Abstract
The reduction of amphotericin B (AmB)-induced renal tubular apoptosis and nephrotoxicity by N-acetylcysteine (NAC) in a murine model was evaluated. Four groups of rats were treated with AmB for 5 days, and each group concomitantly received two doses of 30, 60, or 120 mg of NAC/kg of body weight/day or sterile water for 5 days. Groups that received concomitant NAC at any dose had significantly decreased levels of apoptosis compared to that in animals receiving AmB only (48.8% versus 27.4, 23.6, or 23.5%, respectively; P < 0.001).
Nephrotoxicity is the major dose-limiting side effect of amphotericin B (AmB) deoxycholate (14, 16, 34). AmB-induced nephrotoxicity is usually reversible; however, up to 15% of patients may require dialysis, resulting in extended hospital stays and increased mortality (3, 34). Nephrotoxicty is secondary to renal vasoconstriction, which leads to tubular damage and a decreased glomerular filtration rate (GFR) (11, 14, 15). The mechanism of renal tubular damage has not been fully elucidated; however, AmB has been suggested previously to induce dose-dependent renal tubular cell apoptosis (32).
Several approaches to decreasing the incidence of AmB nephrotoxicity have been proposed. These approaches include prehydrating the drug formulation with saline and prolonging the infusion time (10, 18), infusing the drug in a fat emulsion (Intralipid) solution (7, 19, 21, 25, 27), and coadministering the drug with mannitol (6); however, none of these approaches have proven to be effective. While lipid formulations of AmB were found to be less nephrotoxic than other formulations of the drug, these formulations do not completely eliminate nephrotoxicity (9, 17, 23, 35).
Recently, Varlam et al. demonstrated that AmB causes a dose-dependent apoptotic effect in rat renal tubular cells, with ensuing nephrotoxicity (32). They also showed that AmB-induced apoptosis and the resulting nephrotoxicity can be reduced by the concomitant use of recombinant human insulin-like growth factor 1 (rhIGF-1), an antiapoptotic agent.
N-Acetylcysteine (NAC) is an antiapoptotic and antioxidant drug, and NAC administration prior to the administration of radiocontrast agents prevents the nephrotoxicity associated with these agents (4, 5, 30). The purpose of our study was to evaluate the effect of the concomitant administration of NAC on AmB-induced renal tubular cell apoptosis.
(An abstract describing this study was presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2006 [22].)
Three-week-old male Sprague-Dawley rats weighing 100 g on average were maintained in individual cages. The animals had free access to a standard diet and received water ad libitum. Prior to the experiments, rats were randomized and divided into four groups consisting of 10 animals each. Animals in each of the groups (A, B, C, and D) were treated with 10 mg of intraperitoneal (i.p.) AmB deoxycholate (Bristol-Myers Squibb)/kg of body weight/day for 5 days. In addition to AmB, group A was given i.p. sterile water and groups B, C, and D were treated i.p. with two doses of 30, 60, and 120 mg of NAC/kg/day, respectively, for five consecutive days. The doses of AmB (32) and NAC (1, 12) were determined based on data from the literature. Another five animals (group E) were treated with sterile water only. Rats were weighed daily, and changes in the body weights were recorded. At the end of the study, animals were sacrificed and kidneys were harvested and placed into formaldehyde solutions for histopathological evaluation. Kidneys were paraffinized, and 4-μm tissue sections were prepared and examined for apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) using the ApopTag Plus peroxidase in situ apoptosis detection kit (Chemicon, Millipore).
A pathologist quantified the degree of apoptosis in a blinded fashion. For the estimation of the level of apoptosis, the apoptotic index (AI) was determined by counting the TUNEL-positive renal tubular epithelial cells and the unstained cells (mean, 500 cells in each field) in 10 sequentially selected microscopic fields at a magnification of ×400. The number of apoptotic cells in each field was then divided by the total number of cells in the field. Differences between the groups were analyzed by Student's t test. A P value of <0.05 was considered to reflect statistically significant data.
