Abstract
Staphylococcal sepsis is a major cause of morbidity and mortality in very-low-birth-weight (VLBW) infants. A human chimeric monoclonal antibody, pagibaximab, was developed against staphylococcal lipoteichoic acid. We evaluated the safety, tolerability, and pharmacokinetics of pagibaximab in VLBW neonates. A phase 1/2, randomized, double-blind, placebo-controlled, dose escalation study was conducted in VLBW infants (700 to 1,300 g) 3 to 7 days old. Patients received two doses 14 days apart of intravenous pagibaximab (10, 30, 60, or 90 mg/kg of body weight) or placebo in a 2:1 ratio. Blood and urine samples were obtained pre- and postinfusion for analysis of safety and pharmacokinetics, and data on adverse events were gathered. Staphylococcal organisms causing sepsis were collected and evaluated. Fifty-three patients received at least one dose of pagibaximab or placebo. The average gestational age was 27.6 weeks; the average birth weight was 1,003 g. All serious adverse events were deemed unrelated or probably not drug related. Morbidity and mortality were similar across treatment groups. No evidence of immunogenicity of pagibaximab was detected. Pagibaximab pharmacokinetics was linear. The mean clearance (CL), volume of distribution, and elimination half-life of pagibaximab were independent of dose. The serum half-life was 20.5 ± 6.8 days. Pagibaximab enhanced serum opsonophagocytic activity. All staphylococci causing sepsis were opsonizable by pagibaximab. Two infusions of pagibaximab, administered 2 weeks apart to high-risk neonates appeared safe and tolerable, and pharmacokinetics were linear. Evaluation of more frequent doses, at the highest doses tested, in neonates at high-risk of staphylococcal sepsis, is warranted.
Very-low-birth-weight (VLBW) neonates (<1,500-g birth weight) are at high risk for late-onset (>72 h of life) hospital-acquired sepsis (13, 16, 17). Such infections are a major cause of morbidity, prolong time in the hospital and intensive care unit, increase the need for antibiotics, and further increase the substantial cost of medical care for these infants (8, 17). Staphylococci, including coagulase-negative staphylococci (CONS) and Staphylococcus aureus, are responsible for between 56 and >75% of hospital-acquired, late-onset neonatal sepsis (13, 31). Recent reports (12, 28, 30) show continuing increases in resistance of staphylococci to antimicrobial agents. Frequent and prolonged exposures to antimicrobials have been demonstrated to increase the risk of developing infections with resistant organisms (30, 34). Therapeutic products and strategies that could prevent infections would minimize the need for antimicrobial products (20).
Lipoteichoic acid (LTA) is a highly conserved epitope in the staphylococcal cell wall that inhibits phagocytosis of bacteria in vitro, induces the cytokine cascade through stimulation of Toll-like receptors, and may be necessary for staphylococcal survival (18, 19, 24, 27). An anti-LTA murine/human chimeric monoclonal antibody, pagibaximab, was developed by recombinant DNA technology (J. J. Mond, unpublished data), and its activity was confirmed in vitro and in animal studies against CONS (37; Mond, unpublished) and S. aureus (L. E. Weisman, unpublished data). On the basis of preclinical pagibaximab bactericidal activity against a number of clinical isolates in vitro and in staphylococcal sepsis models in suckling animals, we have selected 500 μg/ml as the putative protective level of this antibody. In summary, we found that pagibaximab resistance bound 24 different strains of CONS and S. aureus and demonstrated increased bacterial killing in vitro against all of these strains. There was a clear dose-response curve with 400 μg/ml being required to show the maximum killing activity on all of the strains tested and lower doses being less bactericidal. In a suckling rat model of sepsis caused by CONS, pagibaximab significantly increased survival at a dose of 80 mg/kg of body weight (P = 0.0007), and the effect of 40 mg/kg was significantly lower. This was associated with suckling rat serum pagibaximab concentrations of approximately 275 to 400 μg/ml. In a lethal suckling rat model of S. aureus sepsis, pagibaximab significantly increased survival at 80 mg/kg/dose (P = 0.02), and protection was lower at doses of 40 mg/kg. This was associated with suckling rat serum pagibaximab concentrations of 400 to 500 μg/ml. In view of the fact that VLBW infants have compromised innate immunity, we hypothesized that we needed to have excess antibody to ensure bactericidal activity under conditions in which the effector system might be compromised as occurs in the VLBW infant. For this reason, we selected 500 μg/ml of antibody as the level which we hypothesized would be protective. It has also been hypothesized that pagibaximab could potentially prevent staphylococcal shock syndrome (15). Thus, pagibaximab appears a promising option in preventing staphylococcal sepsis and its sequelae.
Pagibaximab has been studied in healthy human adults as a single intravenous (i.v.) dose at 3 or 10 mg per kilogram and appeared to be safe and tolerable (38). The current clinical study, the first study of pagibaximab in VLBW neonates, was intended to evaluate the safety, tolerability, and pharmacokinetics of pagibaximab in this high-risk patient population.
(This work was presented in part at the Pediatric Academic Societies' Annual Meetings in Baltimore, MD, May 2003, and San Francisco, CA, May 2004.)
MATERIALS AND METHODS
Study design.
This was a phase 1/2, randomized, double-blind, placebo-controlled, dose escalation study assessing the safety and pharmacokinetic profile of four dose levels of pagibaximab. Based on previous studies of a neonatal monoclonal antibody to prevent infection (33), monoclonal antibodies to treat infection (1, 11), pagibaximab in animal models (37; Mond, unpublished; Weisman, unpublished), neonatal suckling rat toxicity studies (Mond, unpublished), and a pagibaximab study of adults (38), the four dose levels of pagibaximab chosen for the present study were 10, 30, 60, and 90 mg/kg. Based on these in vitro and animal studies, serum pagibaximab levels of 500 μg/ml were anticipated to provide protection against the broadest spectrum of CONS and S. aureus sepsis in VLBW neonates. The study was conducted from October 2001 through May 2003 in three neonatal intensive care units in two medical centers in the United States.
Study entry criteria.
