Abstract
Clarithromycin decreases CYP3A4 activity and thus gradually inhibits its own metabolism as well as that of coadministered drugs. The aim of this study was to obtain an understanding of the time course of these changes. The plasma concentration-time profiles of clarithromycin and its active metabolite, 14(R)-hydroxy-clarithromycin, in 12 young healthy volunteers after oral administration of a clarithromycin suspension (500 mg twice a day [b.i.d.] for seven doses) were modeled by population pharmacokinetic analysis in the NONMEM program. The nonlinearity of clarithromycin metabolism was considered during model development, and the metabolite disposition kinetics were assumed to be linear. The absorption kinetics of clarithromycin were best described by a Weibull function model. The pharmacokinetics of clarithromycin and its 14(R)-hydroxyl metabolite were adequately described by a one-compartment model each for clarithromycin and its metabolite as well as an inhibition compartment that reflects the autoinhibition of clarithromycin metabolism. Up to 90% of the apparent total clarithromycin clearance (60 liters/h) was susceptible to reversible autoinhibition, depending on the concentration in the inhibition compartment. The proposed semimechanistic population pharmacokinetic model successfully described the autoinhibition of clarithromycin metabolism and may be used to adjust the doses of other drugs that are metabolized by CYP3A4 and that are coadministered with clarithromycin. Simulations showed that for the standard dose of 500 mg b.i.d., no further increase in the level of exposure occurs after approximately 48 h of treatment. For a 1,000-mg b.i.d. dose, the achievement of steady state is expected to take several days and to achieve a 3.6-fold higher level of clarithromycin exposure than the 500-mg b.i.d. dose. This evaluation provides a rationale for safer and more effective therapy with clarithromycin.
Clarithromycin is a broad-spectrum macrolide antibiotic and is widely used for the treatment of upper and lower respiratory tract and other infections (11, 19, 30). Clarithromycin interacts with many drugs on the level of CYP3A intestinal and hepatic metabolizing enzymes (15, 29). This may change the efficacy and tolerability of other CYP3A substrates that are coadministered with clarithromycin.
Clarithromycin is rapidly and nearly complete absorbed from the gastrointestinal tract (17). Due to notable first-pass metabolism, approximately 50% to 55% of an oral dose is bioavailable as clarithromycin in the systemic circulation (6, 13, 15). The free fraction of clarithromycin in plasma is about 0.3 in healthy volunteers (7, 33). Clarithromycin is widely distributed throughout the body and has an apparent volume of distribution that ranges from 126 to 306 liters (5, 29, 33). Approximately 22% of an oral dose is recovered as the parent compound, with 18% being recovered in the urine and 4% being recovered in the feces (10). The elimination half-life of clarithromycin is dose and time dependent and ranges from 2.7 to 4.8 h (5, 8, 12, 33). In healthy subjects, the average total body clearance ranges from 29 to 58 liters/h and the average renal clearance ranges from 6.7 to 12.8 liters/h, depending on the amount and the number of doses administered (5, 29). After the administration of single and multiple (seven) doses of 500-mg clarithromycin tablets, the apparent total body clearance of clarithromycin (CLp) was reported to decrease from 42.1 to 18.7 liters/h (5, 33). As the renal clearance does not change under these conditions, the nonlinearity is attributed to nonrenal elimination mediated by cytochrome P450 metabolism (5). Clarithromycin is extensively metabolized into at least eight metabolites via three metabolic pathways; i.e., hydroxylation at the 14 position, N-demethylation, and hydrolysis of the cladinose sugar. Secondary biotransformation was also evident (10, 12, 35).
Clarithromycin hydroxylation at position 14 is stereospecific, yielding the 14-hydroxy-(R)-epimer as the main metabolite, which accounts for 20% of the metabolites from the parent drug (10, 12, 19). Indeed, this metabolite contributes significantly to the overall antimicrobial effect of clarithromycin (20, 35). The formation of this main metabolite is predominantly mediated by CYP3A4 (2, 28, 31, 35) and was suggested to be capacity limited, which may in part account for the nonlinearity of clarithromycin pharmacokinetics that has been observed (5).
Clarithromycin is also a potent inhibitor of intestinal and hepatic CYP3A4 activity (15) in a dose-dependent manner (34). It has been classified as a mechanism-based inhibitor (36). Clarithromycin inhibits the activity (15, 26) but not the level of expression (26) of intestinal wall CYP3A. On the basis of in vitro models, clarithromycin was predicted to cause a reduction in the steady-state concentration of liver CYP3A4 to approximately 39% of the initial level (21). The mechanism of this autoinhibition was reported to be reversible (16, 26), irreversible (2, 15, 25), and mediated by suicide inhibition by the formation of a metabolic intermediate complex (21).
For clarithromycin, the ratio of the area under the free plasma concentration-time curve (fAUC) to the MIC is considered to be the most predictive pharmacokinetic-pharmacodynamic index (9, 32) and is used to link the pharmacokinetic parameters to the most important antimicrobial pharmacodynamic parameter, i.e., the MIC. On the basis of the findings with animal models of infection, a derived target value of the ratio of the fAUC from 0 to 24 h (fAUC0-24) for plasma/MIC was assumed to be 35 for clarithromycin (9, 32). Actually, the clarithromycin dosage regimen for adults of 500 mg twice daily (b.i.d.) achieves an average fAUC0-24 of about 5.85 ± 1.79 mg·h/liter in plasma (33), which may provide sufficient efficacy, as long as the MIC does not exceed 0.25 to 1.0 μg/ml (29). However, this dosing regimen may be ineffective for the treatment of infections caused by pathogens for which the MIC of the drug is greater than 1 μg/ml in plasma or greater than 0.125 μg/ml in tissues, where the fAUC is lower, suggesting the need for the administration of a higher dose (22, 33). Because of the nonlinear kinetics, it is difficult to predict the changes in fAUC achieved by the use of higher doses.
