Abstract
Raltegravir is a human immunodeficiency virus type 1 integrase strand transfer inhibitor that is metabolized by glucuronidation via UGT1A1 and may be affected by inducers of UGT1A1, such as rifampin (rifampicin). Two pharmacokinetic studies were performed in healthy subjects: study 1 examined the effect of administration of 600-mg rifampin once daily on the pharmacokinetics of a single dose of 400-mg raltegravir, and study 2 examined the effect of 600-mg rifampin once daily on the pharmacokinetics of 800-mg raltegravir twice daily compared to 400-mg raltegravir twice daily without rifampin. Raltegravir coadministered with rifampin resulted in lower plasma raltegravir concentrations: in study 1, the geometric mean ratios (GMRs) and 90% confidence intervals (90% CIs) for the plasma raltegravir concentration determined 12 h postdose (C12), area under the concentration-time curve from 0 h to ∞ (AUC0-∞), and maximum concentration of drug in plasma (Cmax) (400-mg raltegravir plus rifampin/400-mg raltegravir) were 0.39 (0.30, 0.51), 0.60 (0.39, 0.91), and 0.62 (0.37, 1.04), respectively. In study 2, the GMRs and 90% CIs for raltegravir C12, AUC0-12, and Cmax (800-mg raltegravir plus rifampin/400-mg raltegravir) were 0.47 (0.36, 0.61), 1.27 (0.94, 1.71), and 1.62 (1.12, 2.33), respectively. Doubling the raltegravir dose to 800 mg when coadministered with rifampin therefore compensates for the effect of rifampin on raltegravir exposure (AUC0-12) but does not overcome the effect of rifampin on raltegravir trough concentrations (C12). Coadministration of rifampin and raltegravir is not contraindicated; however, caution should be used, since raltegravir trough concentrations in the presence of rifampin are likely to be at the lower limit of clinical experience.
Raltegravir (Isentress; formerly MK-0518) is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor, a member of a new class of antiretroviral agent. Integrase is one of three enzymes involved in the process of viral replication. It catalyzes the stepwise process of integration of the HIV-1 DNA into the genome of the host cell (1, 7, 19). Raltegravir has potent in vitro activity against HIV-1 (19; Isentress [raltegravir] prescribing information; Merck and Co., Inc.) and demonstrated robust antiviral activity in the HIV-1-infected patient population (6, 9, 15, 20). In addition, raltegravir has generally been well tolerated with a very favorable safety profile (6, 9, 15, 20).
The principal route of elimination of raltegravir is metabolism via UGT1A1 with a small component of elimination via renal excretion (13). It is not metabolized by the cytochrome P-450 oxidation system and is neither a clinically meaningful inducer nor an inhibitor of enzymes involved in drug metabolism. Consequently, raltegravir has shown relatively minimal interactions when coadministered with other antiviral agents (10; Isentress [raltegravir] prescribing information; Merck and Co., Inc.). The pharmacokinetic (PK) profile of raltegravir may, however, be affected by compounds that inhibit or induce UGT1A1, such as rifampin (rifampicin).
Anti-HIV agents, including raltegravir, are generally given in combination, both with other HIV drugs and other supportive medications commonly used in the HIV patient population. Rifampin is used in the treatment of tuberculosis infection, which is common in this population. Rifampin is known to induce a number of cytochrome P-450 enzymes, and administration of rifampin with drugs that undergo biotransformation through these metabolic pathways accelerates elimination of coadministered drugs. Rifampin increases clearance of a number of antiretroviral agents via this mechanism (2, 3). Furthermore, rifampin also induces phase II enzymes, such as UDP-glucuronosyl transferase, which has been described to act in vitro and in vivo (5, 8, 21), and therefore has potential to affect the pharmacokinetics of raltegravir.