Eligible patients were infants with a birth weight of 700 to 1,300 g, 3 to 7 days of age (inclusive), inpatients in the neonatal intensive care unit with i.v. access, and expected to live at least 1 week following infusion. Patients with any of the following conditions were excluded from eligibility: clinically overt systemic infection; life-threatening hemodynamic instability; severe congenital anomaly or genetic disorder; known or suspected hepatic or renal insufficiency; persistent seizure disorder; immunodeficiency due to reasons other than prematurity; a history of immune globulin administration prior to first study drug infusion; any history (patient or mother) of a hypersensitivity or severe vasomotor reaction to immunoglobulin G (IgG) or blood products; abnormal laboratory findings, including liver function tests, blood urea nitrogen, bilirubin, complete blood count (CBC); concomitant or recent receipt of other investigational agents; expectation that we would not be able to monitor the patient for the duration of the study; mother with serology positive for hepatitis B virus surface antigen or neonate's receipt of hepatitis B virus immune globulin since birth. The institutional review board at each center approved the study.
Evaluation of patients.
After informed consent was obtained from the infant's parents or legal guardian, a baseline evaluation of medical history, physical examination, and laboratory testing was performed. Laboratory evaluations included standard hematology, blood chemistry, liver function, renal function, and urinalysis testing.
Fifteen minutes before administration of the study drug, vital signs, oxygen saturation, and physical examination were obtained. The randomized dose of study drug (pagibaximab or placebo) was administered as an i.v. infusion at 0.01 ml per kilogram per minute. The infusion rate was slowly increased to 0.02, 0.05, 0.1, and 0.125 ml/kg/min every 15 min if there was no physical evidence of an adverse event (AE), including changes in oxygen saturation, heart rate, blood pressure, temperature, and respirations. Additional vital signs and clinical assessment data were collected every 15 min until the infusion was complete and 30 and 60 min postinfusion.
On day 14, patients were rescreened for eligibility for the second dose. Eligible patients had a similar predose evaluation. Administration of the second dose of study drug occurred at the patient's previous randomized dose based on the original treatment assignment, and infusion followed the same procedures described for the initial infusion.
On days 3 and 7, the patients were assessed for safety, medical history, vital signs, and physical examination. On days 14, 28, and 42, the patients were assessed for safety, medical history, vital signs, and physical examination, and the following were obtained: urinalysis, CBC with differential and platelet count, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, and creatinine. If available, data on bilirubin, electrolytes, and glucose were also collected. Additionally, on day 42, blood was drawn for human antimurine antibody/human antichimeric antibody (HAMA/HACA) analysis. On day 56, if still hospitalized, patients were assessed for safety, medical history, vital signs, physical examination, and urinalysis was obtained. If a patient was released from the hospital prior to day 56, the patient's parent or legal guardian was contacted by telephone and asked about the patient's health since hospital discharge. Blood was drawn for anti-LTA antibody levels on days 0 (1 h after infusion), 3, 7, 14 (prior to the second infusion), 14 (1 h after the second infusion), 28, 42, and 56 (if still hospitalized). Patients were monitored for a total of 8 weeks after the start of the first study drug infusion.
Throughout the study, patients were closely monitored for signs of infection. If the attending physician deemed it necessary to evaluate a patient for sepsis, meningitis, or other infection, a workup was performed. The workup included CBC with differential; platelet count; blood culture from a peripheral vein (although physicians were encouraged to collect two samples, a single sample was considered acceptable); cerebrospinal fluid examination, including gram stain, cell count, and culture; urine culture by bladder tap or sterile catheterization; and culture from other sterile sources as indicated. Samples of all staphylococcal bacteria isolated from blood, cerebrospinal fluid, and other sterile sites were sent to a central laboratory for analyses.
Randomization and dose escalation.
Eligible VLBW infants were randomized at a ratio of 2:1 to receive i.v. pagibaximab at 10, 30, 60, and 90 mg/kg or an equal volume of placebo (saline) on days 0 and 14. Two birth weight groups (700 to 1,000 g and 1,001 to 1,300 g) accrued independently, with each dose and birth weight group consisting of four patients receiving pagibaximab and two patients receiving placebo.
Dose escalation to the next higher level occurred within each birth weight group only after the last patient in the previous dose and weight group was monitored for at least 14 days from the first dose by the safety monitoring committee. This committee was composed of the two principal investigators, the independent medical monitor, and three physicians from the study sponsor.
Blinding.
This was a double-blind study. The only persons who knew patient treatment assignments were the statisticians at the contract research organization overseeing the study, who were responsible for assignment of patient identification numbers and study drug allocation, and the pharmacists at the study sites, who were responsible for preparing the infusion.
Safety analyses.
For the purpose of this study, an AE was defined as any adverse change from the patient's baseline condition that occurred following the first administration of the study drug through the end of the study period. Changes from the patient's baseline condition known to be normal physiologic changes associated with the development of premature infants were not considered to be AEs. A protocol-defined toxicity table was used to grade the severity of each AE on a scale of 1 to 4.
A serious adverse event (SAE) was defined as any event that resulted in any of the following outcomes: (i) death; (ii) a life-threatening AE; (iii) inpatient hospitalization or prolongation of hospitalization; (iv) persistent or significant disability or incapacity; (v) an important medical event that required intervention in order to prevent any of the other serious outcomes. All grade 4 AEs, as defined in the toxicity table, were considered SAEs. The investigator assessed each SAE for severity and relationship to study drug in a blinded manner.
Assessment of immunogenicity.
HAMA/HACA levels were determined by radiometric human anti-Hu96-110 HACA assays. Hu96-110, a human/murine chimeric monoclonal antibody (IgG1κ), is the active ingredient of pagibaximab. Polystyrene beads were coated with Hu96-110. Test serum, normal human serum, and goat anti-human IgG (positive control) were added to borosilicate glass culture tubes. A single Hu96-110-coated bead was added to each tube. Each bead was washed. 125I-labeled Hu96-110 was added to all tubes. Each bead was washed again. The beads were transferred to clean tubes, and particle emissions were counted to determine the amount of 125I-labeled Hu96-110 bound to each bead. The assay result was calculated from the bound 125I-labeled Hu96-110 and known concentration of 125I-labeled Hu96-110. The results were expressed as nanograms per milliliter of Hu96-110. During validation of the assay, the mean and standard deviation of the response in 27 normal human serum samples was 25.4 ± 8.7 ng/ml. The upper limit of normal human serum was defined as the mean plus 3 standard deviations or 52 ng/ml. A positive sample would have a value that exceeds the upper limit and or was twice as high as the preinfusion sample.
