, and weighed. The plasma was centrifuged, a volume of 50 μl of plasma was added to 10 ml of Ecolite liquid scintillation fluid, and the drug and metabolite contents in plasma were determined by liquid scintillation counting. The data are expressed as total μmol of HDP-(S)-[8-14C]HPMPA equivalents per liter of plasma (μM) or as nanomoles per gram of tissue. The value for each time point is the average of three replicates. Relative oral bioavailability was calculated by determining the areas under the curve (AUC) for the drug and metabolites and dividing the AUC obtained by the intraperitoneal route of administration by the AUC obtained by the oral route of administration.
Antiviral activity in HBV transgenic mice. (i) Animals and compounds.
Homozygous female and male HBV transgenic mice (weight, 22.2 ± 2.8 g) were used. The transgenic mice (founder strain, 1.3.32) were originally obtained from Frank Chisari (Scripps Research Institute, La Jolla, CA) (9). HDP-(S)-HPMPA, 15M-HDP-(S)-HPMPA, and ODE-(S)-HPMPA were dissolved in distilled water at the concentration suitable for delivery of the correct dosage by oral gavage in a 0.1-ml volume. Adefovir dipivoxil was obtained from Gilead Sciences (Foster City, CA) and prepared in citric acid (0.05 M, pH 2.0). The drugs were administered to the mice by oral gavage in a 0.1-ml volume per 10 g of body weight to obtain the indicated daily doses.
(ii) Liver HBV DNA assay.
Liver HBV DNA was quantified by Southern blot hybridization or quantitative PCR (21). The PCR primers do not distinguish between different replicative intermediates or chromosomal transgenic DNA, but the background PCR signal of transgenic DNA is sufficiently low for the quantitation of extrachromosomal HBV DNA. For both assays, liver tissue (approximately 0.1 g) was homogenized in lysis buffer immediately upon necropsy. For extraction of the DNA, the samples were incubated at 55°C for 2 to 4 h, phenol-chloroform extracted, and alcohol precipitated. For Southern blot analysis, 40 μg DNA was digested with the HindIII enzyme, since HindIII does not cut within the HBV transgene sequence. The blotting procedure involved alkaline transfer onto positively charged nylon filters. The radioactive signals from the hybridization of the 32P-labeled HBV DNA genome were measured by a phosphorimaging method (Optiquant) with a Cyclone storage phosphorscreen (multisensitive medium; Perkin-Elmer). The ratio of the number of viral DNA bands to the number of transgene bands was used to determine the concentration of viral DNA per host DNA. This calculation was based upon the knowledge that there are 1.3 copies of the transgene present per host cell with this line of transgenic mice. A real-time duplex PCR (21) was performed with HBV-specific primers and an HBV-specific probe (forward primer, ATAAAACGCCGCAGACACATC; reverse primer, AACCTCCAATCACTCACCAACC; probe, 6-carboxyfluorescein-AGCGATAACCAGGACAAGTTGGAGGACA-BHQ1a-6-carboxyfluorescein) and mouse GAPDH-specific primers and a mouse GAPDH-specific probe (forward primer, GCATCTTGGGCTACACTGAGG; reverse primer, GAAGGTGGAAGAGTGGGAGTTG; probe, 4,4,7,2′,4′,5′,7′-hexachloro-6-carboxy-fluorescein [HEX]-ACCAGGTTGTCTCCTGCGACTTCAACAG-BHQ1a-HEX). FullVelocity quantitative PCR master mix (Strategene, La Jolla, CA) was used. The assay was run with a series of 10-fold dilutions of pooled liver DNA from HBV transgenic mice to obtain a standard curve. The y axis was the log dilutions of the standard, and the x axis was the threshold cycle (CT) values. R2 values were used to measure the quality of the curve, which was always above 0.098. Mean CT values were obtained for duplicates of each sample. The mean CT values of each sample were used to obtain the relative log DNA value by using a formula of the fit line of the standard curve.