Two studies were performed to characterize the effect of rifampin on the pharmacokinetics of raltegravir. Study 1 evaluated the effect of steady-state rifampin on a single 400-mg dose of raltegravir. Study 2 compared the pharmacokinetics of 800-mg twice-daily raltegravir plus rifampin to that of 400 mg twice-daily raltegravir.
(Portions of the data for study 1 were presented at the 8th International Congress on Drug Therapy in HIV Infection, Glasgow, Scotland, 2006 [4], and portions of the data for study 2 were presented at the 48th Interscience Conference On Antimicrobial Agents and Chemotherapy and the Infectious Diseases Society of America 46th Annual Meeting [a joint meeting], Washington, DC, 25 to 28 October 2008 [11].)
MATERIALS AND METHODS
Subjects.
Healthy men and women between 18 and 55 years of age were eligible for enrollment. Subjects were eligible if they were within 40% of ideal body weight for study 1 and with a body mass index of <33 kg/m2 for study 2. HIV-infected individuals, those bearing a clinically significant medical condition, smokers, and those with a known history of alcohol and/or drug abuse were excluded from the study. Exclusion criteria also included recent participation in an investigational drug study and inability to refrain from the use of prescription and nonprescription medications. Subjects agreed to participate in the studies by giving written informed consent prior to study commencement. The study protocols were approved by the institutional review boards of the study centers. The studies were conducted in accordance with the guidelines on good clinical practice and with the ethical standards for human experimentation established by the Declaration of Helsinki.
Study design.
Study 1 was an open-label, two-period, fixed-sequence study. In period 1, all subjects were administered a single oral dose of 400 mg of raltegravir in the fasted state (subjects fasted for 8 h prior to dosing and remained fasting for 4 h after dosing), followed by at least a 4-day washout period prior to the start of period 2. In period 2, the same subjects were administered 600 mg rifampin once daily for 15 days. On day 14 of period 2, all subjects were administered the daily dose of rifampin in combination with a single oral dose of 400 mg raltegravir in the fasted state. All other doses of rifampin in period 2 were administered either 1 h before or 2 h after a meal.
Study 2 was an open-label, two-period, fixed-sequence study. In period 1, subjects received 400 mg of raltegravir every 12 h for 4 days. Raltegravir was administered without regard to food except for the evening dose on day 3 and both doses on day 4, when subjects consumed a light meal 2 h prior to receiving raltegravir. In period 2, the same subjects received 800 mg of raltegravir every 12 h and 600 mg of rifampin once daily for 14 days; the day 14 raltegravir afternoon dose was not administered. Rifampin was administered either 1 h before or 2 h after a meal. Raltegravir was administered without regard to food except for the evening dose on day 13 and the morning dose on day 14 in period 2, when subjects consumed a light meal 2 h prior to receiving raltegravir.
The safety and tolerability of raltegravir and rifampin were assessed in both studies by clinical evaluation of vital signs, physical examinations, electrocardiograms, and laboratory safety evaluations. Adverse experiences were monitored throughout the study and were evaluated with respect to intensity, duration, seriousness, outcome, and relationship to study drug.
Bioanalytical and PK analyses.
Serial blood samples were collected to determine plasma concentrations of raltegravir. In study 1, samples were collected on day 1 of period 1 and day 14 of period 2 for raltegravir analysis at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 32, and 48 h postdose. In study 2, samples were collected following the morning raltegravir dose on day 4 of period 1 and on day 14 of period 2 at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h postdose.
The analytical method for the determination of raltegravir concentrations in human plasma involves isolation, via 96-well liquid-liquid extraction, of the analyte and internal standard from plasma, followed by reverse-phase high-pressure liquid chromatography tandem mass spectrometry analysis as previously described (18). The lower limit of quantitation for the plasma assay is 2 ng/ml (4.5 nM), and the linear calibration range is 2 to 1,000 ng/ml.