Pharmacokinetic analyses.
The pharmacokinetic profile of pagibaximab was assessed using an antigen capture enzyme-linked immunosorbent assay measuring the concentration of anti-LTA antibodies in serum. In brief, S. aureus LTA was coated onto the bottom of 96-well microtiter plates. After unbound LTA was washed off, test sera diluted in phosphate-buffered saline with Tween 20 and immunoglobulin-depleted human serum was incubated in the wells. The bound anti-LTA antibody was detected by incubation with a horseradish peroxidase-labeled anti-human immunoglobulin antibody and a colorimetric reagent (3,3′,5,5′-tetramethylbenzidine [TMB]). The amount of anti-LTA antibody in serum was determined by comparison to a pagibaximab standard of known amount (38). Noncompartmental analysis was used to estimate clearance, volume of distribution (V), and half-life (t1/2).
Opsonophagocytic activity.
The opsonophagocytic (bacterial killing) activity of pagibaximab in serum was determined using a modified standard assay (14). Specifically, the following components were used: Staphylococcus epidermidis American Type Culture Collection (ATCC) strain 55133 (for measurement of patient serum activity) or clinical isolates (for measurement of pagibaximab activity) as the source of bacteria, HL60 cells (human acute promyelocytic leukemia cell line) as a source of human polymorphonuclear cells (PMNs), and C1q as a source of complement. In brief, patient serum (diluted 1:90) or pagibaximab (at various concentrations), PMNs, and diluted complement were mixed with a suspension of bacteria and incubated in a 96-well plate. Bacterial killing was measured by comparing the number of bacteria present at the time of initial mixing and after 2 h of incubation. Bacteria were enumerated by performing colony counts on tryptic agar plates with 5% sheep blood. Controls included PMNs alone, complement alone, and PMNs plus complement. Using the formula {[number of bacteria (time zero to 2 h)]/number of bacteria at time zero]} × 100, the percent bacterial killing was calculated.
Bacterial analysis.
Frozen stocks of staphylococcal isolates were shipped to a central laboratory for species identification. Clinical isolates of CONS from blood cultures were analyzed. The isolates were thawed and streaked for isolation on blood agar (Remel, Lenexa, KS) to confirm culture purity and presence of staphylococci. The species was determined by using the API Staph Ident system (BioMerieux, Hazelwood, MO) (21). Briefly, isolated staphylococcal colonies were tested in various biochemical assays per the manufacturer's instructions. Two ATCC reference isolates, ATCC 49521 and ATCC 35984, were used as control organisms for S. aureus and S. epidermidis, respectively. Those staphylococcal isolates for which an unequivocal species could not be determined by API Staph Strip, were sent to Accugenix (Newark, DE) for 16S 500-bp sequence identification.
The same isolates were evaluated for genetic relatedness by performing pulsed-field gel electrophoresis (PFGE) following published procedures (26). Briefly, chromosomal DNA was isolated from the various staphylococcal isolates, digested in agarose with SmaI, and then subjected to PFGE using a contour-clamped homogeneous electric field system (Bio-Rad, Hercules, CA). Dendrograms were generated based on the genetic relatedness of the digestion patterns (23).
Analysis of sepsis episodes.
For all patients who had sepsis evaluations, analyses of sepsis caused by CONS were divided into four categories. Each category included signs and symptoms consistent with clinical sepsis. In addition, if two or more peripheral blood cultures grew CONS, it was categorized as definite sepsis. If one peripheral blood culture grew CONS when only one peripheral blood culture was drawn, it was categorized as probable sepsis. If one peripheral blood culture grew CONS when more than one peripheral blood culture was drawn, it was categorized as possible sepsis. If one or more central venous line blood cultures grew CONS in the absence of positive peripheral cultures, it was categorized as line sepsis.
Statistical methods.
The statistical analyses were essentially descriptive. Safety analyses were performed on the intent-to-treat (ITT) population, defined as all randomized patients who received at least one dose of study drug. Continuous variables were summarized by the mean, standard deviation, median, and range. Categorical variables were summarized by the frequency and percentage. There was no formal hypothesis testing planned for primary objectives. However, if differences were observed, appropriate formal hypothesis testing for primary and/or secondary outcomes was performed at the significance level of α = 0.05 (two-sided test).
RESULTS
Patient population.
Fifty-five patients were randomized into the study. Of these, two patients never received the study drug; consent for one patient was withdrawn prior to the first study drug infusion and the other was excluded due to low hemoglobin. These 53 patients (47 from Baylor College of Medicine and 6 from Weill Medical College) were considered the ITT population that formed the basis of our analysis. Fifty-three patients received at least one dose of study drug, and 44 (83%) of 53 received two doses. Nine patients (17%) did not receive the second dose because they met one or more exclusion criteria for the second dose.
Patient baseline characteristics.
Demographic and other baseline characteristics of study patients were generally comparable across the treatment groups (Table 1). The mean gestational age for patients was 27.6 weeks (ranging from 25.0 to 33.0 weeks), and the mean birth weight was 1,003 g (ranging from 702 to 1,300 g).
TABLE 1.
Patient baseline characteristics by treatment group
| Characteristic | Value for treatment groupa
|
|||||
|---|---|---|---|---|---|---|
| Pagibaximab
|
Placebo (n = 20) | Total (all groups) (n = 53) | ||||
| 10 mg/kg (n = 8) | 30 mg/kg (n = 8) | 60 mg/kg (n = 8) | 90 mg/kg (n = 9) | |||
| Gestational age (wk) (mean ± SD) | 27.4 ± 1.7 | 27.5 ± 1.4 | 27.0 ± 1.5 | 28.3 ± 2.2 | 27.6 ± 2.4 | 27.6 ± 2.0 |
| Birth wt (g) (mean ± SD) | 990 ± 170 | 1,030 ± 172 | 1,015 ± 168 | 1,015 ± 209 | 987 ± 159 | 1,003 ± 167 |
| Female gender (%) | 37.5 | 50.0 | 25.0 | 44.4 | 45.0 | 41.5 |
| Race (%) | ||||||
| Black | 25.0 | 25.0 | 12.5 | 11.1 | 35.0 | 24.5 |
| Hispanic | 37.5 | 62.5 | 62.5 | 11.1 | 25.0 | 35.8 |
| White | 25.0 | 12.5 | 25.0 | 66.7 | 30.0 | 32.1 |
| Apgar score (mean ± SD) | ||||||
| 1 min | 5.4 ± 2.0 | 5.6 ± 2.9 | 5.5 ± 1.9 | 5.4 ± 1.9 | 4.9 ± 2.1 | 5.3 ± 2.1 |
| 5 min | 7.4 ± 1.4 | 7.3 ± 2.1 | 7.6 ± 1.1 | 7.3 ± 1.3 | 7.0 ± 2.0 | 7.3 ± 1.6 |
n indicates the number of patients in the treatment group.
