Abstract
Two ampD homologues, ampDI and ampDII, of Stenotrophomonas maltophilia have been cloned and analyzed. Comparative genomic analysis revealed that the genomic context of the ampDII genes is quite different, whereas that of the ampDI genes is more conserved in S. maltophilia strains. The ampD system of S. maltophilia is distinct from that of the Enterobacteriaceae and Pseudomonas aeruginosa in three respects. (i) AmpDI of S. maltophilia is not encoded in an ampDE operon, in contrast to what happens in the Enterobacteriaceae and P. aeruginosa. (ii) The AmpD systems of the Enterobacteriaceae and P. aeruginosa are generally involved in the regulation of ampR-linked ampC gene expression, while AmpDI of S. maltophilia is responsible for the regulation of two intrinsic β-lactamase genes, of which the L2 gene, but not the L1 gene, is linked to ampR. (iii) S. maltophilia exhibits a one-step L1 and L2 gene derepression model involving ampDI, distinct from the two- or three-step derepression of the Enterobacteriaceae and P. aeruginosa. Moreover, the ampDI and ampDII genes are constitutively expressed and not regulated by the inducer and AmpR protein, and the expression of ampDII is weaker than that of ampDI. Finally, AmpDII is not associated with the derepression of β-lactamases, and its role in S. maltophilia remains unclear.
Stenotrophomonas maltophilia, a gram-negative rod, shows increasing prevalence in clinical infections (2, 4). S. maltophilia produces two inducible β-lactamases (the L1 and L2 enzymes) which together confer resistance to all β-lactams (4). L1, a molecular class B and functional group 3 β-lactamase, is a Zn2+-dependent metalloenzyme with a broad substrate profile including penicillins, cephalosporins, and carbapenems (25). L2 displays a hydrolytic ability toward penicillins, cephalosporins, and monobactams (26). A LysR-type transcriptional regulator gene, ampR, is contiguous to the L2 gene but divergently transcribed (10). No putative transcriptional regulator gene has been discovered in the proximity of the L1 gene. AmpR has been shown to be a key regulator for L1 and L2 β-lactamase induction in response to β-lactam challenge (24). Recently, the regulatory characteristics of AmpR have been further elucidated (17).
ampR-ampC systems, homologous to the ampR-L2 gene module, are widely distributed among several species of Enterobacteriaceae and in Pseudomonas aeruginosa (18, 21). A similar induction mechanism involving AmpR has been proposed for the ampR-ampC systems and the ampR-L2 gene module (17). The induction of chromosomal ampC is linked with bacterial cell wall recycling, and several regulatory genes, such as ampR, ampG, and ampD, are involved in the induction course (6). The degraded cell wall product GlcNAc-anhMurNAc-peptide (N-acetylglucosaminyl-1,6-anhydro-N-acetylmuramyl-peptide), including GlcNAc-anhMurNAc-tripeptide, GlcNAc-anhMurNAc-tetrapeptide, and GlcNAc-anhMurNAc-pentapeptide, is transported into the cytosol by the AmpG protein and further cleaved into 1,6-anhydromuramic acid and peptide by AmpD. The peptide is reused by the enzymes of the cell wall-recycling pathway, resulting in the formation of UDP-N-acetylmuramic acid-pentapeptide. AmpR, a transcriptional regulator of ampC expression, is regulated allosterically by two cell wall components, anhMurNAc-peptide and UDP-N-acetylmuramic acid-pentapeptide. The binding between AmpR and anhMurNAc-peptide activates AmpC gene expression. In contrast, AmpR binding with UDP-N-acetylmuramic acid-pentapeptide represses the expression of AmpC.
AmpD, a cytosolic anhydro-N-acetylmuramyl-l-alanine amidase, acts as a key enzyme to balance the concentration of GlcNAc-anhMurNAc-peptide and UDP-N-acetylmuramic acid-pentapeptide in the cytosol. The role of the AmpD protein in the Enterobacteriaceae, including Enterobacter cloacae and Citrobacter freundii, has been well studied (8, 22). In addition, three ampD genes (ampD, ampDh2, and ampDh3) are present in P. aeruginosa, and a new mechanism of stepwise upregulation has been proposed (13). Moreover, a recent study has demonstrated that other regulatory pathways are involved in the derepressed phenotype of P. aeruginosa, in addition to the three known ampD genes (27). Recently, an AmpD-like lipoprotein, AmiD, was identified and characterized in Escherichia coli (30). Apparently, multiple anhydro-N-acetylmuramyl-l-alanine amidase-like genes can be commonly seen in a microorganism. Multiple AmpD homologues may coordinately regulate the expression of the ampC gene in the Enterobacteriaceae and P. aeruginosa, resulting in a two-step or three-step derepression phenotype (13, 19).