The concentrations for raltegravir in plasma were converted into molar units (nanomolar), and actual sampling times, converted to elapsed time relative to dosing times, were used to estimate PK parameters using WinNonlin (Pharsight Corporation, Mountain View, CA) for each treatment in each subject. Values below the plasma raltegravir assay limit of quantitation (BLQ), i.e., <2 ng/ml (<4.5 nM) were replaced according to the following rules: predose BLQ value = 0; first BLQ value in the terminal phase = (1/2)(lower limit of quantitation) = 1 ng/ml = 2.3 nM; second and subsequent BLQ values in the terminal phase = 0. For study 1, the distribution and elimination phases of each plasma raltegravir concentration profile were fit to a biexponential equation (concentration = Ae−αt + Be−βt, where A is the zero time intercept for α phase or the distribution phase, B is the zero time intercept for β phase or the elimination phase, and t is time) using the Gauss-Newton (Levenberg and Hartley) minimization method and a weighting of 1/(predicted concentration)2. The onset of the α phase was determined by inspection. The half-life (t1/2) for each phase was calculated as the quotient of ln(2) and α or β. The area under the concentration-time curve from 0 h to the last dose (AUC0-last) was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. The area under the concentration-time curve from 0 h to ∞ (AUC0-∞) was estimated as the sum of AUC0-last and the extrapolated area given by the quotient of the last measured concentration and β. For study 2, AUC0-12 was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. For both studies, the maximum concentration of drug in plasma (Cmax) and time to maximum concentration of drug in plasma (Tmax) were obtained by inspection of the plasma raltegravir concentration data. Nominal plasma raltegravir sampling times were used to determine Tmax provided that the actual observed time of Tmax did not differ meaningfully from the nominal plasma sampling time. Plasma raltegravir concentration determined 12 h postdose (C12) values were assessed from the plasma raltegravir concentrations determined for the nominal sampling times at 12 h postdose.
Statistical analyses.
The effect of coadministration of rifampin on the PK profile of raltegravir (e.g., C12, AUC, Cmax) was evaluated using a linear mixed-effect model appropriate for a two-period, fixed-sequence design with fixed effect term of treatment and a random subject effect. Two-sided 90% confidence intervals (90% CIs) for the true mean difference (raltegravir plus rifampin versus raltegravir alone) in raltegravir C12 on the log scale were calculated on the basis of the above model. These confidence limits were then exponentiated to obtain a CI for the true geometric mean ratio (GMR) for raltegravir C12 (raltegravir plus rifampin/raltegravir alone). Cmax and AUC of raltegravir were analyzed in a similar fashion as C12, and GMR were reported for these two parameters. The other parameters were analyzed on the raw scale. For study 1, the harmonic means were reported for the apparent half-life, with Hodges-Lehman estimates and the corresponding 90% CIs for the median differences (raltegravir and rifampin − raltegravir) also provided.
RESULTS
Demographics.
Ten healthy male and female subjects were enrolled into study 1 with a mean (range) age and weight of 38.4 years (24 to 52 years) and 79.1 kg (64.1 to 101.0 kg), respectively. Of the 10 subjects enrolled, 7 were Caucasian (70%), 2 were Hispanic (20%), and 1 was African-American (10%). Seven subjects were male (70%). Nine subjects completed the study. One subject did not complete the study due to withdrawal of consent. Pharmacokinetic data for this subject were incomplete and were not included in the final PK analysis. All subjects were included in the safety analysis.
Eighteen healthy male and female subjects were enrolled into study 2 with a mean (range) age and weight of 35.2 years (18 to 54 years) and 76.8 kg (57.5 to 116.0 kg), respectively. Of the 18 subjects enrolled, 13 were Caucasian (72%), 4 were African-American (22%), and 1 was Polynesian (6%). Twelve subjects were male (67%). Four patients did not complete the study due to adverse effects (n = 1) or withdrawal of consent (n = 3). Seventeen subjects had PK data and were included in the final PK analysis. All subjects were included in the safety analysis.