Pharmacokinetics.
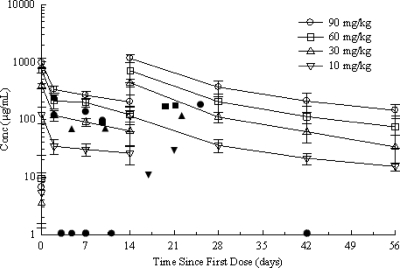
Mean patient preinfusion (endogenous) plasma anti-LTA concentrations were low and ranged from 3.49 to 9.44 μg/ml across the dose groups. Mean plasma anti-LTA concentrations increased in a dose-related manner (Fig. 1). In the 60-mg/kg and 90-mg/kg dose groups, following the second infusion of pagibaximab, an extrapolation of the serum antibody levels suggests that a sustained mean anti-LTA level over 500 μg/ml was observed for a period of approximately 6 and 12 days, respectively (Fig. 1). Six (85.7%) of eight patients in the 60-mg/kg dose group and 8 (100%) of 8 patients in the 90-mg/kg dose group had plasma anti-LTA concentrations over 500 μg/ml immediately after the day 14 pagibaximab infusion. In the 10-mg/kg and 30-mg/kg dose groups, no patient and one (12.5%) of eight patients, respectively, had an antibody concentration over 500 μg/ml immediately after the day 14 infusion. One patient in the 90-mg/kg dose group had an antibody concentration over 500 μg/ml on day 28.
FIG. 1.
Plasma anti-LTA antibody concentrations over time by pagibaximab dose group on a semilogarithmic axis. The values shown are means ± standard deviations. Each error bar represents 1 standard deviation. There were seven patients in the 10-mg/kg dose group with data for six patients included on day 56. There were eight patients in the 30-mg/kg dose group with data for seven patients included on day 42 and data for five patients included on day 56. There were eight patients in the 60-mg/kg dose group with data for seven patients included on day 14, day 28, and day 42 and data for six patients included on day 56. There were eight patients in the 90-mg/kg dose group with data for six patients included on day 56. The individual symbols represent positive staphylococcal blood cultures and are plotted as the concentration (Conc) of pagibaximab in serum (as determined by direct measurement by an enzyme-linked immunosorbent assay) versus the time in days when the positive blood culture was drawn. ✹, placebo treatment group; ▾, 10-mg/kg dose group; ▴, 30-mg/kg dose group; ▪, 60-mg/kg dose group; •, 90-mg/kg dose group. All placebo symbols lie on the x axis, since there were no measurable serum antibody concentrations in this group. All staphylococcal infections occurred at an estimated serum anti-LTA concentration of <500 μg/ml.
Following i.v. infusion of pagibaximab, mean pharmacokinetic values across dose groups were independent of dose. Total plasma CL ranged from 0.32 to 0.43 ml per hour, mean V ranged from 182 to 285 ml, and mean t1/2 ranged from 369 to 599 h or 19 to 25 days. The pharmacokinetics of pagibaximab in premature infants therefore appeared linear at doses ranging from 10 to 90 mg/kg.
Examination of the mean plasma anti-LTA over time profile (Fig. 1) revealed that the decay after the first dose appeared to be similar to that after the second dose. However, this evaluation was limited by the time points available. Blood sampling for pharmacokinetic analysis was restricted by the small volume of blood available from the neonates.
Dose proportionality analysis showed that the linear regression of the log area under the plasma drug concentration-time curve versus the log total dose suggested that for these data, the doses were proportional with the estimated slope of 0.92, the 95% confidence interval of 0.78 to 1.06, and a P value of <0.0001.
One patient in the 90-mg/kg dose group received only the first dose of pagibaximab, but blood samples were collected for the entire 56-day period. This patient's pharmacokinetic parameters for CL (0.347 ml/h), V (241 ml), and t1/2 (481 h) were consistent with the rest of the 90-mg/kg group, suggesting that the pharmacokinetics of pagibaximab were consistent after one or two doses.
Safety analyses.
All safety data were reviewed throughout the study by an independent medical monitor, the safety monitoring committee, and an external data safety monitoring board, and there were no safety concerns.
Adverse events.
Fifty-two (98%) of the 53 patients in the ITT population experienced at least one AE during the study. AEs experienced by patients in this study were consistent with events known to occur with prematurity and in low-birth-weight neonates (2). The AEs most commonly reported in study patients were anemia and hyperkalemia, with 38 (71.7%) and 29 (54.7%) of 53 patients, respectively, experiencing at least one episode. The percentages of patients experiencing the most common AEs were generally similar in the treatment groups (Table 2). There was no trend toward increased frequency of clinical or laboratory AE with increased pagibaximab dose.
TABLE 2.
Adverse events occurring in ≥10% of patients in the intent-to-treat population by treatment group
| Adverse event | No. (%) of patients who experienced the adverse event in treatment groupa
|
||
|---|---|---|---|
| Pagibaximab (10, 30, 60, or 90 mg/kg) (n = 33) | Placebo (n = 20) | Total (all groups) (n = 53) | |
| Anemia | 24 (72.7) | 14 (70.0) | 38 (71.7) |
| Hyperkalemia | 17 (51.5) | 12 (60.0) | 29 (54.7) |
| Apnea | 12 (36.4) | 5 (25.0) | 17 (32.1) |
| Increased serum alkaline phosphatase | 6 (18.2) | 9 (45.0) | 15 (28.3) |
| Respiratory distress | 11 (33.3) | 4 (20.0) | 15 (28.3) |
| Intraventricular hemorrhage | 6 (18.2) | 7 (35.0) | 13 (24.5) |
| Conjugated bilirubinemia | 7 (21.2) | 5 (25.0) | 12 (22.6) |
| Thrombocytopenia | 8 (24.2) | 4 (20.0) | 12 (22.6) |
| Hyponatremia | 5 (15.2) | 5 (25.0) | 10 (18.9) |
| Unconjugated hyperbilirubinemia | 5 (15.2) | 3 (15.0) | 8 (15.1) |
| Hypotension | 6 (18.2) | 2 (10.0) | 8 (15.1) |
| Oxygen desaturation | 5 (15.2) | 3 (15.0) | 8 (15.1) |
| Increased blood urea nitrogen | 2 (6.1) | 4 (20.0) | 6 (11.3) |
| Neutropenia | 5 (15.2) | 1 (5.0) | 6 (11.3) |
n indicates the number of patients in the treatment group.