Statistical analysis.
HBV DNA values and the corresponding doses of adefovir dipivoxil, HDP-(S)-HPMPA, 15M-HDP-(S)-HPMPA, and ODE-(S)-HPMPA in micromoles/kg/day were entered into Prism (version 4.0) software (GraphPad Software, San Diego, CA). The compound doses were log transformed and normalized by setting the value of HBV DNA in control animals to 100%, and then inhibition curves were generated by fitting the data to a sigmoidal dose-response curve (variable slope). The 50% effective doses (ED50s) (±95% confidence interval [CI]) are reported for each curve fit to the data. ED90s (±95% CI) were obtained by using the following formula: log EC50 = [log ECf − (1/Hill slope)]·log[F/(100 − F)], where ECf is the effective concentration producing a fractional response, f, and F is the fraction of the control response (f = 100 − F) and where y = bottom + (top − bottom)/{1 + 10^[(log EC50 − x)·Hill slope]}, where top indicates the upper limit of the response range (set to 100) and bottom indicates the lower limit of the response range (set to 0), and inputting the EC50 and Hill slope from the curve fit for each individual compound. Since inhibition curves are represented as a percentage of the inhibition of the control (taken as 100%), the ED90 corresponds to an F value of 10. All curves were plotted by using Prism (version 4.0) software. The individual data points were expressed as the mean amount of HBV DNA, and error bars correspond to the standard error of the mean.
RESULTS
Antiviral activities in vitro.
The antiviral activities of (S)-HPMPA and three alkoxyalkyl analogs were determined in 2.2.15 cells, which constitutively produce HBV (Table 1). (S)-HPMPA had an EC50 of 1.2 μM, in good agreement with previously reported data (11, 29). HDP-(S)-HPMPA was highly active and had an EC50 and an EC90 in the submicromolar range (0.188 and 0.616 μM, respectively), whereas the EC50 and EC90 of (S)-HPMPA were 1.20 and 5.05 μM, respectively. These results indicate that HDP-(S)-HPMPA has six- to eightfold greater antiviral activity than (S)-HPMPA. The penultimate branch methyl analog 15M-HDP-(S)-HPMPA and ODE-(S)-HPMPA were somewhat more active in vitro, with EC50s of 0.059 and 0.075, respectively, and EC90s of 0.181 and 0.211 μM, respectively, representing antiviral activities 16- to 28-fold greater than the activity of (S)-HPMPA. In transient transfections in Huh7 cells (Table 2), HDP-(S)-HPMPA retained full activity against several lamivudine-resistant HBV polymerase mutants (L180M, M204V, M204I, and L180M/M204V). However, HDP-(S)-HPMPA was highly resistant to an N236T mutant, which is adefovir resistant.
TABLE 1.
Anti-HBV activities of (S)-HPMPA and alkoxyalkyl prodrugs in 2.2.15 cellsa
| Compound | EC50 (μM) | EC90 (μM) | CC50 (μM) | SI |
|---|---|---|---|---|
| (S)-HPMPA | 1.20 ± 0.29 | 5.05 ± 1.34 | >300 | >60 |
| HDP-(S)-HPMPA | 0.188 ± 0.091 | 0.616 ± 0.076 | >300 | >483 |
| 15M-HDP-(S)-HPMPA | 0.059 ± 0.011 | 0.181 ± 0.012 | >300 | >1,657 |
| ODE-(S)-HPMPA | 0.076 ± 0.048 | 0.211 ± 0.048 | >300 | >1,422 |
Extracellular HBV DNA levels were assessed by quantitative blot hybridization (19). CC50, 50% cytotoxic concentration. EC50s, EC90s, and 50% cytotoxic concentrations are means ± standard deviations (n = 3). SI, selective index, which is the 50% cytotoxic concentration/EC90.
TABLE 2.