Pharmacokinetics. (i) Study 1.
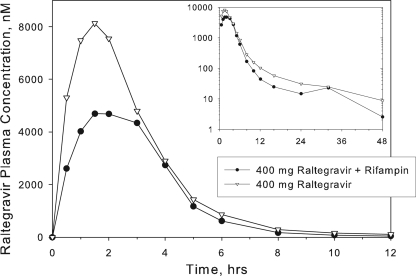
Table 1 summarizes the raltegravir PK parameters observed in study 1, and Fig. 1 shows the corresponding plasma raltegravir concentration profiles with or without multiple doses of rifampin. Rifampin reduced C12 of a single 400-mg dose of raltegravir by approximately 61%, with smaller effects on raltegravir AUC0-∞ (reduced by approximately 40%) and Cmax (reduced by approximately 38%).
TABLE 1.
Study 1: comparison of plasma raltegravir pharmacokinetics following administration of single oral doses of 400-mg raltegravir with or without rifampina
| Pharmacokinetic parameter | Geometric mean
|
GMR (raltegravir + rifampin/raltegravir) (90% CI) | P valueb | |
|---|---|---|---|---|
| Raltegravir + rifampin | Raltegravir alone | |||
| C12 (nM)c | 36.3 | 92.1 | 0.39 (0.30, 0.51) | 0.0002 |
| AUC0-∞ (μM·h)c | 16.51 | 27.57 | 0.60 (0.39, 0.91) | 0.0514 |
| Cmax (μM)c | 5.34 | 8.61 | 0.62 (0.37, 1.04) | 0.1234 |
| Tmax (h)d | 3.0 | 1.5 | ||
| t1/2α (h)e | 0.93 | 1.07 | −0.15 (−0.23, −0.04)f | |
| t1/2β (h)e | 9.6 | 8.5 | −0.1 (−4.5, 4.9)f | |
Single oral doses of 400-mg raltegravir with or without administration of 600-mg rifampin once daily were given to nine healthy male and female subjects who enrolled in and completed study 1.
Corresponding to a test whether the true GMR is equal to 1.0.
Geometric mean computed from least-squares estimate from an analysis of variance performed on the natural-log-transformed values and then back-transformed to the raw scale.
Median on raw scale reported for Tmax.
t1/2α, half-life at the α or distribution phase; t1/2β, half-life at the β or elimination phase. Harmonic mean on raw scale reported for t1/2.
Hodges-Lehman estimate of median treatment difference on raw scale with corresponding 90% CIs for true median treatment difference reported.
FIG. 1.
Study 1: arithmetic mean raltegravir concentrations in plasma following a single oral dose of raltegravir with or without multiple oral doses of rifampin once daily to healthy male and female subjects (note the semilog scale on the inset).
(ii) Study 2.
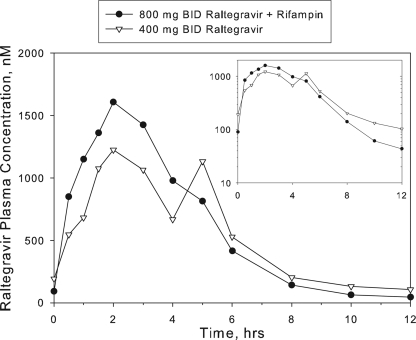
Comparison of plasma raltegravir PK parameter values and summary statistics for 800-mg raltegravir with rifampin versus 400-mg raltegravir alone are provided in Table 2. Figure 2 shows the corresponding concentration profiles of raltegravir in plasma. Coadministration of 800-mg twice-daily raltegravir plus 600-mg once-daily rifampin resulted in an approximately 53% decrease in raltegravir C12 compared to administration of 400-mg twice-daily raltegravir alone. Raltegravir AUC0-12 and Cmax were somewhat higher after administration of 800-mg raltegravir with rifampin relative to 400-mg raltegravir: AUC was increased by an average of approximately 27% and Cmax by approximately 62%. Median Tmax values were slightly shorter after administration of 800-mg raltegravir with rifampin with a median of 1.75 h compared to 3.00 h for 400-mg raltegravir alone.