One AE, moderate oxygen supplementation, was assessed by the investigator as probably related to study drug infusion. This event, experienced by a patient in the 90-mg/kg pagibaximab group, occurred immediately after the second study drug infusion and resolved in 1 h. All other AEs were considered by the investigators as either unrelated or probably not related to study drug.
Serious adverse events.
Twenty-three (43%) of 53 patients in the ITT population experienced at least one SAE during the study. Cholestasis was the most common SAE, with 10 patients (18.9%) reporting at least one episode. Other SAEs occurring in ≥5% of patients in the ITT population included necrotizing enterocolitis (NEC) and sepsis due to an identified organism (seven patients, or 13.2%, each), and hyperkalemia and thrombocytopenia (three patients, or 5.7%, each).
The SAEs experienced by patients in this study were generally similar across treatment groups. No trend toward increased frequency of SAEs with increasing pagibaximab dose was observed. All SAEs reported for patients in this study were recognized comorbidities associated with prematurity, and all were assessed by the investigators as either unrelated or probably not related to study drug.
Significant clinical outcomes.
In order to assess any potential adverse effect of pagibaximab on clinical events known to occur at high frequency in low-birth-weight neonates, the frequency of patients experiencing NEC (Bell's stage 2 or greater) (7), bronchopulmonary dysplasia (oxygen dependency at 36 weeks postmenstrual age) (6), severe intraventricular hemorrhage (Papille's grade 3 or 4) (25), retinopathy of prematurity requiring surgery, and death were summarized by treatment group. The percentages of patients in the pagibaximab and placebo treatment groups experiencing significant clinical outcomes were generally similar for bronchopulmonary dysplasia (57.6% versus 66.6%, respectively), NEC (15.2% versus 11.1%, respectively), retinopathy of prematurity requiring surgery (3.0% versus 10.0%, respectively), and death (9.1% versus 5.0%, respectively). A severe intraventricular hemorrhage was experienced by 3.0% of patients in the pagibaximab group and 20% of patients in the placebo group (P = 0.061). The number of patients experiencing significant clinical outcomes in the individual treatment groups was small; however, no trend toward increased frequency of any significant clinical event with increased pagibaximab dose was observed.
Deaths.
Four (7.5%) of the 53 patients in the ITT population died during the study, including three (9.1%) of 33 patients receiving pagibaximab and one (5.0%) of 20 patients receiving placebo (P = 1.00). A second patient in the placebo group died 7 months after completing the study follow-up period.
One patient in the pagibaximab treatment group died on study day 21 due to NEC and sepsis. This infant received the first dose of pagibaximab (10 mg/kg) on study day 0 and did not receive the second dose because of failure to fulfill the eligibility criteria. A second patient in the pagibaximab treatment group died on study day 5 due to severe hyaline membrane disease and subsequent NEC, and no organism was identified. This infant received the first dose of pagibaximab (10 mg/kg) on study day 0 and died prior to receiving the second dose. A third patient in the pagibaximab treatment group died on study day 11 from sepsis. This infant received the first dose of pagibaximab (60 mg/kg) on study day 0 and died prior to administration of the second dose.
One patient in the placebo group died on study day 36 from sepsis, NEC, and prematurity resulting in multiple organ failure. This infant received the first dose of placebo (as part of the 10-mg/kg dose group) on study day 0 and did not receive the second dose because of failure to fulfill the eligibility criteria. A second patient in the placebo group died 7 months after completing the study follow-up period. The immediate cause of death was cardiopulmonary failure secondary to multiple organ system insufficiency and extreme prematurity.
None of these deaths was considered by the investigators to be attributable to study drug. All of the events resulting in death are known to be associated with premature infants with very low birth weight.
HAMA/HACA analysis.
Concentrations of HAMA/HACA were relatively unchanged for all patients across treatment groups throughout the study and remained well below the upper normal limit (52 ng/ml) from predose to postdose.
Vital signs, physical examinations, and clinical chemistry/hematology/urinalysis.
In all treatment groups, patient infusion vital signs showed normal variability. Noninfusion vital signs showed no indication of a dose response effect. Systolic pressure and diastolic pressure increased with age, as expected for this population, and were similar across treatment groups. Heart rate and respiratory rate showed normal variability for all treatment groups. Temperature was stable over time for all treatment groups. The median body weight increased from approximately 1,000 to 2,140 g over the study period; all dose groups showed the same tendency. Variability in all laboratory results over time was consistent with premature newborn parameters.
Opsonophagocytic activity.
Pagibaximab enhanced the opsonophagocytic (bacterial killing) activity in serum (Table 3). An increase in opsonophagocytic activity was demonstrated at the lowest dose level (10 mg/kg) and was increased at the higher dose levels. There did not appear to be a significant difference in activity between the 30-, 60-, and 90-mg/kg groups; however, only a single serum dilution of these samples was tested. Differences may have been observed at higher dilutions, but not enough serum was available for testing. In contrast, minimal or no opsonophagocytic activity was observed in patients treated with placebo.
TABLE 3.