Results are summarized in Table 1. At the end of the study, the average weight of the animals receiving only AmB was significantly lower than those of the animals receiving concomitant NAC (P < 0.001). During the study period, animals in group A were observed to become less active as the treatment progressed to day 5. For the group treated with AmB only, the mean AI was 48.8%, and the analysis of kidney tissues from rats in the negative control group (receiving sterile water only) revealed an AI of 2.8%. The coadministration of NAC resulted in significantly decreased AIs for groups B (27.4%; P < 0.001), C (23.6%; P < 0.001), and D (23.5%; P < 0.001) compared with that for the group treated with AmB only (Table 1 and Fig. 1). There was no difference in the AI among the three NAC-treated groups.
TABLE 1.
Average AIs for animal groupsa
| Group | Regimen | AI (%) | Average wt (g) |
|---|---|---|---|
| A | AmB only | 48.8 | 159 |
| B | AmB and NAC (twice daily at 30 mg/kg) | 27.4* | 205* |
| C | AmB and NAC (twice daily at 60 mg/kg) | 23.6* | 207* |
| D | AmB and NAC (twice daily at 120 mg/kg) | 23.5* | 211* |
| E | Sterile water | 2.8 | 205 |
*, P < 0.05. P values were calculated by comparing results for group B, C, or D with those for group A.
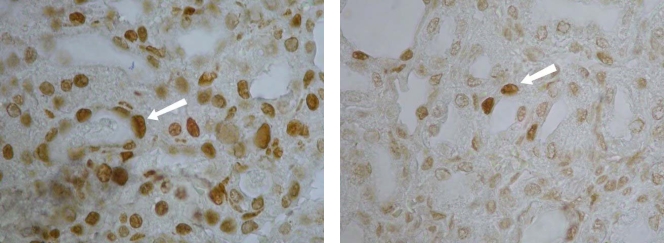
FIG. 1.
Photomicrographs of renal tubular cells (at a magnification of ×400). Apoptotic cells were detected immunohistochemically by in situ end labeling of DNA strand breaks (TUNEL assay). All dark-stained cells (white arrows) are apoptotic. (Left) Tubular epithelial cells from rats treated with 10 mg/kg AmB; (right) cells from rats treated concomitantly with AmB and 60 mg/kg/12 h of NAC for 5 days, showing a significant decrease in apoptosis.
The use of NAC for the prevention of the radiocontrast agent-induced nephrotoxicity led us to evaluate the efficacy of NAC in the prevention of AmB-induced apoptosis and renal injury (28, 31). NAC is usually administered orally at 600 mg twice daily on the day before and the day of administration of the contrast agent. However, in some studies, the intravenous form of the drug has been used in doses as high as 50 mg/kg (13, 29). The mechanism of contrast agent-induced nephropathy has not been completely elucidated, but this result is attributed to a combination of renal ischemia, tubular epithelial cell toxicity, oxidative tissue damage, and apoptosis, effects that are very similar to those of AmB (2, 8, 24, 26, 33).
In our study, we demonstrated that NAC reduced AmB-induced renal tubular apoptosis. Varlam et al. showed that rhIGF-1 decreases the incidence of renal tubular apoptosis (from 43% to 2%) in animals treated with AmB at 5 mg/kg/day and significantly reduces the frequency of apoptosis (from 52% to 18%) in animals receiving AmB at 10 mg/kg/day. In our study, AmB treatment resulted in a mean AI of 48.8% and the concomitant use of NAC decreased the AI to 23.5 to 27.4%. Nitescu et al. showed that treatment with 200 mg/kg NAC decreases the GFR and reduces plasma creatinine levels, hyperkalemia, and systemic oxidative stress in rats subjected to renal ischemic injury (20). In another study, prophylactic NAC prevented decreases in the GFR associated with high doses of AmB (12). Although we could show that NAC significantly reduced the AmB-induced tubular apoptosis, we did not evaluate the electrolytes in serum or urine samples or the serum creatinine levels because of technical unavailability. However, Varlam et al. demonstrated that rhIGF-1 improves the ability of the kidney to concentrate urine and prevents hypokalemia and dehydration.
In summary, we demonstrated that renal tubular apoptosis caused by AmB could be reduced by the concomitant use of the antioxidant NAC. Our findings are promising for the clinical use of NAC for the reduction of AmB-induced nephrotoxicity. Further clinical studies are warranted.
Acknowledgments
This project was supported by a grant from the Scientific and Technological Research Council of Turkey (TUBITAK).