The genome sequences of two S. maltophilia strains, K279a (2; http://www.sanger.ac.uk/Projects/S_maltophilia) and R553-1 (http://www.genome.gov/page.cfm?pageID=10506376), have been determined. A genome-wide search of the sequence databases showed that two AmpD homologues are found in S. maltophilia K279a (Smlt0154 and Smlt1562) and R553-1 (Smal0115 and Smal1320). The exact functions of these AmpD-like proteins in the induction of the L2 β-lactamase (and perhaps of L1) in S. maltophilia are still only partially understood. This study aimed to gain a better understanding of the functions of the AmpD homologues in S. maltophilia.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are listed in Table 1. S. maltophilia KJ is a clinical isolate producing the L1 and L2 β-lactamases, as identified by isoelectric focusing electrophoresis in our previous study (10). S. maltophilia KJ displays a low basal L2 lactamase activity, and expression of L1 and L2 is highly inducible. Moreover, the inducibility of L1 and L2 is AmpR dependent (17).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype, sequence, or properties | Reference |
|---|---|---|
| S. maltophilia | ||
| KJ | Wild type; a clinical isolate from Taiwan | 9 |
| KJΔDI | S. maltophilia KJ ampDI isogenic mutant; deletion of 103-bp internal DNA fragment of ampDI gene | This study |
| KJΔDII | S. maltophilia KJ ampDII isogenic mutant; deletion of 149-bp internal DNA fragment of ampDII gene | This study |
| KJΔDIΔDII | S. maltophilia KJ double mutant of ampDI and ampDII genes; deletion of 103-bp and 149-bp internal DNA fragments of ampDI and ampDII genes, respectively | This study |
| KJΔR | S. maltophilia KJ ampR isogenic mutant; deletion of 468-bp internal DNA fragment of ampR gene | This study |
| E. coli | ||
| DH5α | λ− ϕ80dlacZ&Dgr;M15 &Dgr;(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Invitrogen |
| S17-1 | λ pir+ mating strain | |
| Plasmids | ||
| pEX18Tc | sacB oriT Tcr | 6 |
| pAmpDI | pEX18Tc vector with an 820-bp DNA fragment of S. maltophilia KJ containing the intact ampDI gene and the 50-bp upstream region; Tcr | This study |
| pAmpDII | pEX18Tc vector with a 2,172-bp DNA fragment of S. maltophilia KJ containing the intact ampDII gene and the 858-bp upstream region; Tcr | This study |
| pAmpDIIDI | pEX18Tc vector with a 2,502-bp DNA fragment containing the intact ampDI and ampDII genes; Tcr | This study |
| pΔDI | pEX18Tc vector with a 1,918-bp DNA fragment of S. maltophilia KJ containing the ampDI gene with an internal 103-bp deletion, the 841-bp upstream region, and 349-bp downstream region of ampDI; Tcr | This study |
| pΔDII | pEX18Tc vector with a 2,089-bp DNA fragment of S. maltophilia KJ containing the ampDII gene with an internal 149-bp deletion, the 889-bp upstream region, and 523-bp downstream region of ampDI; Tcr | This study |
| Primersa | ||
| AmpDI-F | 5′-CGAAGCTTCGACAAGGAAAGGGAAGGCAG-3′ | This study |
| AmpDI-R | 5′-CAAGATCTGCACCCACCAACAGCGGCAG-3′ | This study |
| AmpDII-F | 5′-CACTTCCACTGTCCTCGTTC-3′ | This study |
| AmpDII-R | 5′-CCCTTGCCCTTCAGTTCC-3 | This study |
| DIN-F | 5′-TGAAGCTTCCAATGGTGGCAGTGG-3′ | This study |
| DIN-R | 5′-GGTCTAGAAGTGGCAGGCGGTCTTC-3′ | This study |
| DIC-F | 5′-GGTCTAGACAACAGCGGGCATTTCTAC-3′ | This study |
| DIC-R | 5′-CTGAATTCCGCACGCATCTACGCCGAC-3′ | This study |
| 16rDNAQ-F | 5′-GACCTTGCGCGATTGAATG-3′ | This study |
| 16rDNAQ-R | 5′-CGGATCGTCGCCTTGGT-3′ | This study |
| L1Q-F | 5′-ACCCCTGGCAGATCGGCAC-3′ | This study |
| L1Q-R | 5′-CAGCAGCACCGCCGTTTC-3′ | This study |
| L2Q-F | 5′-AACGCACCCACCGATGCC-3′ | This study |
| L2Q-R | 5′-CGCCTGTCCAGCAATGCC-3′ | This study |
| AmpDIQ-F | 5′-CTACGAAGACCGCCTGCC-3′ | This study |
| AmpDIQ-R | 5′-GAAATGCCCGCTGTTGCC-3′ | This study |
| AmpDIIQ-F | 5′-CCACCACACCGAGCAGAAG-3′ | This study |
| AmpDIIQ-R | 5′-ATCTGCGCCGCACTGAAC-3′ | This study |
Underlining indicates the restriction sites introduced for cloning.