Antiviral activities of HDP-(S)-HPMPA, lamivudine, and adefovir against drug-resistant HBVa
| HBV strain | HDP-(S)-HPMPA
|
Lamivudine
|
Adefovir
|
|||
|---|---|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | EC50 (μM) | EC90 (μM) | EC50 (μM) | EC90 (μM) | |
| Wild type | 0.8 ± 0.1 | 3.0 ± 0.4 | 0.2 ± 0.04 | 3.0 ± 0.4 | 2.0 ± 0.3 | 7.0 ± 0.8 |
| L180M | 1.0 ± 0.2 | 3.5 ± 0.5 | 16 ± 1.9 | 41 ± 5.1 | 2.6 ± 0.3 | 7.3 ± 0.9 |
| M204V | 0.9 ± 0.1 | 3.2 ± 0.4 | >100 | >100 | 1.5 ± 0.2 | 7.2 ± 0.8 |
| M204I | 1.2 ± 0.2 | 4.0 ± 0.5 | >100 | >100 | 2.4 ± 0.3 | 8.0 ± 0.9 |
| L180M/M204V | 0.7 ± 0.1 | 2.8 ± 0.3 | >100 | >100 | 2.5 ± 0.3 | 7.6 ± 0.9 |
| N236T | >100 | >100 | 0.3 ± 0.05 | 1.5 ± 0.2 | 14 ± 1.7 | 52 ± 6.0 |
Transient transfection in Huh7 cells. The values are means ± standard deviations (n = 4).
Drug metabolism in vitro.
To determine the reason for the increased antiviral activity of HDP-(S)-HPMPA, we studied the uptake and conversion of [8-14C]-(S)-HPMPA and HDP-(S)-[8-14C]HPMPA to HPMPApp in HepG2 cells (Table 3). At 24 and 48 h of exposure, (S)-HPMPA gave rise to 10 and 13 picomoles/flask of HPMPApp, respectively, whereas HDP-(S)-HPMPA gave rise to 393 and 444 picomoles/flask of HPMPApp, respectively. Other differences included the more rapid conversion of HPMPA to HPMPAp when HDP-(S)-HPMPA was metabolized by HepG2 cells. However, the ratio of HPMPAp to HPMPApp ranged from 2.0 to 2.6 and was similar in cells exposed to (S)-HPMPA and HDP-(S)-HPMPA. Finally, an unidentified metabolite was also present in much greater amounts when the cells were exposed to HDP-(S)-HPMPA. HPMPApp is proposed to be the active antiviral agent, and the higher levels in HepG2 cells probably account for its increased antiviral activity in vitro (Table 1).
TABLE 3.
Uptake and conversion of [8-14C]-(S)-HPMPA and HDP-(S)-[8-14C]HPMPA to HPMPApp in HepG2 cells in vitro
| Compound | Amt taken up (pmol/flask)
|
|||
|---|---|---|---|---|
| (S)-HPMPA
|
HDP-(S)-HPMPA
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| UMa | 33 | 27 | 596 | 255 |
| HPMPA | 62 | 70 | 402 | 344 |
| HPMPAp | 26 | 34 | 1007 | 888 |
| HPMPApp | 10 | 13 | 393 | 444 |
UM, unidentified metabolite.
Pharmacokinetics after oral and intraperitoneal administration in mice.