The aim of this study was to quantitatively describe the nonlinearity in the pharmacokinetics of clarithromycin in the presence of its active metabolite, 14(R)-hydroxy-clarithromycin, and to better understand the time course of inhibition by population pharmacokinetic modeling. These data are important for obtaining a better understanding of the underlying processes and assessing the dose adjustments that may be needed for clarithromycin and coadministered CYP3A4 substrates.
MATERIALS AND METHODS
Subjects and treatments.
A total of 12 healthy Caucasian volunteers (7 men, 5 women) were enrolled in a single-center, open, randomized steady-state clinical study which had been approved by the Ethics Committee of the Ministry of Health Clinic Hospital of the Republic of Moldavia, Chisinau, Republic of Moldavia. The study was conducted according to the revised version of the Declaration of Helsinki. All participants gave their written informed consent. The volunteers were nonsmokers or former smokers between the ages of 19 and 41 years (mean ± standard deviation, 28 ± 8 years), their weights ranged from 45.1 to 86.1 kg (mean ± standard deviation, 66.5 ± 11.8 kg), and their body heights ranged from 150.0 to 186.0 cm (mean ± standard deviation, 168.4 ± 9.7 cm).
The participants were judged to be healthy on the basis of their medical histories; vital signs; the findings of a complete physical examination, neurological assessment, and 12-lead electrocardiography; and the results of clinical chemistry, hematologic, urine, and virological tests.
Each subject took an oral dose of 500 mg clarithromycin every 12 h for four consecutive days (a total of seven doses; each dose was 500 mg per 10 ml of clarithromycin suspension equivalent to 500 mg clarithromycin [Klacid; Abbott B.V., Hoofddorp, The Netherlands]). Subjects took the drug in the fasting state with 240 ml low-carbonation calcium-poor mineral water. After each study drug administration, the subjects lay in bed for at least 3 h. The consumption of alcohol was prohibited starting at 2 days predosing, the consumption of beverages or foods containing methylxanthines was prohibited starting at 2 days predosing, and the consumption of grapefruit products was prohibited starting at 7 days predosing. No food was allowed from approximately 8 h before until approximately 4 h after each morning dosing and from approximately 2.5 h before until approximately 3 h after each evening dosing. Identical food was given on all study days. No fatty food was given during the entire study. No fluid intake from 1 h before until 2 h after dosing (with the exception of the fluid for study drug administration) was allowed. Thereafter, 120 ml of low-carbonation calcium-poor mineral water (room temperature) was given every hour until 11 h after the morning administration. No extensive fluid intakes (>120 ml/h) were allowed during the nights.
Sampling and assay.
Blood samples were collected for the quantification of clarithromycin and its 14(R)-hydroxyl metabolite immediately before administration and 0.33, 0.67, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 8, and 12 h after the administration of the first, third, and seventh doses. Additionally, one sample each was taken immediately prior to the administration of the fifth and the sixth doses. Plasma samples were analyzed by a validated liquid chromatography-tandem mass spectrometry assay.
Briefly, plasma samples (0.1 ml) were precipitated by addition of 200 μl of acetonitrile containing the internal standard. After the samples were thoroughly mixed, the samples were centrifuged at 3,280 × g for 5 min at approximately +4°C and the supernatant was diluted (1:1) with buffer. Fifteen microliters of each sample was chromatographed on a reversed-phase column (Waters Symmetry C8 column), eluted with an isocratic solvent system consisting of ammonium acetate buffer and acetonitrile (65/35, vol/vol, pH 4), and monitored by liquid chromatography-tandem mass spectrometry by a selected reaction monitoring method, as follows: precursor → product ion m/z 749 → m/z 158 for clarithromycin, m/z 765 → m/z 158 for 14(R)-hydroxy-clarithromycin, and m/z 838 → m/z 679 for the internal standard. All analyses were performed in the positive mode. Under these conditions, clarithromycin, 14(R)-hydroxy-clarithromycin, and the internal standard were eluted after approximately 1.8, 0.8, and 2.0 min, respectively. MacQuan software (version 1.6, 1991 to 1998; PE Sciex, Thornhill, Ontario, Canada) was used for evaluation of the chromatograms.
The results for the plasma samples were measured by comparison with the results for plasma in a calibration row prepared by adding defined amounts of the standard solution to drug-free human plasma. Spiked quality controls were prepared for determination of interassay variation by the addition of defined amounts of the stock solution or the control spiked with a higher concentration to defined amounts of test drug-free human plasma. No interference was observed in plasma for clarithromycin, 14(R)-hydroxy-clarithromycin, or the internal standard. Weighted linear regression (1/concentration2) was performed for calibration. The linearity of the calibration curve in human plasma could be shown between 0.00992 μg/ml and 3.98 μg/ml for clarithromycin and between 0.0101 and 4.04 μg/ml for 14(R)-hydroxy-clarithromycin. The lower limits of quantification were identical to the lowest calibration levels.
The interday precision and the analytical recovery of the spiked quality control standards of clarithromycin in human plasma ranged from 3.0 to 4.4% and were 99.9% for 3.07 μg/ml, 100.4% for 1.02 μg/ml, 100.3% for 0.0941 μg/ml, and 100.9% for 0.0267 μg/ml. The interday precision and the analytical recovery of the spiked quality control standards of 14(R)-hydroxy-clarithromycin in human plasma ranged from 6.3 to 6.8% and were 100.1% for 3.04 μg/ml, 97.2% for 1.01 μg/ml, 95.5% for 0.0930 μg/ml, and 96.8% for 0.0264 μg/ml.
Population pharmacokinetic analysis. (i) Model development.
A total of 624 samples with clarithromycin and 624 samples with its 14-(R)-hydroxyl metabolite from 12 subjects were comodeled by using the nonlinear mixed-effects approach implemented in the NONMEM program (version V, level 1.1; NONMEM Project Group, University of California at San Francisco, 1998). First-order conditional estimation with the interaction estimation option was used. The model was specified as a set of differential equations by use of the ADVAN6 subroutine and was parameterized by use of the clearance and volume of distribution parameters.