Cloning and sequencing of the ampDI and ampDII genes.
In order to PCR amplify the two ampD genes of S. maltophilia KJ, two sets of primers derived from the K279a genome sequence (2), AmpDI-F/AmpDI-R for ampDI and AmpDII-F/AmpDII-R for ampDII, were used (Table 1). The AmpD homologues of Smlt1562 and Smlt0154 from S. maltophilia KJ were designated AmpDI and AmpDII, respectively. All the PCR amplifications were performed using the following program: 5 min at 94°C and 30 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C, followed by a final extension step of 10 min at 72°C. The amplified products contained the putative promoter region and structural ampD genes, with amplicons of 820 bp for the ampDI gene and 2,172 bp for the ampDII gene. The PCR amplicons were treated with the restriction enzymes HindIII and XbaI and then cloned into the plasmid pEX18Tc (7) treated with the same enzymes. Both strands of the cloned DNA were sequenced. The resultant plasmids were named pAmpDI and pAmpDII. A 1.4-kb ampDII gene fragment obtained from pAmpDII was inserted into the HindIII-treated pAmpDI, resulting in the recombinant plasmid pAmpDIIDI.
Construction of ampDI, ampDII, and ampDIDII mutants.
A gene replacement strategy was used for the construction of the ampDI, ampDII, and ampDIDII mutants. A 944-bp PCR amplicon containing the upstream and N-terminal regions of the ampDI gene was obtained by PCR using the primer sets DIN-F/DIN-R (Table 1) and cloned into the yT&A vector (Yeastern Biotech Co.) (pTDIN). Similarly, the recombinant plasmid pTDIC, which contained the 793-bp C-terminal and downstream regions of the ampDI gene, was obtained using the PCR primer sets DIC-F/DIC-R (Table 1). pTDIN was digested with HindIII and XbaI and cloned into pEX18Tc, and then the 793-bp XbaI-EcoRI fragment cut from pTDIC was further ligated. The resultant plasmid, pΔDI, with an internal 103-bp deletion of the ampDI gene, was used for the construction of mutant KJΔDI. A similar strategy was employed for constructing the recombinant plasmid pΔDII, which had an internal 149-bp deletion of the ampDII gene. The resultant plasmids, pΔDI and pΔDII, were transformed into E. coli S17-1 and further introduced into S. maltophilia KJ by conjugation (10). Transconjugants were selected on LB plates containing norfloxacin (2.5 μg/ml) and tetracycline (30 μg/ml). For mutant selection, the transconjugants were further transferred onto 10% sucrose-containing plates. The authenticity of the mutants was checked by colony PCR amplification (16) and sequencing. The double mutant was constructed sequentially from the single mutant by the same procedure.
Complementation studies.
The plasmids pAmpDI, pAmpDII, and pAmpDIIDI were introduced into strain KJΔDIΔDII by conjugation. The transconjugants were selected on LB plates containing 2.5 μg/ml norfloxacin and 30 μg/ml tetracycline and further confirmed by colony PCR (16) using AmpDIQ-F/AmpDIQ-R and AmpDIIQ-F/AmpDIIQ-R as the primers.
Preparation of β-lactamase extracts.
Cells were grown to mid-log phase, and an inducer at a specific concentration was added for the production of β-lactamase. After further incubation for 2 h, the cells were harvested by centrifugation and washed with 10 mM sodium phosphate buffer at pH 7.0. Following resuspension of the pellet in 2 ml of the same buffer, the cells were disrupted by sonication to yield the cell extract, which was ready for isoelectric focusing and β-lactamase activity assays.
Determination of β-lactamase activity.