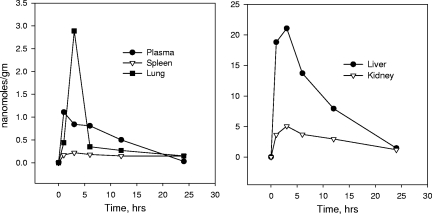
Before attempting to treat HBV transgenic mice, we administered 10 mg/kg of radioactive HDP-(S)-HPMPA to mice by the oral or the intraperitoneal route and measured the plasma and tissue levels of the drug at times ranging from 1 to 24 h (Fig. 1). The plasma levels of the drug and the metabolites were lower with the oral administration of HDP-(S)-HPMPA. The maximum plasma concentration (Cmax) of the drug and its metabolites [primarily intact drug and some (S)-HPMPA formed in tissues] with the intraperitoneal administration of HDP-(S)-HPMPA was 2.23 nmol/ml, whereas it was 1.11 nmol/ml after oral administration (Table 4). On the basis of the AUC from time zero to 24 h, the relative oral bioavailability could be estimated to be 74%. With oral administration, plasma drug and metabolite levels peaked at 1 h, giving rise to peak levels in the lung, liver, kidney, and spleen at 3 h and declining to low levels of 0.1 to 1.4 nmol/g at 24 h (Fig. 1). The liver and the kidney had the greatest exposure to HDP-(S)-HPMPA and its metabolites, with AUCs from time zero to 24 h of 222 and 68.8 nmol·h/g. The lung, spleen, heart, and brain had AUCs of 10, 3.9, 2.7, and 0.68 nmol·h/g, respectively (Table 4). The level of exposure of the liver to the drug was excellent with orally administered HDP-(S)-HPMPA.
FIG. 1.
Plasma and tissue levels of 14C-labeled drug and metabolites were determined at various times after oral administration of 10 mg/kg HDP-(S)-[8-14C]HPMPA to mice, and the results are expressed as nanomoles per gram of tissue. The results are the averages of three separate determinations.
TABLE 4.
Pharmacokinetics of HDP-(S)-[8-14C]HPMPA after oral and intraperitoneal administration to micea
| Fluid or tissue | Orally administered HDP-(S)-[8-14C]HPMPA
|
Intraperitoneally administered HDP-(S)-[8-14C]HPMPA
|
||||
|---|---|---|---|---|---|---|
| Tmaxb (h) | Cmax (nmol/g)c | AUCd (nmol·h/g) | Tmax (h) | Cmax (nmol/g) | AUCd (nmol·h/g) | |
| Plasma | 1 | 1.11 | 12.1 | 3 | 2.23 | 16.4 |
| Liver | 3 | 21.1 | 222.2 | 1 | 131.5 | 545.1 |
| Kidney | 3 | 5.07 | 68.8 | 1 | 22.9 | 195.7 |
| Lung | 3 | 2.89 | 12.8 | 1 | 2.84 | 21.5 |
| Spleen | 3 | 0.22 | 3.90 | 1 | 1.58 | 16.3 |
| Heart | 1 | 0.20 | 2.69 | 1 | 0.57 | 3.80 |
| Brain | 3 | 0.07 | 0.68 | 1 | 0.16 | 0.98 |
An oral or intraperitoneal dose of 10 mg/kg was administered.
Tmax, time to Cmax.
For plasma, the units of Cmax are nanomoles/ml.
AUC from time zero to 24 h.
Oral antiviral studies with HBV transgenic mice.
We compared the effects of orally administered HDP-(S)-HPMPA, ODE-(S)-HPMPA, and 15M-HDP-(S)-HPMPA versus those of orally administered adefovir dipivoxil in HBV transgenic mice. A preliminary oral toxicology study with HDP-(S)-HPMPA in rats indicated that 7 days of treatment with oral doses of less than 10 mg/kg/day was not associated with any morbidity, mortality, or histopathologic evidence of toxicity (data not shown). Doses of 30 or 100 mg/kg produced some morbidity and mortality, which appeared to be related to gastrointestinal toxicity, but there was no evidence of increased liver enzyme levels or abnormal kidney function (data not shown).