TABLE 2.
Study 2: comparison of plasma raltegravir pharmacokinetics following administration of multiple oral doses of raltegravir with or without rifampina
| Pharmacokinetic parameter | Geometric mean
|
GMR (800-mg raltegravir + rifampin/400-mg raltegravir) (90% CI) | P valueb | |
|---|---|---|---|---|
| 800-mg raltegravir + rifampin | 400-mg raltegravir alone | |||
| C12 (nM)c | 43.51 | 92.47 | 0.47 (0.36, 0.61) | <0.0001 |
| AUC0-12 (μM·h)c | 5.85 | 4.61 | 1.27 (0.94, 1.71) | 0.1862 |
| Cmax (μM)c | 1.95 | 1.21 | 1.62 (1.12, 2.33) | 0.0329 |
| Tmax (h)d | 1.75 | 3.00 | ||
Multiple oral doses of 400-mg raltegravir twice daily for 4 days and multiple oral doses of 800-mg raltegravir twice daily plus 600-mg rifampin once daily for 14 days were given to healthy male and female subjects. Fourteen subjects received 800-mg raltegravir plus rifampin. Seventeen subjects received 400-mg raltegravir alone.
Corresponding to a test whether the true GMR is equal to 1.0.
Geometric mean computed from least-squares estimate from an analysis of variance performed on the natural-log-transformed values and then back-transformed to the raw scale.
Median on raw scale reported for Tmax.
FIG. 2.
Study 2: arithmetic mean raltegravir concentrations in plasma following administration of multiple oral doses of 400-mg raltegravir twice daily (BID) for 4 days and multiple oral doses of 800-mg raltegravir twice daily plus 600-mg rifampin once daily for 14 days to healthy male and female subjects (note the semilog scale on the inset).
Safety and tolerability.
Coadministration of rifampin and raltegravir was generally well tolerated in both studies. In study 1, no serious clinical or serious laboratory adverse experiences were reported, and no subject did not complete the study because of an adverse experience. Ten subjects reported a total of 19 nonserious clinical adverse experiences, 15 of which were determined by the investigator to be possibly drug related. The most commonly reported drug-related clinical adverse experiences (reported by two or more subjects) were urine discoloration, headache, and upper respiratory tract infection.
In study 2, no serious clinical or serious laboratory adverse experience was reported. One subject did not complete the study due to an adverse experience considered not drug related by the investigator. Eighteen subjects reported a total of 37 clinical adverse experiences, 9 of which were considered by the investigator as drug related. The most commonly reported drug-related clinical adverse experiences (reported by two or more subjects) were urine discoloration, headache, and flatulence.
In both studies, over half of the drug-related adverse experiences were discolored urine (a commonly seen adverse experience with rifampin administration) which was seen only in the study periods with rifampin administration. For both studies, all clinical adverse experiences reported were generally transient in nature and rated mild to moderate in intensity, and there were no laboratory adverse experiences reported.
DISCUSSION
Since its marketing approval, raltegravir has been of significant benefit to the HIV-1-infected patient population. As with other anti-HIV agents, raltegravir will be given in combination with other antiretrovirals as well as other supportive medications. As such, characterization of drug interactions with raltegravir is important. Rifampin coadministration is anticipated in the target population. Rifampin is a known potent broad inducer of drug-metabolizing enzymes including UGT1A1, the enzyme primarily responsible for the metabolism of raltegravir. Characterization of the effect of rifampin on the pharmacokinetics of raltegravir is important both to guide coadministration of the two drugs and to determine the potential for raltegravir pharmacokinetics to be altered by other potent and less potent inducers of UGT1A1.