A one-compartment open model with first-order absorption with or without a lag time was tested to describe the clarithromycin plasma concentration profiles after the first dose with first-order elimination. A compartment for the hydroxyl metabolite was included. The absorption behavior could not be described properly by first-order absorption; therefore, a single-phase Weibull function (WB) was tested. This function was coded as WB = 1 − exp[−(kw·Tw)λ], where kw is the absorption rate constant, Tw is the time after the administration of the previous dose, and λ is the shape parameter (24). This model appropriately described the absorption phase, and the use of more complicated input models was not successful.
The process of distribution of the parent and its metabolite was assumed to follow linear pharmacokinetics. This model was selected as a base structural model. CLp was modeled by the use of linear and/or nonlinear kinetics, including the use of a Michaelis-Menten model. Elimination of the metabolite was assumed to follow linear pharmacokinetics.
Individual body weights were related to a standard weight, i.e., 70 kg, and used as the covariate for the apparent volume of distribution and total clearance parameters of clarithromycin and its metabolite as CLi = C̄L̄·(BW/70 kg)0.75 and Vi = V̄·(BW/70 kg), respectively, where CLi and Vi stand for the individual apparent total clearance and the volume of distribution, respectively; BW is the individual body weight (expressed in kg); and C̄L̄ and V̄ represent the mean clearance and the mean volume of distribution, respectively, for typical subjects weighing 70 kg (1).
(ii) Statistical model.
An exponential-variability model was included to describe between-subject variability for all pharmacokinetic parameters, and stepwise cumulative inclusion of the respective error terms was used. The model was θj = θp·exp(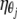 ), where θj is the estimate for a pharmacokinetic parameter in the jth individual, θp is the population mean of the pharmacokinetic parameter, and ηθj represents the random deviation of a random variable with zero mean and variance ω2 that distinguishes the jth individual pharmacokinetic parameter from the population mean.
), where θj is the estimate for a pharmacokinetic parameter in the jth individual, θp is the population mean of the pharmacokinetic parameter, and ηθj represents the random deviation of a random variable with zero mean and variance ω2 that distinguishes the jth individual pharmacokinetic parameter from the population mean.
Two separate residual variability terms, one for clarithromycin and the second for its hydroxyl metabolite, were modeled by using proportional and/or additive error models.
(iii) Model selection.
A visual inspection of individual curve fits, NONMEM's objective function, and standard diagnostic plots were used to determine the best model. Additionally, the precisions of the pharmacokinetic parameter estimates and the variability estimates were considered during model development. The predictive performance was evaluated by the use of visual predictive checks to assess whether a model adequately described the central tendency and variability of the observations. The medians and the 5th and 95th percentiles of the concentration-time curves were calculated by the use of validated Perl scripts (Active Perl, version 5.10.0; ActiveState, Vancouver, British Columbia, Canada) (4).
(iv) Simulation.
On the basis of the final parameter estimates of the selected population model, simulations were performed with the NONMEM (version V) program to predict the concentration profiles for multiple doses of 250, 500, 750, or 1,000 mg b.i.d. The simulation was done for periods of time sufficient to achieve theoretical steady state. For these doses, fAUC0-24, the fAUC0-24/MIC ratio, and the probability of attainment of the pharmacodynamic target of an fAUC0-24/MIC ratio of >35 (9, 32) across various MICs were calculated at steady state and by assuming no significant change in the level of protein binding (23, 33).
RESULTS
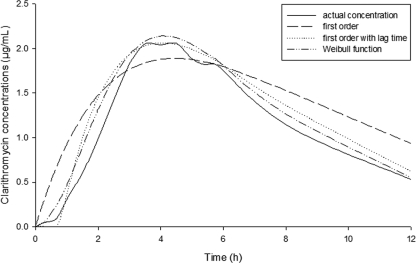
The absorption profiles of clarithromycin were best described by a Weibull absorption model that resulted in a marked improvement in the objective function value (OFV; change in OFV [ΔOFV], −86) compared with that achieved with the first-order absorption model. A plot of the mean plasma concentrations of clarithromycin versus time after administration of the first dose is shown in Fig. 1. Figure 1 shows how the Weibull model best described the absorption phase in comparison with how a standard first-order absorption model described the absorption phase.
FIG. 1.
Geometric mean plasma concentration-time profile of clarithromycin after administration of the first dose: Weibull versus first-order absorption by use of the linear elimination pathway.
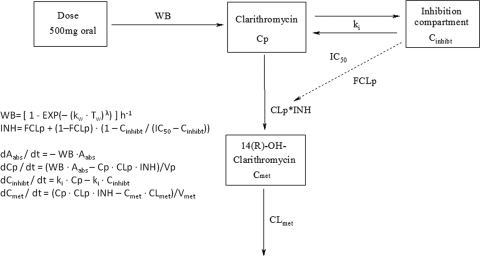
On the basis of the administration of multiple doses of 500 mg, CLp was found to decrease with time in a nonlinear fashion, as shown by noncompartmental methods (3). As expected, a linear two-compartment model could not describe this nonlinearity. Addition of a nonlinear elimination pathway with Michaelis-Menten kinetics considerably improved the model fit (ΔOFV, −252); however, the overall nonlinearity was still not properly explained, as this model was not able to capture the maximum plasma clarithromycin concentrations. Finally, the inhibitory effect of clarithromycin on its CLp was modeled mechanistically by addition of a hypothetical inhibition compartment. The concentration of the parent drug was used to derive the extent of (reversible) inhibition in this compartment. Here, CLp was split into two components. The first component was inhibited by the hypothetical clarithromycin concentration in the inhibition compartment (Cinhibt), whereas the second component was not affected by Cinhibt. The fraction of CLp that was not inhibited by Cinhibt was described as the fraction of the clearance not subject to inhibition, and its estimate was allowed to range from zero (i.e., 100% inhibition of clearance) to unity (i.e., no inhibition of clearance) (27). A transfer rate constant between the parent and the inhibition compartments (ki), in addition to the concentration in this compartment that yielded 50% inhibition of CLp, was included to explain the inhibition of clearance over time. This model is outlined in Fig. 2. Inclusion of the inhibition compartment side by side with the drug model adequately described the nonlinear time-dependent autoinhibition pharmacokinetics of clarithromycin. This model led to a significant improvement in the objective function compared to that achieved with the Michaelis-Menten elimination model (ΔOFV, −752).