Specific β-lactamase activity was determined spectrophotometrically on crude sonic cell extracts (17). The differential L1 and L2 β-lactamase activities were determined by the modified nitrocefin-EDTA method (9). The specific activity (U/mg) was expressed as nanomoles of nitrocefin hydrolyzed per minute per milligram of protein, using an extinction coefficient (Δɛ) of 20,500 M−1 cm−1 for nitrocefin at 486 nm, as suggested by the manufacturer (Oxoid, United Kingdom). The protein concentration was determined using the Bio-Rad protein assay reagent, with bovine serum albumin as a standard.
QRT-PCR.
Total RNA from cultures grown to log phase with and without inducers was isolated by using the PureLink Total RNA Purification System (Invitrogen, Carlsbad, CA) and treated with 1 unit of RNase-free DNase I (Invitrogen, Carlsbad, CA) for 15 min to eliminate DNA contamination. cDNA was generated from equal amounts of RNAs of the assayed strains by using an MMLV Reverse Transcriptase 1st Strand cDNA Synthesis Kit (Epicentre Biotechnologies, Taiwan). Quantitative real-time PCR (QRT-PCR) was performed with Smart Quant Green Master Mix (Protech Technology Enterprise Co., Ltd.) using the programmed ABI Prism 7000 Sequence Detection System (Applied Biosystems) (11). The primers used for QRT-PCR are listed in Table 1. The level of gene expression was calculated using the comparative ΔΔCT method (20). The expression of assayed genes was normalized to the endogenous 16S rRNA gene for variation in RNA quantity and quality. Each experiment was performed three times.
Antimicrobial susceptibility test.
MICs were determined in triplicate by a standard twofold serial agar dilution method according to the guidelines of the Clinical Laboratory Standards Institute (1). All antibiotics were purchased from Sigma. The MICs of cefepime, imipenem, and meropenem were quantified using Etest strips (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions.
Nucleotide sequence accession numbers.
The nucleotide sequences of S. maltophilia KJ ampDI and ampDII have been deposited in GenBank under accession no. EJ447465 and EJ447466.
RESULTS AND DISCUSSION
The ampDI gene and its genomic context organization are conserved in different S. maltophilia strains.
Sequence analysis of the 820-bp PCR amplicon from strain KJ revealed a putative ampDI gene. The predicted 188-amino-acid AmpDI protein exhibited 98, 94, 67, 32, 33, 33, and 31% identity, respectively, to Smlt1562 of S. maltophilia K279a (accession no. NC_010943), Smal1320 of S. maltophilia R551-3 (accession no. NC_011071), XCC1539 of Xanthomonas campestris pv. campestris ATCC 33913 (accession no. NC_003902), PA4522 (AmpD) of P. aeruginosa PAO1 (accession no. NC_002516), AmpD of E. cloacae 14 (accession no. Z14003), AmpD of C. freundii OS60 (accession no. Z14002), and AmpD of E. coli K-12 (accession no. AAC73221). The intraspecies diversity in the AmpDI protein was at least 6%, as revealed by comparison among S. maltophilia strains KJ, K279a, and R551-3. This diversity value is consistent with those of E. cloacae (22) and C. freundii (29). A highly conserved “common core region” of the AmpD protein and seven strictly conserved residues revealed by Jacobs et al. (12) were also identified in the AmpDI protein of S. maltophilia KJ. Furthermore, the AmpDI protein of S. maltophilia KJ conserved the four residues essential for C. freundii AmpD activity proposed by Genereux et al. (5).
The AmpDI protein is likely a cytoplasmic protein, according to the SignalP3.0 Server prediction (http://www.cbs.dtu.dk/services/SignalP/).
Two whole genomic sequences of S. maltophilia strains K279a and R551-3 are available in the public database. The genomic organizations surrounding ampDI in strains K279a and R551-3 were compared. Highly conserved genetic contexts were found to surround the ampDI gene. A 528-bp open reading frame (ORF) encoding a hypothetical protein was located upstream of the ampDI gene, while a putative sulfurtransferase-encoding gene was observed downstream. However, no ampE homologue was present in the neighborhood of the ampDI gene. This structure is different from the genomic organization of the ampD-ampE operon of the Enterobacteriaceae and P. aeruginosa (8, 15).
The ampDII gene and its genomic context are more divergent in different S. maltophilia strains.