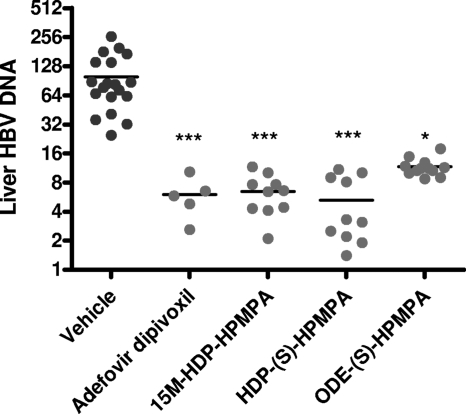
The oral administration of 4 mg/kg/day of HDP-(S)-HPMPA, ODE-(S)-HPMPA, and 15M-HDP-(S)-HPMPA, a second-generation, metabolically stable analog, for 14 days reduced the liver HBV DNA level compared with that in the vehicle-treated control (Fig. 2). The reduction in the total liver HBV DNA load compared with that in the vehicle-treated control was roughly 1 log unit (P < 0.001) and was similar to that produced by treatment with adefovir dipivoxil at the same dosage. Treatment with ODE-(S)-HPMPA was also more active than treatment with the vehicle (P < 0.05). There was no significant difference in the activities of the four compounds by analysis of variance. Evaluation by Southern blotting gave essentially the same results (data not shown). Since the dynamic range for the liver HBV DNA level in transgenic mice is small, further comparisons were done by assessment of the dose-responses to various compounds in vivo after the administration of a series of lower doses.
FIG. 2.
HBV transgenic mice were treated by oral gavage with 7 μmol/kg (4 mg/kg) of the test compounds daily for 14 days. The numbers of animals were as follows: controls, 8 per group; adefovir dipivoxil, 5 per group; HDP-(S)-HPMPA and 15M-HDP-(S)-HPMPA, 10 per group. The liver HBV DNA level was determined by quantitative PCR. The liver HBV DNA level is expressed in relative units/μg cell DNA. Statistical significance was determined by analysis of variance and Dunn's multiple-comparison test (Prism, version 5). ***, P < 0.001 compared with the results obtained for the animals treated with the vehicle control; *, P < 0.05 compared with the results obtained for the animals treated with the vehicle control. The results for the different compounds did not differ significantly from each other.
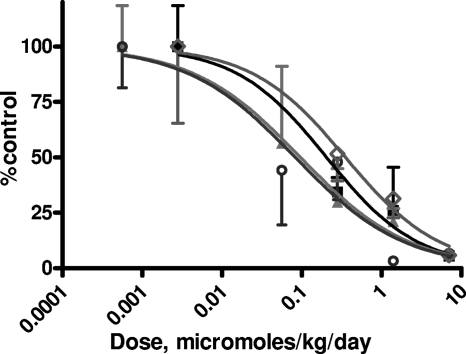
HBV transgenic mice were treated with vehicle, HDP-(S)-HPMPA, 15M-HDP-(S)-HPMPA, ODE-(S)-HPMPA, or adefovir dipivoxil for 14 days by oral gavage at doses of 0.056, 0.28, 1.4, and 7 μmol/kg/day and the liver HBV DNA level was determined by Southern blotting analysis (Fig. 3). The amount of liver HBV DNA expressed in transgenic mice was variable between animals, and the results for the lower drug concentrations were less reliable because of the background of the assay (21). Nevertheless, the dynamic range of the HBV DNA assay is sufficient to detect dose-response relationships. All four compounds reduced the liver HBV DNA level progressively to more than 1 log unit at the highest concentration tested. The ED50s of the compounds ranged from 0.082 to 0.35 μmol/kg/day (Table 5). HDP-(S)-HPMPA and 15M-HDP-(S)-HPMPA appeared to be slightly more active than ODE-(S)-HPMPA and adefovir dipivoxil at low doses, but these differences were not statistically significant. The ED90s ranged from 2.96 to 6.91 μmol/kg day (Table 5). The ED90s of HDP-(S)-HPMPA and 15M-HDP-(S)-HPMPA were 1.7 and 1.8 mg/kg/day, respectively, whereas they were 1.8 and 4.1 mg/kg/day for adefovir and ODE-(S)-HPMPA, respectively. There was no statistically significant difference between the EC90s of the four compounds. Orally administered (S)-HPMPA was not tested, but it is known to be inactive in animal models of CMV infection (25).