Opsonophagoctic activity (bacterial killing) in serum of neonates over time against S. epidermidis ATCC strain 55133
| Pagibaximab treatment group | Mean opsonophagoctic activity (% bacterial killing) (SEM) on:
|
|||||
|---|---|---|---|---|---|---|
| Study day 0
|
Study day 14
|
Study day 28 | Study day 42 | |||
| Before the first infusion | 1 h after the first infusion | Before the second infusion | 1 h after the second infusion | |||
| 10 mg/kg/dose | 26.7 (24.7) | 37.2 (18.2) | 24.1 (7.3) | 46.9 (14.1) | 29.4 (9.1) | 20.9 (6.2) |
| 30 mg/kg/dose | 0 (0) | 69.4 (7.9) | 42.1 (10.5) | 68.4 (10.6) | 48.5 (10) | 35.3 (7.7) |
| 60 mg/kg/dose | 0 (0) | 60 (11.5) | 44.8 (9.1) | 56.7 (13.2) | 50.1 (10.7) | 38.6 (7.9) |
| 90 mg/kg/dose | 12 (12) | 71.1 (9.2) | 46.4 (9.4) | 51.7 (14.5) | 49.3 (11.1) | 45.4 (9) |
Clinical signs and symptoms leading to evaluation of sepsis.
Sepsis evaluations were performed in 51 of the 53 patients in the ITT population. The most common signs and symptoms leading to evaluation for sepsis were similar across treatment groups. The most common clinical signs and symptoms leading to evaluation of sepsis were similar for patients in the pagibaximab and placebo treatment groups, with apnea/bradycardia accounting for 23.7% and 26.2% of events, respectively, and cyanosis accounting for 18.3% and 18.5% of events, respectively. Overall, no dose response effect upon the frequency of signs and symptoms leading to evaluation of sepsis was observed.
Sepsis.
Twenty-seven (50.9%) of the 53 patients, including 16 of 33 (48.5%) patients in the pagibaximab treatment group and 11 of 19 (55%) patients in the placebo treatment group, experienced at least one sepsis episode. Three patients each in the pagibaximab treatment group (9.1%) and the placebo treatment group (15%) experienced a second episode of sepsis. Four patients each in the pagibaximab treatment group (12.1%) and the placebo treatment group (20%) experienced sepsis with multiple organisms. Coagulase-negative staphylococcus was the most common organism (40.5%) isolated from blood cultures in patients with sepsis in both the pagibaximab and placebo treatment groups, and only one patient in the 60-mg/kg pagibaximab group experienced Staphylococcus aureus (2.4%) sepsis as part of a mixed infection with CONS (Fig. 1). Sixteen nonstaphylococcal sepsis events occurred in both the pagibaximab (n = 7; 21.2%) and placebo (n = 9; 45%) treatment groups (P = 0.12). The organisms isolated from these blood cultures were Enterococcus (14.3%), Candida (7.1%), Escherichia coli (7.1%), Klebsiella (7.1%), Pseudomonas (7.1%), Enterobacter (4.8%), Serratia (4.8%), Acinetobacter (2.4%), and Streptococcus agalactiae (2.4%) and did not differ significantly between groups.
Sepsis caused by CONS.
Sixteen (31%) of 53 patients experienced sepsis caused by CONS, including 11 (33.3%) of 33 patients receiving pagibaximab and five (25%) of 20 patients receiving placebo (P = 0.76). One patient in the 30-mg/kg pagibaximab group (9.1%) experienced a second episode of sepsis caused by CONS. Although analysis by pagibaximab dose level showed a slightly greater proportion of patients in the 90-mg/kg pagibaximab group experiencing sepsis caused by CONS (4 of 9 patients; 44%) compared with those in the other treatment groups, statistical testing using Fisher's exact test showed no overall difference between dose groups (P value of 0.9).
Of the 16 patients with sepsis caused by CONS, 15 experienced definite (10 patients, 63%) or probable (5 patients, 31.3%) sepsis. No patient experienced possible sepsis caused by CONS. One patient receiving pagibaximab at the 90-mg/kg dose level experienced line sepsis caused by CONS. In all cases, estimated or observed plasma anti-LTA levels were below the putative protective level of 500 μg/ml at the time of diagnosis of CONS-caused sepsis. The species identification of the isolates in the 16 patients with sepsis caused by CONS revealed substantial variation with sepsis caused by S. epidermidis in 11 patients, and in 1 patient each by Staphylococcus simulans, Staphylococcus caprae, mixed infection of S. epidermidis and Staphylococcus hominus, mixed infection of S. epidermidis and Staphylococcus haemolyticus, and mixed infection of S. epidermidis and S. aureus.
Age at diagnosis of first episode of sepsis caused by CONS.
The mean age of patients at the diagnosis of the first episode of sepsis caused by CONS ranged from 11.5 to 22.5 days across treatment groups. In the 10-mg/kg pagibaximab treatment group, the mean age at diagnosis of the first episode was 22.5 days, in the 30-mg/kg treatment group, it was 11.5 days, in the 60-mg/kg treatment group, it was 16.0 days, and in the 90-mg/kg treatment group, it was 16.0 days. In the placebo treatment group, the mean age at first diagnosis of CONS sepsis was 17.8 days.
Opsonizability of CONS by pagibaximab.
Of 25 staphylococcal isolates recovered from the blood cultures of 16 patients with staphylococcal infection, pagibaximab demonstrated bacterial killing (opsonophagocytic assay) against all the isolates. However, there was distinct heterogeneity in the ability of antibody to opsonize the different isolates. Whereas some isolates were opsonized at a concentration of less than 50 μg/ml, others required 400 μg/ml. At pagibaximab concentrations of 400 μg/ml, 18 (67%) isolates demonstrated >90% bacterial killing, 21 (78%) isolates demonstrated >80% bacterial killing, and 24 (89%) isolates demonstrated >70% bacterial killing.
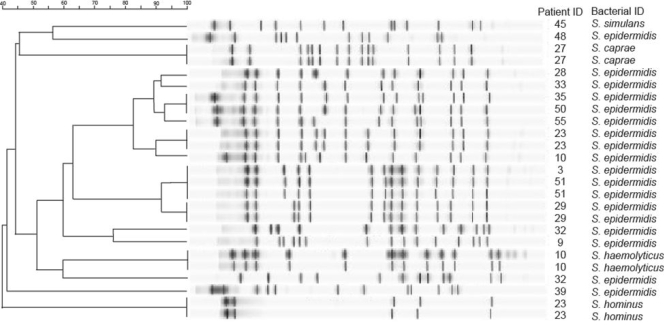
Dendrogram of CONS.