FIG. 2.
Outline and differential equations for the first model. WB, Weibull absorption function from the gastrointestinal tract; Aabs, amount of drug in the absorption compartment; t, time; kw, absorption rate constant; Tw, time after administration of the previous dose; λ, shape parameter of the Weibull function; Vp and Vmet, apparent volumes of distribution of clarithromycin and its hydroxy metabolite, respectively; Cp and Cmet, plasma concentrations of clarithromycin and its hydroxy metabolite, respectively; ki, transfer rate constant between parent and inhibition compartments. CLp is inhibited on the basis of Cinhibt, and IC50 is the concentration in the inhibition compartment yielding 50% of maximum inhibition of clearance of clarithromycin. INH, overall inhibition parameter; FCLp, fraction of the clearance not subject to inhibition; Cinhibt, concentration of clarithromycin effective in the inhibition compartment; CLmet, apparent total clearance of 14(R)-hydroxy-clarithromycin.
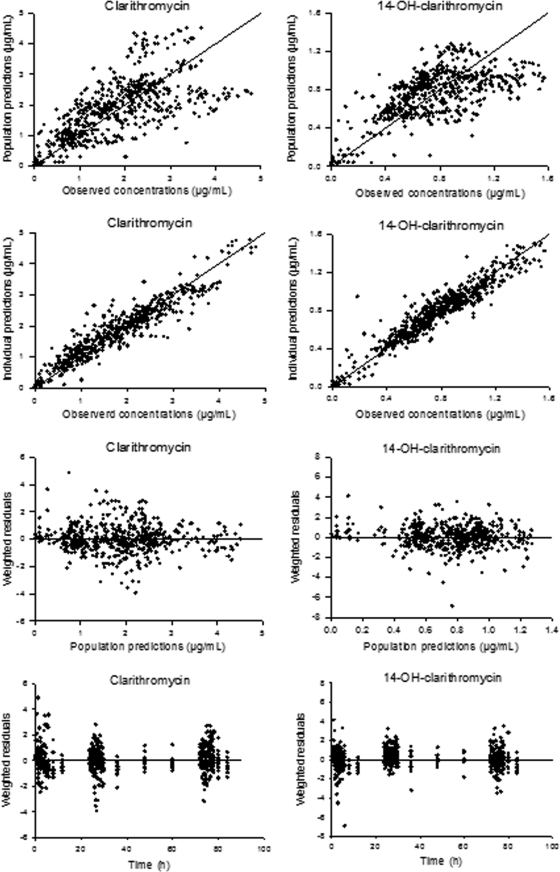
In the final model and for the available data, four between-subject variability terms were identified. In addition, residual additive error models resulted in the best model convergence compared with that achieved with the proportional and the combined-error models. The pharmacokinetic parameter estimates of the final model are shown in Table 1. Standard goodness-of-fit plots of the final model of clarithromycin and its 14-(R)-hydroxy metabolite are given in Fig. 3. These plots suggest that the autoinhibition model selected adequately described the plasma concentration-time profiles of both clarithromycin and its 14-(R)-hydroxy metabolite simultaneously.
TABLE 1.
Estimates of population pharmacokinetic parameters of clarithromycin and 14(R)-hydroxy-clarithromycin
| Parameter (abbreviation, units) | Point estimate | 95% confidence interval | % Relative standard error of estimated variance |
|---|---|---|---|
| Weibull absorption rate constant (kw, h−1) | 0.56 | 0.42-0.69 | |
| Weibull shape parameter (λ) | 2.23 | 1.67-2.77 | |
| Apparent vol of distribution of clarithromycin (Vp, liters) | 172 | 145-198 | |
| Apparent total clearance of clarithromycin (CLp, liters/h) | 60 | 40-80 | |
| Noninhibited fraction of clarithromycin clearance (FCLp) | 0.10 | 0.02-0.17 | |
| Transfer rate constant into and from inhibition compartment (ki, h−1) | 2.01 | 0.09-3.93 | |
| Concn in inhibition compartment yielding 50% inhibition of maximum clearance (IC50, μg/ml) | 0.77 | 0.23-1.28 | |
| Apparent total clearance of 14-OH-clarithromycin (CLmet, liters/h) | 50.2 | 42.3-58.1 | |
| Apparent vol of distribution of 14-OH-clarithromycin (Vmet, liters) | 34 | 12-56 | |
| Between-subject variability (%) in: | |||
| kw | 45.3 | 7.93 | |
| Vp | 25.3 | 2.83 | |
| CLp | 17.4 | 1.15 | |
| CLmet | 27.9 | 2.11 | |
| Residual variability | |||
| Additive error of clarithromycin (μg/ml) | 0.12 | 2.40 | |
| Additive error of 14-OH-clarithromycin (μg/ml) | 0.01 | 0.18 |
FIG. 3.
Goodness-of-fit plots for the final model. Unity and zero lines for clarithromycin and for its hydroxyl metabolite are shown in the left and right panels, respectively.
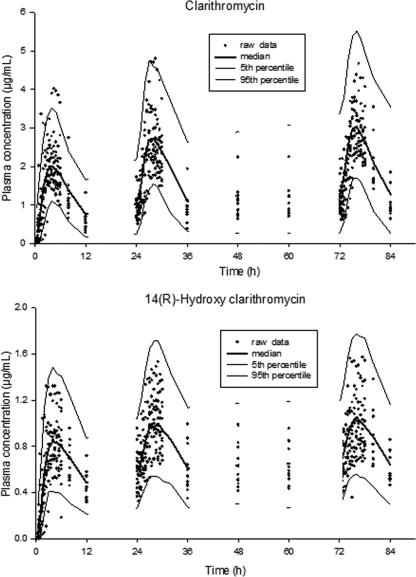
The visual predictive check of clarithromycin and its metabolite indicated that the final model could predict the observations, including the maximum concentrations in plasma (Fig. 4). The simulated 90% prediction interval closely reflected the observed plasma concentrations of clarithromycin and 14-(R)-hydroxy-clarithromycin.