FIG. 3.
Transgenic mice were treated orally for 14 days with vehicle or with the indicated doses of adefovir dipivoxil (closed squares), HDP-(S)-HPMPA (closed triangles), 15M-HDP-(S)-HPMPA (open circles), or ODE-(S)-HPMPA (open diamonds). Adefovir dipivoxil-treated group had 3 to 5 animals per group, while the groups treated with HDP-(S)-HPMPA and 15M-HDP-(S)-HPMPA had 4 to 13 animals per group. The liver HBV DNA level was determined by Southern blot hybridization and is expressed as a percentage of the level for the untreated controls.
TABLE 5.
Comparison of liver HBV DNA levels in HBV transgenic mice after oral administration of adefovir dipivoxil and the alkoxyalkyl esters of (S)-HPMPAa
| Compound | ED50 (μmol/kg/day) | ED90 (μmol/kg/day) |
|---|---|---|
| HDP-(S)-HPMPA | 0.096 (0.013-0.69) | 2.97 (0.11-81.8) |
| 15M-HDP-(S)-HPMPA | 0.082 (0.010-0.66) | 2.96 (0.069-126) |
| ODE-(S)-HPMPA | 0.35 (0.071-1.74) | 6.91 (0.21-224) |
| Adefovir dipivoxil | 0.20 (0.012-3.43) | 3.60 (0.053-246) |
HBV DNA was measured by Southern blotting, and the ED50s and ED90s were determined from the data in Table 3, as noted in Materials and Methods. The values in parentheses are the 95% CIs.
DISCUSSION
HDP-(S)-HPMPA is more active than unmodified (S)-HPMPA in vitro (Table 1), and this appears to be due to the greatly increased cellular uptake and conversion to its active metabolite, HPMPApp (Table 3). A similar mechanism of enhanced in vitro activity for HDP-cidofovir compared with that of unmodified cidofovir has been reported (1). HDP-(S)-HPMPA is cross resistant to an adefovir-resistant HBV mutant (N236T), but the degree of resistance (>100 times) is substantially greater than that of adefovir (5 to 8 times). HPMPA and adefovir are similar except that HPMPA has a hydroxymethyl group in the acyclic side chain, whereas the adefovir phosphonomethoxyethyl acyclic chain has no free hydroxyl.
The mechanism of action of HPMPApp on HBV DNA production is unknown, as there have been no detailed studies of the effects of HPMPApp on the HBV DNA polymerase. Regarding other viral polymerases, workers previously found that (S)-HPMPA blocked adenovirus DNA polymerase at the level of chain elongation (22). The mechanism of action against the vaccinia virus E9L DNA polymerase has recently been reported (20). We found that HPMPApp is an excellent substrate for the E9L polymerase, with Km and Vmax values similar to those of dATP. It is readily incorporated into the growing DNA strand and does not slow chain extension but blocks 3′-to-5′ exonuclease activity when it is in the penultimate position. When it is at the primer terminus, HPMPA can still be excised. However, when it is incorporated into the template strand, these templates cannot be extended across the (S)-HPMPA residues, blocking further rounds of replication and leading to template strand inhibition (20).
HDP-(S)-HPMPA is highly orally bioavailable (Table 4), as has previously been shown with HDP-cidofovir (4) and HDP-tenofovir (23). Excellent liver exposure is also a benefit of oral administration, which seems to be particularly useful for the treatment of HBV and hepatitis C virus (HCV) infections. The toxicity of oral HDP-(S)-HPMPA is primarily to the gastrointestinal tract and is characterized by weight loss and diarrhea in mice (K. Y. Hostetler and J. Trahan, unpublished data), while (S)-HPMPA itself is toxic to the liver and the kidney when it is given parenterally (27). Smeijsters et al. (27) studied (S)-HPMPA given parenterally to mice infected with Plasmodium berghei. They found that a cumulative dose of 28 mg/kg given via an osmotic minipump was lethal and that liver damage and kidney damage were the prominent toxicities (27). In this study, we gave an oral cumulative dose of 56 mg/kg over 14 days and did not detect any observable morbidity or mortality. Thus, HDP-(S)-HPMPA given orally appears to be less toxic than (S)-HPMPA given parenterally. Surprisingly, HDP-(S)-HPMPA and ODE-(S)-HPMPA have also been shown to have activity against HCV genotype 1a, 1b, and 2a replicons at low micromolar concentrations (28).