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Appenroth, D., K. Winnefeld, H. Schroter, and M. Rost. 1993. Beneficial effect of acetylcysteine on cisplatin nephrotoxicity in rats. J. Appl. Toxicol. 13:189-192. [DOI] [PubMed] [Google Scholar]
- 2.Bakris, G. L., N. Lass, A. O. Gaber, J. D. Jones, and J. C. Burnett, Jr. 1990. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am. J. Physiol. 258:F115-F120. [DOI] [PubMed] [Google Scholar]
- 3.Bates, D. W., L. Su, D. T. Yu, G. M. Chertow, D. L. Seger, D. R. Gomes, E. J. Dasbach, and R. Platt. 2001. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 32:686-693. [DOI] [PubMed] [Google Scholar]
- 4.Briguori, C., A. Colombo, A. Violante, P. Balestrieri, F. Manganelli, P. Paolo Elia, B. Golia, S. Lepore, G. Riviezzo, P. Scarpato, A. Focaccio, M. Librera, E. Bonizzoni, and B. Ricciardelli. 2004. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur. Heart J. 25:206-211. [DOI] [PubMed] [Google Scholar]
- 5.Briguori, C., F. Manganelli, P. Scarpato, P. P. Elia, B. Golia, G. Riviezzo, S. Lepore, M. Librera, B. Villari, A. Colombo, and B. Ricciardelli. 2002. Acetylcysteine and contrast agent-associated nephrotoxicity. J. Am. Coll. Cardiol. 40:298-303. [DOI] [PubMed] [Google Scholar]
- 6.Bullock, W. E., R. G. Luke, C. E. Nuttall, and D. Bhathena. 1976. Can mannitol reduce amphotericin B nephrotoxicity? Double-blind study and description of a new vascular lesion in kidneys. Antimicrob. Agents Chemother. 10:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caillot, D., G. Reny, E. Solary, O. Casasnovas, P. Chavanet, B. Bonnotte, L. Perello, M. Dumas, F. Entezam, and H. Guy. 1994. A controlled trial of the tolerance of amphotericin B infused in dextrose or in Intralipid in patients with haematological malignancies. J. Antimicrob. Chemother. 33:603-613. [DOI] [PubMed] [Google Scholar]
- 8.Caldicott, W. J., N. K. Hollenberg, and H. L. Abrams. 1970. Characteristics of response of renal vascular bed to contrast media. Evidence for vasoconstriction induced by renin-angiotensin system. Investig. Radiol. 5:539-547. [DOI] [PubMed] [Google Scholar]
- 9.Carrigan Harrell, C., and L. Hanf-Kristufek. 2001. Comparison of nephrotoxicity of amphotericin B products. Clin. Infect. Dis. 32:990-991. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson, U., B. Seifert, and A. Schaffner. 2001. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. BMJ 322:579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanos, V., and L. Cataldi. 2000. Amphotericin B-induced nephrotoxicity: a review. J. Chemother. 12:463-470. [DOI] [PubMed] [Google Scholar]
- 12.Feldman, L., S. Efrati, V. Dishy, L. Katchko, S. Berman, M. Averbukh, M. Aladjem, Z. Averbukh, and J. Weissgarten. 2005. N-Acetylcysteine ameliorates amphotericin-induced nephropathy in rats. Nephron Physiol. 99:p23-p27. [DOI] [PubMed] [Google Scholar]
- 13.Fishbane, S. 2008. N-Acetylcysteine in the prevention of contrast-induced nephropathy. Clin. J. Am. Soc. Nephrol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 14.Girmenia, C., G. Gentile, A. Micozzi, and P. Martino. 2001. Nephrotoxicity of amphotericin B desoxycholate. Clin. Infect. Dis. 33:915-916. [DOI] [PubMed] [Google Scholar]
- 15.Groll, A. H., D. Mickiene, V. Petraitis, R. Petraitiene, R. M. Alfaro, C. King, S. C. Piscitelli, and T. J. Walsh. 2003. Comparative drug disposition, urinary pharmacokinetics, and renal effects of multilamellar liposomal nystatin and amphotericin B deoxycholate in rabbits. Antimicrob. Agents Chemother. 47:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbarth, S., S. L. Pestotnik, J. F. Lloyd, J. P. Burke, and M. H. Samore. 2001. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am. J. Med. 111:528-534. [DOI] [PubMed] [Google Scholar]