The ampDII gene from strain KJ was located on a 2,172-kb PCR amplicon. Sequencing of the amplicon revealed a complete 747-bp ORF and two truncated ORFs. The complete ORF encoded AmpDII, which showed 99, 90, 67, 38, 33, and 40% homology, respectively, to Smlt0154 of S. maltophilia K279a (accession no. CAQ_43763), Smal0115 of S. maltophilia R551-3 (accession no. ACF_49820), XCC3805 of X. campestris pv. campestris ATCC 33913 (accession no. AAM_43051), PA5485 (AmpDh2) (accession no. NP_254172) and PA0807 (AmpDh3) (accession no. NP_249498) of P. aeruginosa PAO1, and AmiD of E. coli (accession no. NP_415388). AmpDI and AmpDII of S. maltophilia KJ displayed 29% identity. As predicted by the LipoP 1.0 server (14; http://www.cbs.dtu.dk/services/LipoP/), a putative signal peptidase II cleavage site was detected between the 18th Ala and the 19th Cys residues, indicating that AmpDII could be a lipoprotein. According to the “+2 rule” of lipoprotein sorting (28), AmpDII is likely to be transferred to the outer membrane.
Interestingly, the genetic contexts of ampDII in S. maltophilia strains K279a and R551-3 were different. In K279a, ampDII was flanked by a putative ammonia transporter gene (Smlt0153) and a putative murein-degrading transglycosylase gene (Smlt0156), while in R551-3, there was an approximately 17.5-kb DNA fragment inserted between the putative ammonia transporter (Smal 0111) and ampDII (Smal0115) genes, as well as an extra 4-kb DNA fragment inserted between ampDII and the putative murein-degrading transglycosylase (Smal0119) gene. The ampDII genomic organization of strain KJ is more similar to that of strain K279a.
AmpDI, but not AmpDII, is involved in regulation of β-lactamase expression.
To investigate the biological functions of AmpDI and AmpDII, the isogenic mutants KJΔDI, KJΔDII, and KJΔDIΔDII were constructed. It has been found that inactivation of ampD of the Enterobacteriaceae and P. aeruginosa generally leads to a derepression phenotype (12, 19). Therefore, induction assays and β-lactamase activity measurements were performed with the wild-type strain KJ and the isogenic mutants KJΔDI, KJΔDII, and KJΔDIDII. Cefoxitin (50 μg/ml) was used as the inducer, and the nitrocefin-EDTA method (9) was used to differentially determine the L1 and L2 β-lactamase activities. Strain KJΔDII displayed an induction pattern (low basal level and high induced activity) similar to that of the wild-type strain KJ, whereas strains KJΔDI and KJΔDIΔDII had a fully derepressed phenotype (constitutive hyperexpression) with an equivalent fully derepressed β-lactamase activity (Table 2). Furthermore, the mutants KJΔDI and KJΔDIΔDII expressed both the L1 and L2 β-lactamases in the basal condition (Table 2), indicating that AmpDI is involved in the expression of both the L1 and L2 genes.
TABLE 2.
Basal and induced β-lactamase activities of S. maltophilia strains
| Strain | β-Lactamase activity (Ua/mg)
|
|||||
|---|---|---|---|---|---|---|
| Basal
|
Inducedb
|
|||||
| (L1 + L2)c | L1d | L2e | (L1+L2)c | L1d | L2e | |
| KJ | 20 | 0 | 20 | 1,958 | 605 | 1,437 |
| KJΔDI | 4,478 | 1,155 | 3,323 | 4,379 | 1,064 | 3,315 |
| KJΔDII | 16 | 1 | 15 | 2,105 | 624 | 1,481 |
| KJΔDIΔDII | 4,431 | 896 | 3,535 | 4,478 | 1,006 | 3,472 |
| KJΔDIΔDII (pKJAmpDI) | 20 | 1 | 19 | 1,186 | 282 | 904 |
| KJΔDIΔDII (pKJAmpDII) | 4,706 | 1,272 | 3,434 | 4,539 | 1,475 | 3,064 |
| KJΔDIΔDII (pKJAmpDIIDI) | 14 | 2 | 12 | 743 | 366 | 377 |
One unit of β-lactamase activity is defined as 1 nanomole of nitrocefin hydrolyzed per minute. The results are means of three independent determinations. Standard derivations were within 10% of the means in all cases.
With 50 μg/ml cefoxitin as the inducer.
The total β-lactamase activity (L1 and L2) was determined by the nitrocefin method.
The L1 β-lactamase activity was calculated by subtracting the L2 β-lactamase activity from the total β-lactamase activity.
The L2 β-lactamase activity was determined by the nitrocefin-EDTA method.
Isoelectric focusing analysis of β-lactamases in the cell extracts revealed two identical bands, corresponding to L1 and L2, in the induced strain KJ and in the uninduced strain KJΔDI. This finding confirmed that both the L1 and L2 genes are coderepressed in the AmpDI-null mutant.