FIG. 4.
Visual predictive check of the final model for original plasma clarithromycin concentrations and hydroxy-clarithromycin after the administration of multiple doses.
The concentrations of clarithromycin in the theoretical inhibition compartment as well as the extent of inhibition over time are shown in Fig. 5. The plot suggests that inhibition started rapidly and that the 500-mg b.i.d. dose inhibited enzyme activity by approximately 50% to 70% of the initial values during steady state.
FIG. 5.
Simulated clarithromycin concentration in the inhibition compartment (A) for the 500-mg b.i.d. dose and its autoinhibition effect with time (B). The value of the overall inhibition parameter is 100% if there is no inhibition, whereas maximal potential inhibition at high clarithromycin concentrations in the effect compartment would yield a value of 10% and would represent the noninhibitable fraction of CLp.
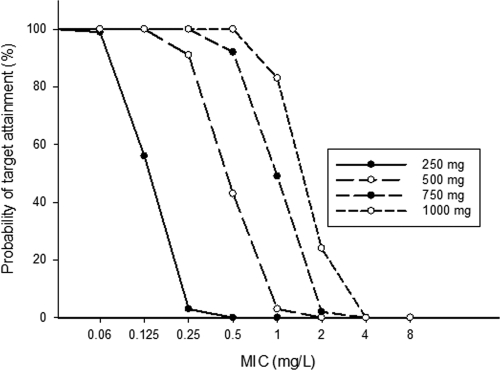
On the basis of the pharmacokinetic parameter estimates of the final model, the simulated medians for four dose levels, i.e., 250, 500, 750, and 1,000 mg b.i.d. (Fig. 6), suggested that the extent of autoinhibition and the emerging changes in pharmacokinetics with time were much more pronounced for the higher doses. For the 500-mg b.i.d. standard dose, no further increase in the level of exposure occurred after the second day of treatment, because it is expected to take several days for a 1,000-mg b.i.d. dose to reach steady state. The AUCs at steady state for the 1,000-mg b.i.d. dose would exceed those for the 500-mg b.i.d. dose by 3.6-fold (Table 2). For the parent drug at steady state, the probabilities that the target concentrations for pathogens for which the MIC is 0.5 mg/liter would be achieved are 0%, 43%, 94%, and 100% for the 250-, 500-, 750-, and 1,000-mg doses, respectively (Table 2 and Fig. 7).
FIG. 6.
Simulated median plasma concentrations of clarithromycin after the administration of 12 doses of clarithromycin b.i.d. at 250, 500, 750, and 1,000 mg.
TABLE 2.
Simulation of main pharmacokinetic-pharmacodynamic parameters of clarithromycin and 14-(R)-hydroxy-clarithomycin after administration of multiple ascending doses by use of population model estimatesa
| Compound | Dose (mg) | fAUCinitial, 0-24 (μg·h/ml) | fAUCss, 24 (μg·h/ml) | Probability (%) of target attainment (%) at steady state at MIC (mg/liter) ofb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | ||||
| Clarithromycin | 250 | 4.64 (1.06-9.20)c | 5.1 (1.19-10.18) | 100 | 99 | 56 | 3 | 0 | 0 | 0 | 0 |
| 500 | 11.11 (5.10-20.0) | 15.10 (6.9-31.9) | 100 | 100 | 100 | 91 | 43 | 3 | 0 | 0 | |
| 750 | 19.74 (10.9-34.7) | 33.4 (17.4-62) | 100 | 100 | 100 | 100 | 92 | 49 | 2 | 0 | |
| 1,000 | 28.71 (18.2-51.2) | 54.3 (30.4-95.0) | 100 | 100 | 100 | 100 | 100 | 83 | 24 | 0 | |
| 14-OH-Clarithromycin | 250 | 2.83 (1.16-5.07) | 3.17 (1.29-5.57) | NAd | NA | NA | NA | NA | NA | NA | NA |
| 500 | 5.0 (2.45-8.39) | 6.29 (3.29-10.25) | NA | NA | NA | NA | NA | NA | NA | NA | |
| 750 | 6.81 (3.75-11.14) | 9.42 (5.34-15.10) | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1,000 | 8.29 (4.46-13.63) | 12.48 (7.24-19.19) | NA | NA | NA | NA | NA | NA | NA | NA | |
Values were calculated on the basis of oral dosing b.i.d. and represent medians and 90% nonparametric prediction intervals. fAUCinitial, 0-24 and fAUCss, 24, fAUCs over 24 h on the first day and at steady state at 24 h, respectively. The fAUC was calculated by correcting the total AUC for the free fraction (f), which was 0.3 if a nonsignificant change in the level of protein binding was assumed (23, 33).
It is assumed that a fAUC24 at steady state for plasma/MIC ratio target of at least 35 is desirable in humans for pathogen eradication (32).
Values in parentheses are 90% confidence intervals.
NA, not applicable.
FIG. 7.
Probability of target attainment for free clarithromycin (i.e., fAUC24/MIC, >35) at steady state for various MICs upon b.i.d. administration of the respective clarithromycin doses.