In summary, we compared the antiviral activities of HDP-(S)-HPMPA, ODE-(S)-HPMPA, and 15M-HDP-(S)-HPMPA administered orally with that of adefovir dipivoxil administered orally in the HBV transgenic mouse. All compounds were active in this animal model of HBV infection, and there was no statistically significant difference between the four treatments. Given their activities against both HBV and HCV, these compounds appear to be worthy of further investigation as possible treatments for HBV and HCV infections.
Acknowledgments
This work was funded in part by NIAID contract NO1-AI-50036 (to J.D.M.); NIAID contract NO1-AI-30046 (to B.E.K.); and NIAID grants AI-076558, AI-066499, AI-071803, and AI-074057 (to K.Y.H.) and AI-069989 (to D.L.W.).
Dr. Hostetler has an equity interest and serves as a consultant to Chimerix Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. The increased antiviral activity of 1-O-hexadecyloxypropyl-cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Holý, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethyoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beadle, J. R., W. B. Wan, S. L. Ciesla, K. A. Keith, C. Hartline, E. R. Kern, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine against cytomegalovirus and orthpoxviruses, in vitro. J. Med. Chem. 49:2010-2015. [DOI] [PubMed] [Google Scholar]
- 4.Ciesla, S. L., J. Trahan, K. L. Winegarden, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq, E. 1991. Chemotherapy of the acquired immune deficiency syndrome (AIDS): acyclic nucleoside phosphonate analogs. Int. J. Immunopharmacol. 13(Suppl. 1):91-98. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E. 2007. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antivir. Res. 75:1-13. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq, E., A. Holý, I. Rosenberg, T. Sakuma, J. Balzarini, and P. C. Maudgal. 1986. A novel selective broad-spectrum anti-DNA virus agent. Nature 323:464-467. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holý. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartline, C. B., K. M. Gustin, W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Activity of ether lipid ester prodrugs of acyclic nucleoside phosphonates against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 11.Heijtink, R. A., J. Kruining, G. A. de Wilde, J. Balzarini, E. de Clercq, and S. W. Schalm. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holý, A. 2003. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharm. Des. 9:2567-2592. [DOI] [PubMed] [Google Scholar]
- 13.Hostetler, K. Y., K. A. Aldern, W. B. Wan, S. L. Ciesla, and J. R. Beadle. 2006. Alkoxyalkyl esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine are potent inhibitors of the replication of wild-type and drug-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 50:2857-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huffman, J. H., R. W. Sidwell, A. G. Morrison, J. Coombs, and E. J. Reist. 1994. Structure-activity relationship of phosphonic acid analogs of acyclovir or ganciclovir against human cytomegalovirus in MRC-5 cells. Nucleosides Nucleotides 13:607-613. [Google Scholar]
- 15.Iyer, R. P., A. Roland, Y. Jin, S. Mounir, B. E. Korba, J. G. Julander, and J. D. Morrey. 2004. Anti-hepatitis B virus activity of ORI-9020, a novel phosphorothioate dinucleotide, in a transgenic mouse model. Antimicrob. Agents Chemother. 48:2318-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer, R. P., Y. Jin, A. Roland, J. D. Morrey, S. Mounir, and B. E. Korba. 2004. Phosphorothioate di- and trinucleotides as a novel class of anti-hepatitis B virus agents. Antimicrob. Agents Chemother. 48:2199-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julander, J. G., R. W. Sidwell, and J. D. Morrey. 2002. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antivir. Res. 55:27-40. [DOI] [PubMed] [Google Scholar]