The RNA transcripts of the L1 and L2 genes from strains KJ and KJΔDI were quantified by QRT-PCR. Under the uninduced condition, the transcript ratio of KJΔDI to KJ was 89 for the L1 gene and 184 for the L2 gene. However, this ratio was 3 for the L1 gene and 21 for the L2 gene under the induced condition. Consequently, the AmpDI protein has a stronger repressor effect on L1 and L2 β-lactamase expression regardless of the presence of β-lactams.
Complementation assays were performed by introducing pAmpDI, pAmpDII, and pAmpDIIDI into strain KJΔDIΔDII, and the basal and induced β-lactamase activities of these transconjugants were quantified. Strains KJΔDIΔDII(pAmpDI) and KJΔDIΔDII(pAmpDIIDI) exhibited the wild-type β-lactamase expression pattern, i.e., low basal level and inducibility, as observed in the wild-type strain KJ, while complementation with pAmpDII did not restore the inducible phenotype in KJΔDIΔDII (Table 2).
These results confirmed that AmpDI is a functional anhydro-N-acetylmuramyl-l-alanine amidase in S. maltophilia and is associated with the regulation of L1 and L2 gene expression. Although strains KJ, KJΔDIΔDII(pAmpDI), and KJΔDIΔDII(pAmpDIIDI) displayed β-lactamase inducibility (Table 2), the induced β-lactamase activities were notably different, since KJΔDIΔDII(pAmpDI) showed an induced β-lactamase activity lower than that of the wild-type strain, KJ (Table 2). This difference could be related to the copy number of plasmid pAmpDI. More AmpDI proteins in KJΔDIΔDII(pAmpDI) could result in a stronger repressor effect.
As for AmpDII, results obtained with KJΔDII suggested that AmpDII has no significant effect on the expression of the L1 and L2 genes, and this hypothesis was confirmed by data from the complementation assays. However, it is noteworthy that the induced β-lactamase activity of strain KJΔDIΔDII(pAmpDIIDI) was lower than that of strain KJΔDIΔDII(pAmpDI) (743 versus 1,186 [Table 2]), suggesting that the AmpDII protein could exhibit a synergistic effect on the function of the AmpDI protein. The synergistic effect of AmpDII on the AmpDI function was not detectable with mutants, which could be due to lower expression of the ampDII gene when carried in the chromosomal context than when carried on the multicopy plasmid.
The effect of AmpD inactivation on β-lactam resistance was also determined. All tested β-lactams exhibited the same MICs for the wild-type strain, KJ, and the ampD-associated mutants except cefepime, which was slightly less active against the KJΔDI and KJΔDIΔDII mutants (Table 3). These results suggest that ampDI inactivation likely does not have clinical relevance.
TABLE 3.
MICs of β-lactam antibiotics for S. maltophilia KJ and its derived ampD-associated mutants
| Antibiotic | MIC (μg/ml) of antibiotic for strain:
|
|||
|---|---|---|---|---|
| KJ | KJΔDI | KJΔDII | KJΔDIΔDII | |
| Penicillins | ||||
| Piperacillin | 1,024 | 1,024 | 1,024 | 1,024 |
| Carbenicillin | 1,024 | 1,024 | 1,024 | 1,024 |
| Cephalosporins | ||||
| Cefuroxime | 2,048 | 2,048 | 2,048 | 2,048 |
| Cefoxitin | 1,024 | 1,024 | 1,024 | 1,024 |
| Ceftriaxone | 256 | 256 | 256 | 256 |
| Cefepime | 64 | 96 | 64 | 96 |
| Carbapenems | ||||
| Imipenem | >32 | >32 | >32 | >32 |
| Meropenem | >32 | >32 | >32 | >32 |
| Monobactam | ||||
| Aztreonam | >2,048 | >2,048 | >2,048 | >2,048 |
The ampDI and ampDII genes are constitutively expressed.
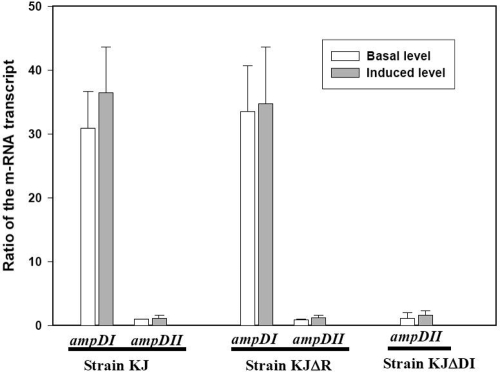
The expression of the ampDI and ampDII genes under different conditions, such as the basal state, induced state, and AmpDI-null state, was studied using QRT-PCR (Fig. 1). The expression of the ampDI and ampDII genes was constitutive and was hardly influenced by the addition of the inducer. Under basal or induced conditions, the expression of ampDI was higher than that of ampDII, suggesting that in wild-type S. maltophilia, AmpDI could play a significant role in the cell wall-recycling process. Since strains KJΔDI and KJ expressed equivalent amounts of ampDII transcript (Fig. 1), no compensatory expression of the ampDII gene occurs in the absence of the AmpDI protein.