DISCUSSION
This study proposes a population pharmacokinetic model in which the nonlinearity in the clarithromycin pharmacokinetic profile was quantitatively described in terms of an inhibitable fraction of the total clearance. This was modeled by addition of an inhibition compartment to the model, in which the change in CLp over time in the drug compartment is dependent on the concentration in the inhibition compartment. This study supports the idea of explaining the autoinhibition effect of a drug on its metabolizing enzyme by inclusion of the inhibition compartment side by side with the drug compartment. This model was successively used to describe the inhibitory effect of linezolid on its own metabolizing enzyme and the nonlinear increase in the extent of bioavailability, which leads to the appearance of side effects with time (27). The new features of our model were the incorporation of a Weibull function input, the allometric scaling approach for the covariate body weight on clearance and the volume of distribution, the ability to describe parent and metabolite plasma concentration profiles, as well as the autoinhibition process of elimination of the parent compound over time.
A single Weibull function successfully described the clarithromycin absorption process. The adequacy of the Weibull function emerges in its ability to reflect the change in the absorption process from linearity with time, probably as a consequence of intestinal CYP inactivation. Thus, an adequate empirical description of the absorption phase greatly contributed to the successful modeling of nonlinear elimination.
In the final model, the distribution of clarithromycin throughout the body was assumed to follow linear kinetics and a one-compartment model was sufficient. This is in accordance with the findings of a previous study (5) and with data on pharmacokinetics in tissue obtained by microdialysis that showed that the elimination process rather than the distribution process governs the concentration-versus-time profiles (33). Clarithromycin was estimated to enter the inhibition compartment rapidly and had an average half-life of approximately 0.35 h (Table 1). A component of CLp was subjected to inhibition by its concentration in the inhibition compartment. The inhibitable component expresses the function of the activity of clarithromycin-metabolizing enzymes, mainly CYP3A4, which is assumed to be inhibited with time by clarithromycin itself. This inhibition was quantified by incorporating an inhibition compartment into the drug model. The fraction of clarithromycin clearance that was inhibited over time was a function of the clarithromycin concentration in this compartment. As shown by the individual prediction-versus-observation plot for clarithromycin (Fig. 3), the observed peak concentrations were slightly higher than the fitted concentrations. This might have been caused by the presence of a second disposition compartment. Attempts to estimate a model with two disposition compartments for clarithromycin, however, were unsuccessful.
The active metabolite of clarithromycin, 14-(R)-hydroxy-clarithromycin, may be formed in part in the enterocytes as a result of first-pass metabolism. This process could not be modeled separately here because the metabolite was not administered separately and data for clarithromycin after intravenous administration were not available for this study. Thus, the final model assumption was that all clarithromycin molecules are converted to the metabolite. The modeling of additional elimination pathways was not possible because the metabolite formation fraction was not known and the metabolite was not administered intravenously. This assumption should not affect the quality of the model fits or the predictive performance of the model. The modeling of the elimination process for the hydroxyl metabolite with a saturable kinetic model did not improve the model fit; however, circumstantial evidence for nonlinearity has been reported (5, 12), as this metabolite undergoes further biotransformation (12, 35).
For the final model, the apparent volume of distribution of clarithromycin was 172 liters (95% confidence interval, 145 to 198), which is in agreement with the previously reported range of 126 to 306 liters (5, 33). The CLp was 60 liters/h, and it could be inhibited to 10% of its original value (Table 1). This point estimate of clearance after oral administration is the basic clearance value and decreases over time with the subsequent doses until steady state is achieved. Hence, it should not be compared directly with the previously reported range for apparent total clearances at steady state of 19 to 105 liters/h obtained with similar regimens (5, 15, 33). This decrease in the CLp reflects the increasing inhibition of the metabolizing enzyme, CYP3A, during long-term clarithromycin intake, which is important for the optimization of dosage regimens when other CYP3A substrates are coadministered with clarithromycin. However, more complex inhibition models are probably required if other compounds that modify CYP3A4 activities or expression are present. Nevertheless, the model may still be considered, as long as no specific data for these substrates are available. Furthermore, an approximately 60% decrease in CYP3A activity was observed at steady state with a dose of 500 mg clarithromycin b.i.d. (Fig. 5B). Therefore, the concentrations of CYP3A substrates coadministered with clarithromycin may be more than doubled, which may reach clinical relevance (14). For higher doses, a further pronounced decrease in the level of CYP3A activity is to be expected (Fig. 6; Table 2).
The application of the fAUC/MIC concept for the current study assumes that the model adequately describes the pharmacokinetics of clarithromycin, including its metabolism, and that clarithromycin mediates the main part of the antimicrobial activity, while the metabolite makes only a minor contribution. The appropriateness of the former assumption was confirmed by the use of visual predictive checks. Given that the AUC of clarithromycin is approximately three times as high as that for its metabolite, the latter assumption seems reasonable (18, 20). The calculated fAUC24 values for plasma at steady state after the administration of different doses indicated that the usual adult dose of 500 mg b.i.d. is sufficient to produce effective antibacterial activity (fAUC/MIC > 35 [33]), as long as the desired MIC for the pathogen is approximately 0.25 mg/liter or lower (Table 2), and a probability for target attainment of 91% (Fig. 7). The model presented here suggests that increasing the dose twofold from 500 mg b.i.d. to 1,000 mg b.i.d. would increase the level of exposure to clarithromycin at steady state by almost fourfold, suggesting that infections with pathogens with MICs of up to 1 mg/liter could successfully be treated. However, further clinical studies are required to support this conclusion.
Because of both the rapid elimination and the rapid exchange with tissues, including the inhibition compartment defined here, in subjects without hepatic or renal failure, no further increase in exposure upon chronic administration of the standard 500-mg dose is expected after the second day of treatment (Fig. 6). Accordingly, both the therapeutic action and inhibitory effects on coadministered drugs should have reached the maximum on the second day. This provides some reassurance that no late augmentation of any drug interactions would occur, although depending on the drug coadministered, it may take longer than 2 days to subsequently reach its new steady state. Also, the hope that concentrations may subsequently be increased to support the continuation of an unsuccessful antimicrobial therapy beyond a few days of treatment does not seem to be justified for the 500-mg dose. In contrast, it may take several days until steady state is reached with the 1,000-mg b.i.d. dose (Fig. 6); thus, changes in the extent of inhibition and in bacterial susceptibility, e.g., due to the emergence of resistance, need to be taken into account during this period. The theoretical benefit of a large increase in plasma concentrations with an increase in the dose to obtain better clinical outcomes remains to be assessed in clinical trials. Because it will take some time for the autoinhibition of clearance to result in notably higher AUCs and clinical success probably depends on antimicrobial exposure during the first day(s) of therapy, the clinical benefit of autoinhibition might be less pronounced than that predicted by the steady-state AUCs. For the same reasons, it might be reasonable to consider the administration of a clarithromycin loading dose.