We also analyzed these 25 CONS bacterial isolates for genetic relatedness. The dendrogram (Fig. 2) of these isolates (using a similarity coefficient of 80%) suggests that the strains of CONS varied substantially and were generally unrelated between patients who were infected, even in the same hospital (data not shown). There appeared to be two clusters that were closely related: patients 28, 33, 35, 50, 55, 23, and 10 from the same hospital and patients 3, 51, and 29 from two different hospitals in New York, NY, and Houston, TX. When paired cultures for the same sepsis episode were tested (n = 5), all of these pairs appeared to be related. Although one patient (patient 23) appeared to have two species in each culture, the strains in the two cultures appeared related. The other patient (patient 10) appeared to have two species in one culture and one species in the other culture, and the species in the two cultures appeared to be related.
FIG. 2.
PFGE patterns for genetic relatedness of the 25 CONS isolates. In the far right columns, the patient identification (ID) number and bacterial species identification for each staphylococci were listed for each isolate's dendrogram. The degree of strain relatedness from 40 to 100% is shown by the scale bar.
DISCUSSION
Mean preinfusion (endogenous) plasma anti-LTA antibody levels were found to be negligible in the VLBW neonates in this study. This may be because premature infants do not receive their normal transplacental passage of antibody which occurs predominantly in the final weeks of pregnancy (5), or the immaturity of the premature neonatal immune system makes it unlikely that they would mount a significant antibody response following exposure in utero or in the first few days of life (32). A direct correlation between low serum levels of IgG and an increased risk of late-onset neonatal sepsis has been shown (13, 16). Thus, VLBW neonates are unlikely to possess functional opsonophagocytic activity to staphylococci at birth or during hospitalization, making them a population at high risk of staphylococcal sepsis. Therefore, passive immunization with pagibaximab could be a potentially important step in preventing all neonatal staphylococcal infection. This may be especially important in methicillin (meticillin)-resistant (community and hospital acquired) and methicillin-sensitive S. aureus infections, which although these infections occur less frequently than CONS infections, they result in greater morbidity and mortality than CONS infection in the premature infant (10, 11, 31).
The pharmacokinetics of pagibaximab in premature neonates was dose proportional over doses ranging from 10 to 90 mg/kg. The mean t1/2 ranged from 19 to 25 days across the dose groups and is consistent with previous reports of IgG infusions in neonates (35, 36). This is also consistent with results from a previous study of pagibaximab in healthy human adults (38), with other studies of human/mouse chimeric or full human IgG1 antibodies administered i.v. in adults (4, 9, 22, 29), and commercially available intravenous immunoglobulin in neonates (35, 36). Moreover, after the second infusion of pagibaximab at 60 or 90 mg/kg, mean anti-LTA levels greater than 500 μg/ml, the putative protection level for staphylococcal sepsis, were observed. With the 90-mg/kg dose achieving more sustained levels greater than 500 μg/ml, it suggests that higher or more frequent doses of pagibaximab may be appropriate for further study.
HAMA/HACA levels remained low in the neonates receiving i.v. pagibaximab at 10, 30, 60, and 90 mg/kg at study days 0 and 42, suggesting that pagibaximab did not elicit an antibody response to itself even after repeated doses. In addition, the AEs, SAEs, and clinical outcomes across study groups were not significantly different. This is also similar to previous reports of IgG safety and tolerability in neonates (35, 36). This study suggests that the first use of pagibaximab in VLBW neonates at 10, 30, 60, and 90 mg/kg administered i.v. twice 2 weeks apart appeared safe and well tolerated.
CONS was the most common cause of sepsis in the VLBW neonates in this study, with an incidence of 30.2% across treatment groups, and less than 2% of patients developed S. aureus sepsis. These findings are consistent with the results of previous larger studies of late-onset sepsis in VLBW infants (3, 10, 31) that demonstrated CONS in 14 to 23% of patients and S. aureus in 1.6 to 5% of patients. The majority (63%) of sepsis cases caused by CONS in this study were confirmed by two or more peripheral blood cultures growing CONS. There was no significant difference in incidence rates of sepsis caused by CONS across dose levels of pagibaximab and placebo, overall or by category of infection. Given the small number of patients in each treatment group in this study, no definitive conclusions can be reached regarding the effect of pagibaximab. Larger studies of pagibaximab in VLBW neonates are needed to demonstrate any potential effect for prevention of staphylococcal sepsis in the target population.
Patients receiving 60 or 90 mg/kg of pagibaximab were observed to have sustained plasma anti-LTA levels above the putative protection level of 500 μg/ml following the second dose, so further evaluation of the product with larger and/or more frequent doses should be considered. However, at the time of diagnosis of sepsis caused by CONS, all affected patients had estimated or observed plasma anti-LTA levels below 500 μg/ml. Thus, further evaluation of pagibaximab should focus on dosing regimens that can produce plasma anti-LTA levels over 500 μg/ml for the potential prevention of staphylococcal sepsis.
Acknowledgments
L. E. Weisman, J. A. Garcia-Prats, J. H. Schneider, and M. Nesin received research support, W. G. Kramer received a consulting fee, and L. E. Weisman is a consultant for the sponsor Biosynexus Incorporated.
We thank Karen Adams, George T. Mandy, and Karen E. Johnson (Department of Pediatrics, Baylor College of Medicine, Houston, TX) for their clinical and administrative assistance; Martin Ottolini (Department of Pediatrics, Uniformed Services University of Health Sciences, Bethesda, MD) for his work as study medical monitor; Bonnie LaFleur (Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN) for her statistical assistance and work on the data safety monitoring board; and Gerald B. Merenstein (University of Colorado of the Health Sciences, Aurora, CO) and Laurence B. Givner (Bowman Gray Medical School, Winston-Salem, NC) for their work on the data safety monitoring board.
Footnotes
Published ahead of print on 20 April 2009.
REFERENCES
- 1.Angus, D. C., M. C. Birmingham, R. A. Balk, et al. 2000. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. JAMA 283:1723-1730. [DOI] [PubMed] [Google Scholar]
- 2.Avery, G. B., M. A. Fletcher, and M. G. MacDonald. 1999. Neonatology: pathophysiology and management of the newborn, 5th ed., p. 1501-1534. Lippincott Williams & Wilkins, Philadelphia, PA.