For this new class of antiretroviral agents, there are currently insufficient clinical data to define the target pharmacokinetic parameter associated with efficacy; however, for other classes of retroviral agents, there is a reasonable, but imperfect, association of efficacy with doses that achieve drug trough concentrations that exceed the protein-adjusted 95% inhibitory concentration in the HIV spread assay. The results from the PK/pharmacodynamic analyses of viral response measures did not identify any clinically meaningful correlations with any available measure of plasma raltegravir concentrations in patients receiving raltegravir in combination with additional active antiretroviral agents (22). The PK/pharmacodynamic analyses of the short-term viral response during 10-day monotherapy identified a potential association of day 10 HIV RNA and slope of decline in HIV RNA with drug trough concentration (17). Therefore, the primary PK parameter of interest in these studies was raltegravir C12. Multiple doses of rifampin reduced C12 of a single 400-mg dose of raltegravir by approximately 61%. Raltegravir AUC and Cmax were also decreased by an average of approximately 40% and 38%, respectively. A follow-up study was conducted to see whether doubling the dose of raltegravir to 800 mg twice daily could overcome the effect of rifampin on raltegravir trough concentrations. In this second study, doubling of the raltegravir dose resulted in distinct increases in AUC and Cmax, similar to what would be predicted from the single-dose study. However, a similar increase in raltegravir C12 was not observed (C12 for 800-mg raltegravir plus rifampin was approximately 53% lower compared to the C12 for 400-mg raltegravir alone).
Multiple-dose administration of raltegravir results in minimal accumulation of AUC and Cmax with minor accumulation in C12 (12). Consequently, the effect of doubling the dose of raltegravir on C12 would not necessarily be expected to double C12 values, especially in the presence of a potent inducer, such as rifampin. Induction likely increases clearance and shortens half-life, negatively impacting the extent of accumulation. Thus, the resulting decrease in accumulation with multiple dosing in the presence of rifampin was not expected to result in a simple twofold conversion.
Rifampin is a very potent broad inducer, possibly the most potent inducer of concern for antiretroviral therapies, and has clinically significant effects on many antiretroviral drugs. For example, rifampin decreases indinavir AUC by an average of 92%, and concomitant administration is not recommended (16). Rifampin also substantially reduces the levels of atazanvir, fosamprenavir, lopinavir, and saquinavir in plasma, resulting in recommendations against concomitant administration (3). In this context, the effect of rifampin on raltegravir observed in these studies was fairly modest. This suggests that the level of raltegravir in plasma can be altered by very potent induction of UGT1A1, but the magnitude of the effect does not appear to be as great as that seen for substrates of CYP3A4, such as the protease inhibitors mentioned above.
To date, no threshold C12 has been identified for raltegravir which is known to be associated with reduced efficacy. Based on results of these two drug interaction studies, raltegravir trough concentrations for a regimen including twice-daily raltegravir and rifampin would be expected to be similar to slightly below those achieved at the lowest doses studied in phase II dose escalation studies (9, 14, 15), as well as those achieved when raltegravir is codosed with ritonavir-boosted tipranavir (23). The lowest studied doses in the phase II trials and coadministration of 400-mg twice-daily raltegravir with ritonavir-boosted tipranavir have been shown to be as efficacious as the recommended clinical dose of 400 mg twice daily. Since trough concentrations for rifampin plus raltegravir are likely to be at the lower limits of clinical experience and the pivotal phase III safety and efficacy studies excluded use of rifampin, caution should be used with coadministration, particularly for the subset of patients with no other active agents (aside from raltegravir) in their antiretroviral combination therapy. However, rifampin coadministration with raltegravir is not contraindicated. Clinical efficacy data on the coadministration of rifampin and raltegravir at 400 mg twice daily versus 800 mg twice daily may shed some light on the more appropriate dosing recommendation, and at least one such study is currently in progress.