Thus, the semimechanistic population pharmacokinetic model developed in the present study has the advantage of the ability to describe both the concentration-time course of various clarithromycin concentrations, the inhibitory action of clarithromycin on CYP3A, and especially, the change in the level of CLp over time. The model does not incorporate all underlying mechanisms, such as clarithromycin first-pass metabolism and a separate time course for the inhibition of intestinal and hepatic CYP3A enzymes. However, the model was still useful for the assessment of a possible dose adjustment, as well as for the purpose of guiding further pharmacokinetic-pharmacodynamic considerations and model development activities.
In conclusion, this study identified and quantified a time-dependent decrease in the level of CLp in a nonlinear fashion, which was completed on the second day of treatment for the 500-mg b.i.d. dose. The achievement of steady state was predicted to take several days for a 1,000-mg b.i.d. dose. A semimechanistic population pharmacokinetic model for clarithromycin and its 14-(R)-hydroxy metabolite was developed and provides an adequate description of the time course of clearance inhibition with the regimen used. The seemingly atypical absorption process could be the result of saturable or inhibited first-pass metabolism and may contribute to the overall nonlinear profiles of clarithromycin. The model presented here serves as a useful tool for prediction of plasma clarithromycin concentrations and provides a rationale that may be used to improve its safety with regard to drug-drug interactions and its efficacy on the basis of pharmacokinetic-pharmacodynamic considerations. The applicability of the model in the presence of additional CYP3A ligands (inhibitors or substrates) and the potential clinical benefit of the pronounced increase in plasma concentrations with an increase in the clarithromycin dose remain to be assessed further in clinical trials.
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Anderson, B. J., A. D. McKee, and N. H. Holford. 1997. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin. Pharmacokinet. 33:313-327. [DOI] [PubMed] [Google Scholar]
- 2.Bruce, M. A., S. D. Hall, B. D. Haehner-Daniels, and J. C. Gorski. 2001. In vivo effect of clarithromycin on multiple cytochrome P450s. Drug Metab. Dispos 29:1023-1028. [PubMed] [Google Scholar]
- 3.Bulitta, J., S. Horkovics-Kovats, M. Borek, A. Skott, M. Illauer, M. Rodamer, M. Kinzig-Schippers, and F. Sörgel. 2003. Self-inhibition of clarithromycin's (CLA) metabolism in humans at steady-state, poster A-1625. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Bulitta, J. B., S. B. Duffull, M. Kinzig-Schippers, U. Holzgrabe, U. Stephan, G. L. Drusano, and F. Sorgel. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, S., D. S. Wilson, R. L. Deaton, A. V. Mackenthun, C. N. Eason, and J. H. Cavanaugh. 1993. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J. Clin. Pharmacol. 33:719-726. [DOI] [PubMed] [Google Scholar]
- 6.Chu, S. Y., R. Deaton, and J. Cavanaugh. 1992. Absolute bioavailability of clarithromycin after oral administration in humans. Antimicrob. Agents Chemother. 36:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, S. Y., G. R. Granneman, P. J. Pichotta, J. P. Decourt, J. Girault, and J. B. Fourtillan. 1993. Effect of moderate or severe hepatic impairment on clarithromycin pharmacokinetics. J. Clin. Pharmacol. 33:480-485. [DOI] [PubMed] [Google Scholar]
- 8.Chu, S. Y., L. T. Sennello, S. T. Bunnell, L. L. Varga, D. S. Wilson, and R. C. Sonders. 1992. Pharmacokinetics of clarithromycin, a new macrolide, after single ascending oral doses. Antimicrob. Agents Chemother. 36:2447-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, W. A., S. Kiem, and D. R. Andes. 2002. Free drug 24-hr AUC/MIC is the PK/PD target that correlates with in vivo efficacy of macrolides, azalides, ketolides and clindamycin, abstr. A-1264. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Davey, P. G. 1991. The pharmacokinetics of clarithromycin and its 14-OH metabolite. J. Hosp. Infect. 19(Suppl. A):29-37. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes, P. B., R. Bailer, R. Swanson, C. W. Hanson, E. McDonald, N. Ramer, D. Hardy, N. Shipkowitz, R. R. Bower, and E. Gade. 1986. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob. Agents Chemother. 30:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrero, J. L., B. A. Bopp, K. C. Marsh, S. C. Quigley, M. J. Johnson, D. J. Anderson, J. E. Lamm, K. G. Tolman, S. W. Sanders, J. H. Cavanaugh, and R. C. Sonders. 1990. Metabolism and disposition of clarithromycin in man. Drug Metab. Dispos 18:441-446. [PubMed] [Google Scholar]
- 13.Fraschini, F., F. Scaglione, and G. Demartini. 1993. Clarithromycin clinical pharmacokinetics. Clin. Pharmacokinet. 25:189-204. [DOI] [PubMed] [Google Scholar]
- 14.Fuhr, U. 2008. Improvement in the handling of drug-drug interactions. Eur. J. Clin. Pharmacol. 64:167-171. [DOI] [PubMed] [Google Scholar]
- 15.Gorski, J. C., D. R. Jones, B. D. Haehner-Daniels, M. A. Hamman, E. M. O'Mara, Jr., and S. D. Hall. 1998. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin. Pharmacol. Ther. 64:133-143. [DOI] [PubMed] [Google Scholar]