FIG. 1.
The relative increase in ampDI and ampDII mRNA of the basal and induced S. maltophilia strains KJ, KJΔR, and KJΔDI. Each pair of bars indicates the ratio of the mRNA transcript of a specific gene to that of the strain KJ basal ampDII, as measured by QRT-PCR. The induced condition is 30 μg/ml cefoxitin for 2 h. The mean value is based on three independent experiments. The error bars indicate the standard deviations.
AmpR is not involved in the expression of ampDI and ampDII.
In our previous study, AmpR was shown to act as an important regulator in the induction of the L1 and L2 genes (10). To investigate the role of AmpR in the expression of the ampDI and ampDII genes, the ampDI and ampDII transcripts of strains KJ and KJΔR were evaluated under the basal and induced conditions. Figure 1 shows that the expression of ampDI and ampDII was unaffected by ampR inactivation, suggesting that AmpR is not involved in the expression of the ampDI and ampDII genes.
Concluding remarks.
Following the release of whole genomic sequences, several bacteria have been found to have multiple ampD-like alleles, such as ampD and amiD in E. coli (30) and ampD, ampDh2, and ampDh3 in P. aeruginosa (13). S. maltophilia, like the Enterobacteriaceae, has genes for two AmpD homologues, i.e., AmpDI and AmpDII, in its genome. However, in the present study, the previously mentioned semiconstitutive β-lactamase hyperproduction phenotypes in E. cloacae (19) and the moderate-level (or high-level) hyperinducible phenotypes in P. aeruginosa (13) were not observed in the ampD-associated mutants of S. maltophilia. Recently, Okazaki and Avison have isolated 12 β-lactamase hyperexpression S. maltophilia mutants, 2 of which exhibit the phenotype of semiconstitutive overexpression (24). The ampDII genes of those mutants have been sequenced, and mutations in the ampDII gene were not observed. However, ampDI sequence results were not reported. Nevertheless, the finding of the semiconstitutive overexpression mutants suggests that, in addition to the regulation involving ampD, alternative mechanisms may contribute to the L1 and L2 gene derepression, which is consistent with the results of Schmidtke and Hanson for P. aeruginosa (27).
For some specific enterobacterial strains, such as E. cloacae NOR-1 and Proteus vulgaris, a second set of ampR class A β-lactamase modules exists, in addition to the ampR-ampC module (3, 23). AmpD of E. cloacae NOR-1 has been shown to be involved in the expression of both of the ampR-linked β-lactamases, NmcA and AmpC (22). In this study, AmpDI in S. maltophilia was found to play a key role, not only in the expression of the ampR-linked L2 gene, but also in the expression of the non-ampR-linked L1 gene. This, together with our previous findings (17), indicates that the L1 and L2 genes may be under the control of the ampD-ampR-associated regulatory system.
Acknowledgments
This research was supported by grants CMU-95-152 and CMU-95-102 from the China Medical University and grant NSC 97-2320-B-039-028 from the National Science Council.
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Clinical Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing, 16th informational supplement. CLSI document M100-S16. Clinical Laboratory Standards Institute, Wayne, PA.