- 18.Kini, G. D., J. R. Beadle, H. Xie, K. A. Aldern, D. D. Richman, and K. Y. Hostetler. 1997. Alkoxy propane prodrugs of foscarnet: effect of alkyl chain length on in vitro antiviral activity in cells infected with HIV-1, HSV-1 and HCMV. Antivir. Res. 36:43-53. [DOI] [PubMed] [Google Scholar]
- 19.Korba, B. E., and J. L. Gerin. 1992. Use of a standardized cell culture assay to determine activities of nucleoside analogs against hepatitis B virus replication. Antivir. Res. 19:55-70. [DOI] [PubMed] [Google Scholar]
- 20.Magee, W. C., K. A. Aldern, K. Y. Hostetler, and D. H. Evans. 2008. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob. Agents Chemother. 52:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrey, J. D., N. E. Motter, B. Taro, M. Lay, and J. Fairman. 2008. Efficacy of cationic lipid-DNA complexes (CLDC) on hepatitis B virus in transgenic mice. Antivir. Res. 79:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mul, Y. M., R. T. Van Miltenburg, E. De Clercq, and P. C. Van der Vliet. 1989. Mechanism of inhibition of adenovirus DNA replication by the acyclic nucleoside triphosphate analog (S)-HPMPApp: influence of the adenovirus DNA binding protein. Nucleic Acids Res. 17:8917-8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Painter, G. R., M. R. Almond, L. C. Trost, B. M. Lampert, J. Neyts, E. De Clercq, B. E. Korba, K. A. Aldern, J. R. Beadle, and K. Y. Hostetler. 2007. Evaluation of hexadecyloxypropyl-9-R-[2-(phosphonomethoxy)propyl]adenine, CMX157, a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob. Agents Chemother. 51:3505-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quenelle, D. C., D. J. Collins, B. P. Herrod, K. A. Keith, J. Trahan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2007. Effect of oral treatment with hexadecyloxypropyl-[(S)-9-(3-hydroxy-2-phosphonylmethoxy-propyl)adenine] [(S)-HPMPA] or octadecyloxyethyl-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antimicrob. Agents Chemother. 51:3940-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quenelle, D. C., D. J. Collins, L. R. Pettway, C. B. Hartline, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2008. Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus or human cytomegalovirus in animal models. Antivir. Res. 79:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg, I., and A. Holý. 1987. Acyclic nucleotide analogs. Part II. Synthesis of potential prodrugs and metabolites of 9-(S)-(3-hydroxy-2-phophonylmethoxypropyl)adenine. Coll. Czech. Chem. Commun. 52:2792-2800. [Google Scholar]
- 27.Smeijsters, L. J., H. Nieuwenhuijs, R. C. Hermsen, G. M. Dorrestein, F. F. Franssen, and J. P. Overdulve. 1996. Antimalarial and toxic effects of the acyclic nucleoside phosphonate (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenines in Plasmodium berghei-infected mice. Antimicrob. Agents Chemother. 40:1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyles, D. L., K. A. Kaihara, B. E. Korba, R. T. Schooley, J. R. Beadle, and K. Y. Hostetler. 16 March 2009. ODE-(S)-HPMPA is a potent and selective inhibitor of hepatitis C virus replication in genotype 1a, 1b and 2a replicons. Antimicrob. Agents Chemother. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 29.Yokota, T., S. Mochizuki, K. Konno, S. Mori, S. Shigeta, and E. De Clercq. 1991. Inhibitory effects of selected antiviral compounds on human hepatitis B virus DNA synthesis. Antimicrob. Agents Chemother. 35:394-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuasa, Y., and H. Tsuruta. 2004. Convenient syntheses of iso-methyl-branched long-chain aliphatic aldehydes, known to contribute significantly to meat flavour. Flavour Fragrance J. 19:199-204. [Google Scholar]