- 16.Hung, I. F., A. K. Wu, V. C. Cheng, B. S. Tang, K. W. To, C. K. Yeung, P. C. Woo, S. K. Lau, B. M. Cheung, and K. Y. Yuen. 2005. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective study. Clin. Infect. Dis. 41:291-300. [DOI] [PubMed] [Google Scholar]
- 17.Ishii, K., Y. Saito, S. Itai, M. Nemoto, K. Takayama, and T. Nagai. 1998. Comparative study of pharmacokinetic parameters between clarithromycin and erythromycin stearate in relation to their physicochemical properties. Drug Dev. Ind. Pharm. 24:129-137. [DOI] [PubMed] [Google Scholar]
- 18.Jones, R. N., M. E. Erwin, and M. S. Barrett. 1990. In vitro activity of clarithromycin (TE-031, A-67268) and 14OH-clarithromycin alone and in combination against Legionella species. Eur. J. Clin. Microbiol. Infect. Dis. 9:846-848. [DOI] [PubMed] [Google Scholar]
- 19.Langtry, H. D., and R. N. Brogden. 1997. Clarithromycin. A review of its efficacy in the treatment of respiratory tract infections in immunocompetent patients. Drugs 53:973-1004. [DOI] [PubMed] [Google Scholar]
- 20.Martin, S. J., C. G. Garvin, C. R. McBurney, and E. G. Sahloff. 2001. The activity of 14-hydroxy clarithromycin, alone and in combination with clarithromycin, against penicillin- and erythromycin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:581-587. [DOI] [PubMed] [Google Scholar]
- 21.Mayhew, B. S., D. R. Jones, and S. D. Hall. 2000. An in vitro model for predicting in vivo inhibition of cytochrome P450 3A4 by metabolic intermediate complex formation. Drug Metab. Dispos. 28:1031-1037. [PubMed] [Google Scholar]
- 22.Noreddin, A. M., D. Roberts, K. Nichol, A. Wierzbowski, D. J. Hoban, and G. G. Zhanel. 2002. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob. Agents Chemother. 46:4029-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters, D. H., and S. P. Clissold. 1992. Clarithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic potential. Drugs 44:117-164. [DOI] [PubMed] [Google Scholar]
- 24.Petricoul, O., V. Cosson, E. Fuseau, and M. Marchand. 2007. Population models for drug absorption and enterohepatic recycling, p. 345-382. In E. I. Ette and P. J. Williams (ed.), Pharmacometrics: the science of quantitative pharmacology. John Wiley & Sons, Inc., Hoboken, NJ.
- 25.Pinto, A. G., J. Horlander, N. Chalasani, M. Hamman, A. Asghar, D. Kolwankar, and S. D. Hall. 2005. Diltiazem inhibits human intestinal cytochrome P450 3A (CYP3A) activity in vivo without altering the expression of intestinal mRNA or protein. Br. J. Clin. Pharmacol. 59:440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto, A. G., Y. H. Wang, N. Chalasani, T. Skaar, D. Kolwankar, J. C. Gorski, S. Liangpunsakul, M. A. Hamman, M. Arefayene, and S. D. Hall. 2005. Inhibition of human intestinal wall metabolism by macrolide antibiotics: effect of clarithromycin on cytochrome P450 3A4/5 activity and expression. Clin. Pharmacol. Ther. 77:178-188. [DOI] [PubMed] [Google Scholar]
- 27.Plock, N., C. Buerger, C. Joukhadar, S. Kljucar, and C. Kloft. 2007. Does linezolid inhibit its own metabolism? Population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab. Dispos. 35:1816-1823. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues, A. D., E. M. Roberts, D. J. Mulford, Y. Yao, and D. Ouellet. 1997. Oxidative metabolism of clarithromycin in the presence of human liver microsomes. Major role for the cytochrome P4503A (CYP3A) subfamily. Drug Metab. Dispos. 25:623-630. [PubMed] [Google Scholar]
- 29.Rodvold, K. A. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385-398. [DOI] [PubMed] [Google Scholar]
- 30.Sturgill, M. G., and R. P. Rapp. 1992. Clarithromycin: review of a new macrolide antibiotic with improved microbiologic spectrum and favorable pharmacokinetic and adverse effect profiles. Ann. Pharmacother. 26:1099-1108. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, A., I. Iida, M. Hirota, M. Akimoto, S. Higuchi, T. Suwa, M. Tani, T. Ishizaki, and K. Chiba. 2003. CYP isoforms involved in the metabolism of clarithromycin in vitro: comparison between the identification from disappearance rate and that from formation rate of metabolites. Drug Metab. Pharmacokinet. 18:104-113. [DOI] [PubMed] [Google Scholar]
- 32.Tessier, P. R., M. K. Kim, W. Zhou, D. Xuan, C. Li, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traunmuller, F., M. Zeitlinger, P. Zeleny, M. Muller, and C. Joukhadar. 2007. Pharmacokinetics of single- and multiple-dose oral clarithromycin in soft tissues determined by microdialysis. Antimicrob. Agents Chemother. 51:3185-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ushiama, H., H. Echizen, S. Nachi, and A. Ohnishi. 2002. Dose-dependent inhibition of CYP3A activity by clarithromycin during Helicobacter pylori eradication therapy assessed by changes in plasma lansoprazole levels and partial cortisol clearance to 6beta-hydroxycortisol. Clin. Pharmacol. Ther. 72:33-43. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, F., S. Harada, T. Mitsuyama, Y. Harada, Y. Kitahara, M. Yoshida, and Y. Nakanishi. 2004. Concentration of clarithromycin and 14-R-hydroxy-clarithromycin in plasma of patients with Mycobacterium avium complex infection, before and after the addition of rifampicin. Jpn. J. Antibiot. 57:124-133. [PubMed] [Google Scholar]
- 36.Zhou, S., S. Y. Chan, B. C. Goh, E. Chan, W. Duan, M. Huang, and H. L. McLeod. 2005. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 44:279-304. [DOI] [PubMed] [Google Scholar]