- 3.Aziz, K., D. D. McMillan, W. Andrews, et al. 2005. Variations in rate of nosocomial infection among Canadian neonatal intensive care units may be practice-related. BMC Pediatr. 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma, J., T. Kurimoto, S. Tsuji, et al. 1991. Phase I study on human monoclonal antibody against cytomegalovirus: pharmacokinetics and immunogenicity. J. Immunother. 10:278-285. [DOI] [PubMed] [Google Scholar]
- 5.Ballow, M., K. L. Cates, J. C. Rowe, et al. 1986. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr. Res. 20:899-904. [DOI] [PubMed] [Google Scholar]
- 6.Bancalari, E., N. Claure, and I. R. Sosenko. 2003. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin. Neonatol. 8:63-71. [DOI] [PubMed] [Google Scholar]
- 7.Bell, M. J., J. L. Ternberg, R. D. Feigin, et al. 1978. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie, S. B., K. E. Sands, J. E. Gray, et al. 2000. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr. Infect. Dis. J. 19:56-62. [DOI] [PubMed] [Google Scholar]
- 9.Chow, F. S., L. J. Benincosa, S. B. Sheth, et al. 2002. Pharmacokinetic and pharmacodynamic modeling of humanized anti-factor IX antibody (SB 249417) in humans. Clin. Pharmacol. Ther. 71:235-245. [DOI] [PubMed] [Google Scholar]
- 10.DeJonge, M., D. Burchfield, B. Bloom, et al. 2007. Clinical trial of safety and efficacy of IHN-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J. Pediatr. 151:260-265. [DOI] [PubMed] [Google Scholar]
- 11.Derkx, B., J. Wittes, R. McCloskey, and the European Pediatric Meningococcal Septic Shock Trial Study Group. 1999. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. Clin. Infect. Dis. 28:770-777. [DOI] [PubMed] [Google Scholar]
- 12.Eady, E. A., and J. H. Cove. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16:103-124. [DOI] [PubMed] [Google Scholar]
- 13.Fanaroff, A. A., S. B. Korones, L. L. Wright, et al. 1998. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. Pediatr. Infect. Dis. J. 17:593-598. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, G. W., T. J. Cieslak, S. R. Wilson, L. E. Weisman, and V. G. Hemming. 1994. Opsonic antibodies to Staphylococcus epidermidis: in vitro and in vivo studies using human intravenous immune globulin. J. Infect. Dis. 169:324-329. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2:171-179. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone, I. M., R. A. Ehrenkranz, S. C. Edberg, et al. 1990. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr. Infect. Dis. J. 9:819-825. [DOI] [PubMed] [Google Scholar]
- 17.Gray, J. E., D. K. Richardson, M. C. McCormick, et al. 1995. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use and outcome. Pediatrics 95:225-230. [PubMed] [Google Scholar]
- 18.Grundling, A., and O. Schneewind. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 104:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann, C., I. Spreitzer, N. W. J. Schröder, et al. 2002. Cytokine induction by purified lipoteichoic acids from various bacterial species-role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur. J. Immunol. 32:541-551. [DOI] [PubMed] [Google Scholar]
- 20.Jones, R. N. 1996. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn. Microbiol. Infect. Dis. 25:153-161. [DOI] [PubMed] [Google Scholar]
- 21.Kloos, W. E., and J. F. Wolfshohl. 1982. Identification of Staphylococcus species with the API STAPH-IDENT system. J. Clin. Microbiol. 16:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LoBuglio, A. F., R. H. Wheeler, J. Trang, et al. 1989. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc. Natl. Acad. Sci. USA 86:4220-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papile, L. A., J. Burstein, R. Burstein, and H. Koffler. 1978. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92:529-534. [DOI] [PubMed] [Google Scholar]
- 26.Raimundo, O., H. Heussler, J. B. Bruhn, et al. 2002. Molecular epidemiology of coagulase negative staphylococcal bacteremia in a newborn intensive care unit. Hosp. Infect. 51:33-42. [DOI] [PubMed] [Google Scholar]
- 27.Raynor, R. H., D. F. Scott, and G. K. Best. 1981. Lipoteichoic acid inhibition of phagocytosis of Staphylococcus aureus by human polymorphonuclear leukocytes. Clin. Immunol. Immunopathol. 19:181-189. [DOI] [PubMed] [Google Scholar]
- 28.Smith, T. L., M. L. Pearson, K. R. Wilcos, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 29.Solomon, A., T. A. Waldmann, and J. L. Fahey. 1963. Clinical and experimental metabolism of normal 6.6s gamma-globulin in normal patients and in patients with macroglobulinemia and multiple myeloma. J. Lab. Clin. Med. 62:1-17. [PubMed] [Google Scholar]
- 30.Srinivasan, A., J. D. Dick, and T. M. Perl. 2002. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 15:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll, B. J., N. Hansen, A. A. Fanaroff, et al. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 32.Strunk, T., P. Richmond, K. Simmer, A. Currie, O. Levy, and D. Burgner. 2007. Neonatal immune responses to coagulase-negative staphylococci. Curr. Opin. Infect. Dis. 20:370-375. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian, K. N., L. E. Weisman, T. Rhodes, et al. 1998. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 17:110-115. [DOI] [PubMed] [Google Scholar]
- 34.Tegnell, A. 2003. Study of developed resistance due to antibiotic treatment of coagulase-negative staphylococci. Microb. Drug Resist. 9:1-6. [DOI] [PubMed] [Google Scholar]
- 35.Weisman, L. E., G. W. Fischer, V. G. Hemming, et al. 1986. Pharmacokinetics of intravenous immunoglobulin (Sandoglobulin) in neonates. Pediatr. Infect. Dis. J. 5(3 Suppl.):S185-S188. [PubMed] [Google Scholar]
- 36.Weisman, L. E., G. W. Fischer, P. Marinelli, et al. 1989. Pharmacokinetics of intravenous immunoglobulin in neonates. Vox Sang. 57:243-248. [DOI] [PubMed] [Google Scholar]
- 37.Weisman, L. E., R. F. Schuman, E. Lukomska, J. R. Stinson, O. Parks, and G. W. Fischer. 2001. Effectiveness and pharmacokinetics of an anti-lipoteichoic acid humanized mouse chimeric monoclonal antibody. Pediatr. Res. 49:301A. [Google Scholar]
- 38.Weisman, L. E., G. W. Fischer, H. M. Thackray, et al. 2009. Safety and pharmacokinetics of a chimerized anti-lipoteichoic acid monoclonal antibody in healthy adults. Int. Immunopharmacol. 9:639-644. [DOI] [PubMed] [Google Scholar]