Acknowledgments
We thank all the subjects who participated in these studies. We thank K. C. Lantz, N. Azrolan, K. J. Davis, S. Y. Liou, and S. S. Keshavarz of Merck & Co., Inc., for their assistance with the study and/or preparation of the manuscript.
This study was funded by Merck & Co., Inc., which has reviewed and approved the manuscript.
L.A.W., W.D.H., D.M.B., A.S.P., K.G., B.J., E.M., J.A.C., J.A.S., K.M.G., J.A.W., and M.I. are employees of Merck & Co., Inc., and may hold stock and/or stock options in the company. T.C.M. and J.K.B. have been investigators for Merck & Co., Inc.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Asante-Appiah, E., and A. M. Skalka. 1999. HIV-1 integrase: structural organization, conformational changes, and catalysis. Adv. Virus Res. 52:351-369. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, K. A., and L. Jauregui. 1993. Use of single sample clearance estimates of cytochrome-P450 substrates to characterize human hepatic CYP status in vivo. Xenobiotica 23:307-315. [DOI] [PubMed] [Google Scholar]
- 3.Baciewicz, A. M., C. R. Chrisman, C. K. Finch, and T. H. Self. 2008. Update on rifampin and rifabutin drug interactions. Am. J. Med. Sci. 335:126-136. [DOI] [PubMed] [Google Scholar]
- 4.Brainard, D. M., A. Petry, W. D. Hanley, L. A. Wenning, B. Jin, S. E. Breidinger, J. Berg, J. A. Stone, J. A. Wagner, and M. Iwamoto. 2008. Doubling the dose of raltegravir (RAL) does not increase trough levels in the presence of rifampin (RIF), abstr. A-964, p. 18. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. and Infect. Dis. Soc. Am. 46th Annu. Meet. (ICAAC/IDSA) (a joint meeting), Washington, DC, 2008.
- 5.Chung, J. Y., J. Y. Cho, K. S. Yu, J. R. Kim, H. R. Jung, K. S. Lim, I. J. Jang, and S. G. Shin. 2005. Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin. Pharmacol. Ther. 77:486-494. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, D., J. Gatell, J. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, X. Xu, R. Isaacs, H. Teppler, and B.-Y. Nguyen. 2008. 48-Week results from BENCHMRK-1, a phase III study of raltegravir (RAL) in patients failing antiretroviral therapy (ART) with triple-class resistant HIV-1, abstr. J-114. 15th Conf. Retrovir. Opportun. Infect., Boston, MA, 3 to 6 February 2008.
- 7.Esposito, D., and R. Craigie. 1999. HIV integrase structure and function. Adv. Virus Res. 52:319-333. [DOI] [PubMed] [Google Scholar]
- 8.Gallicano, K. D., J. Sahai, V. K. Shukla, I. Seguin, A. Pakuts, D. Kwok, B. C. Foster, and D. W. Cameron. 1999. Induction of zidovudine glucuronidation and amination pathways by rifampicin in HIV-infected patients. Br. J. Clin. Pharmacol. 48:168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto, M., K. Kassahun, M. D. Troyer, W. D. Hanley, P. Lu, A. Rhoton, A. S. Petry, K. Ghosh, E. Mangin, E. P. DeNoia, L. A. Wenning, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J. Clin. Pharmacol. 48:209-214. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto, M., L. A. Wenning, S. Y. Liou, J. T. Kost, E. Mangin, K. M. Strohmaier, T. C. Marbury, J. Stone, K. M. Gottesdiener, and J. A. Wagner. 2006. Rifampin (RIF) modestly reduces plasma levels of MK-0518, abstr. P299. 8th Int. Cong. Drug Ther. HIV Infect., Glasgow, Scotland, 12 to 16 November 2006.