- 17.Herbrecht, R., S. Natarajan-Ame, Y. Nivoix, and V. Letscher-Bru. 2003. The lipid formulations of amphotericin B. Expert Opin. Pharmacother. 4:1277-1287. [DOI] [PubMed] [Google Scholar]
- 18.Imhof, A., R. B. Walter, and A. Schaffner. 2003. Continuous infusion of escalated doses of amphotericin B deoxycholate: an open-label observational study. Clin. Infect. Dis. 36:943-951. [DOI] [PubMed] [Google Scholar]
- 19.Moreau, P., N. Milpied, N. Fayette, J. F. Ramee, and J. L. Harousseau. 1992. Reduced renal toxicity and improved clinical tolerance of amphotericin B mixed with Intralipid compared with conventional amphotericin B in neutropenic patients. J. Antimicrob. Chemother. 30:535-541. [DOI] [PubMed] [Google Scholar]
- 20.Nitescu, N., S. E. Ricksten, N. Marcussen, B. Haraldsson, U. Nilsson, S. Basu, and G. Guron. 2006. N-Acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusion. Nephrol. Dial. Transplant. 21:1240-1247. [DOI] [PubMed] [Google Scholar]
- 21.Nucci, M., M. Loureiro, F. Silveira, A. R. Casali, L. F. Bouzas, E. Velasco, N. Spector, and W. Pulcheri. 1999. Comparison of the toxicity of amphotericin B in 5% dextrose with that of amphotericin B in fat emulsion in a randomized trial with cancer patients. Antimicrob. Agents Chemother. 43:1445-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odabasi, Z., A. Karaalp, H. Cermik, J. Mohr, O. Ergonul, Z. Agalar, B. Sumer, L. Mulazimoglu, and V. Korten. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1100, p. 22. American Society for Microbiology, Washington, DC.
- 23.Olson, J. A., J. P. Adler-Moore, J. Schwartz, G. M. Jensen, and R. T. Proffitt. 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 50:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson, P. B., and M. Tepel. 2006. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int. Suppl. 2006:S8-S10. [DOI] [PubMed] [Google Scholar]
- 25.Schoffski, P., M. Freund, R. Wunder, D. Petersen, C. H. Kohne, H. Hecker, U. Schubert, and A. Ganser. 1998. Safety and toxicity of amphotericin B in glucose 5% or intralipid 20% in neutropenic patients with pneumonia or fever of unknown origin: randomised study. BMJ 317:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon, R. 1998. Contrast-medium-induced acute renal failure. Kidney Int. 53:230-242. [DOI] [PubMed] [Google Scholar]
- 27.Sorkine, P., H. Nagar, A. Weinbroum, A. Setton, E. Israitel, A. Scarlatt, A. Silbiger, V. Rudick, Y. Kluger, and P. Halpern. 1996. Administration of amphotericin B in lipid emulsion decreases nephrotoxicity: results of a prospective, randomized, controlled study in critically ill patients. Crit. Care Med. 24:1311-1315. [DOI] [PubMed] [Google Scholar]
- 28.Tepel, M. 2003. Acetylcysteine for the prevention of radiocontrast-induced nephropathy. Minerva Cardioangiol. 51:525-530. [PubMed] [Google Scholar]
- 29.Tepel, M. 2006. Preventing nephropathy induced by contrast medium. N. Engl. J. Med. 354:1853-1855. [PubMed] [Google Scholar]
- 30.Tepel, M., M. van der Giet, C. Schwarzfeld, U. Laufer, D. Liermann, and W. Zidek. 2000. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N. Engl. J. Med. 343:180-184. [DOI] [PubMed] [Google Scholar]
- 31.van den Berk, G., S. Tonino, C. de Fijter, W. Smit, and M. J. Schultz. 2005. Bench-to-bedside review: preventive measures for contrast-induced nephropathy in critically ill patients. Crit. Care 9:361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varlam, D. E., M. M. Siddiq, L. A. Parton, and H. Russmann. 2001. Apoptosis contributes to amphotericin B-induced nephrotoxicity. Antimicrob. Agents Chemother. 45:679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg, L. S., P. B. Kurnik, and B. R. Kurnik. 1994. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 45:259-265. [DOI] [PubMed] [Google Scholar]
- 34.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 35.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]