- 2.Crossman, L. C., V. C. Gould, J. M. Dow, G. S. Vernikos, A. K. Okazaki, M. Sebaihia, D. Saunders, C. Arrowsmith, T. Carver, N. Peters, E. Adlem, A. Kerhornou, A. Lord, L. Murphy, K. Seeger, R. Squares, S. Rutter, M. A. Quail, M.-A. Rajandrea, D. Harris, C. Churcher, S. D. Bentley, J. Parkhill, N. R. Thomson, and M. B. Avison. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Gen. Biol. 9:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datz, M., B. Joris, E. A. M. Azab, M. Galleni, J. Van Beeumen, J.-M. Frere, and H. H. Martin. 1994. A common system controls the induction of very different genes. The class-A β-lactamase of Proteus vulgaris and the enterobacterial class-C β-lactamse. Eur. J. Biochem. 226:149-157. [DOI] [PubMed] [Google Scholar]
- 4.Denton, M., and K. G. Kerr. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11:57-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genereux, C., D. Dehareng, B. Devreese, J. van Becumen, J. M. Frere, and B. Joris. 2004. Mutational analsyis of the catalytic centre of the Citrobacter freundii AmpD N-acetylmuramyl-l-alanne amidase. Biochem. J. 377:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson, N., and C. C. Sanders. 1999. Regulation of inducible AmpC beta-lactamase expression among Enterobacteriaceae. Curr. Pharm. Des. 5:881-894. [PubMed] [Google Scholar]
- 7.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 8.Honore, N., M. H. Nicolas, and S. T. Cole. 1989. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol. Microbiol. 3:1121-1130. [DOI] [PubMed] [Google Scholar]
- 9.Hu, R.-M., K.-H. Chiang, C.-W. Lin, and T.-C. Yang. 2008. Modified nitrocefin-EDTA method to differentially quantify the induced L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Lett. Appl. Microbiol. 47:457-461. [DOI] [PubMed] [Google Scholar]
- 10.Hu, R.-M., K.-J. Huang, L.-T. Wu, Y.-J. Hsiao, and T.-C. Yang. 2008. Induction of L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 52:1198-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, S.-H., T.-C. Yang, M.-H. Tsai, I.-S. Tsai, H.-C. Lu, P.-H. Chuang, L. Wan, Y.-J. Lin, C.-H. Lai, and C.-W. Lin. 2008. Gold nanoparticle-based RT-PCR and real-time quantitative RT-PCR assays for detection of Japanese encephalitis virus. Nanotechnology 19:405101. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, C., B. Joris, M. Jamin, K. Klarsov, J. Van Beeumen, D. Mengin-Lecreulx, J. van Heijenoort, J. T. Park, S. Normark, and J.-M. Frere. 1995. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol. Microbiol. 15:553-559. [DOI] [PubMed] [Google Scholar]
- 13.Juan, C., B. Moya, J. L. Perez, and A. Oliver. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high-level β-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langaee, T. Y., M. Dargis, and A. Huletsky. 1998. An ampD gene in Pseudomonas aeruginosa encodes a negative regulator of AmpC β-lactamase expression. Antimicrob. Agents Chemother. 42:3296-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, C.-W., C.-S. Chiou, Y.-C. Chang, and T.-C. Yang. 2008. Comparison of pulsed-field gel electrophoresis and three rep-PCR methods for evaluating the genetic relatedness of Stenotrophomonas maltophilia isolates. Lett. Appl. Microbiol. 47:393-398. [DOI] [PubMed] [Google Scholar]
- 17.Lin, C.-W., Y.-W. Huang, R.-M. Hu, K.-H. Chiang, and T.-C. Yang. 2009. The role of AmpR in regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Res. Microbiol. 160:152-158. [DOI] [PubMed] [Google Scholar]
- 18.Lindberg, F., L. Westman, and S. Normark. 1985. Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc. Natl. Acad. Sci. USA 82:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg, F., S. Lindquist, and S. Normark. 1987. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii β-lactamase. J. Bacteriol. 169:1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 21.Lodge, J. M., S. D. Minchin, L. J. V. Piddock, and S. J. W. Busby. 1990. Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC β-lactamase. Biochem. J. 272:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naas, T., S. Massuard, F. Garnier, and P. Nordmann. 2001. AmpD is required for regulation of expression of NmcA, a carbapenem-hydrolyzing β-lactamase of Enterobacter cloacae. Antimicrob. Agents Chemother. 45:2908-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann, P., S. Mariotte, T. Naas, R. Labia, and M.-H. Nicolas. 1993. Biochemical properties of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and cloning of its gene into Escherichia coli. Antimicrob. Agents Chemother. 37:939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki, A., and M. B. Avison. 2008. Induction of L1 and L2 β-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob. Agents Chemother. 52:1525-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saino, Y., F. Kobayashi, M. Inoue, and S. Mitsuhashi. 1982. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicrob. Agents Chemother. 22:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saino, Y., M, Inoue, and S. Mitsuhashi. 1984. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob. Agents Chemother. 25:362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidtke, A. J., and N. D. Hanson. 2008. Role of ampD homologs in overproduction of AmpC in clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3922-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seydel, A., P. Gounon, and A. Pugsley. 1999. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 34:810-821. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton, P., K. Shannon, and I. Phillips. 1995. DNA sequence differences of ampD mutants of Citrobacter freundii. Antimicrob. Agents Chemother. 39:2494-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara, T., and J. T. Park. 2007. An anhydro-N-acetylmuramyl-l-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J. Bacteriol. 189:5634-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]