- 12.Iwamoto, M., L. A. Wenning, A. S. Petry, M. Laethem, M. De Smet, J. T. Kost, S. A. Merschman, K. M. Strohmaier, S. Ramael, K. C. Lasseter, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293-299. [DOI] [PubMed] [Google Scholar]
- 13.Kassahun, K., I. McIntosh, D. Cui, D. Hreniuk, S. Merschman, K. Lasseter, N. Azrolan, M. Iwamoto, J. A. Wagner, and L. A. Wenning. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab. Dispos. 35:1657-1663. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz, M., J. O. Morales-Ramirez, B.-Y. Nguyen, C. M. Kovacs, R. T. Steigbigel, D. A. Cooper, R. Liporace, R. Schwartz, R. Isaacs, L. R. Gilde, L. A. Wenning, and J. Zhao. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509-515. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz, M., B. Y. Nguyen, E. Gotuzzo, F. Mendo, W. Ratanasuwan, C. Kovacs, G. Prada, J. O. Morales-Ramirez, C. S. Crumpacker, R. D. Isaacs, L. R. Gilde, H. Wan, M. D. Miller, L. A. Wenning, and H. Teppler. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection—results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125-133. [DOI] [PubMed] [Google Scholar]
- 16.McCrea, J., D. Wyss, J. Stone, A. Carides, S. Kusma, C. Kleinbloesem, Y. AlHamdan, K. Yeh, and P. Deutsch. 1997. Pharmacokinetic interaction between indinavir and rifampin. Clin. Pharmacol. Ther. 61:I62. [Google Scholar]
- 17.Merck and Co., Inc. 2007. Isentress (raltegravir) 400 mg for treatment of HIV (NDA 22-145). FDA Antiviral Drugs Advisory Committee Meeting briefing document. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4314b1-01-Merck.pdf.
- 18.Merschman, S. A., P. T. Vallano, L. A. Wenning, B. K. Matuszewski, and E. J. Woolf. 2007. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J. Chromatogr. B 857:15-24. [DOI] [PubMed] [Google Scholar]
- 19.Miller, M. D., M. Witmer, K. Stillmock, P. Felcock, L. Ecto, J. Flynn, G. Dornadula, R. Danovich, and D. Hazuda. 2006. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor, abstr. THAA0302. AIDS 2006 - 16th Int. AIDS Conf., Toronto, Canada, 13 to 16 August 2006.
- 20.Steigbigel, R., P. Kumar, J. Eron, M. Markowitz, M. Loufty, J. Zhao, R. Isaacs, B.-Y. Nguyen, and H. Teppler. 2008. 48-Week results from BENCHMRK-2, a phase III study of raltegravir (RAL) in patients (pts) failing antiretroviral therapy (ART) with triple-class resistant HIV, abstr. J-117. 15th Conf. Retrovir. Opportun. Infect., Boston, MA, 3 to 6 February 2008.
- 21.Sugatani, J., S. Nishitani, K. Yamakawa, K. Yoshinari, T. Sueyoshi, M. Negishi, and M. Miwa. 2005. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol. Pharmacol. 67:845-855. [DOI] [PubMed] [Google Scholar]
- 22.Wenning, L., B.-Y. Nguyen, X. Sun, E. Hwang, Y. Chen, H. Teppler, C. Harvey, R. Rhodes, D. Ryan, N. Azrolan, and J. Stone. 2008. Pharmacokinetic/pharmacodynamic (PK/PD) analyses for raltegravir (RAL) in phase II and III studies in treatment experienced HIV-infected patients, abstr. O21. 9th Int. Workshop Clin. Pharmacol. HIV Ther., New Orleans, LA, 7 to 9 April 2008.
- 23.Wenning, L. A., W. Hanley, J. Stone, A. Moreau, J. T. Kost, E. Mangin, T. Shamp, N. Azrolan, K. M. Gottesdiener, J. A. Wagner, and M. Iwamoto. 2006. Effect of tipranavir + ritonavir (tpv + rtv) on pharmacokinetics of MK-0518, abstr. A-374, p. 8. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006.