Abstract
We investigated the activities of telavancin and vancomycin against biofilm-producing Staphylococcus and Enterococcus strains. At clinically attainable concentrations, telavancin was active against bacteria embedded in biofilm (minimal biofilm eradication concentration [MBEC], 0.125 to 2 μg/ml) and inhibited biofilm formation at concentrations below the MIC. Vancomycin did not demonstrate the same activity (MBEC, ≥512 μg/ml) against Staphylococcus aureus and Enterococcus faecalis. Telavancin may have a unique role in biofilm-associated infections.
Staphylococci and enterococci account for a large proportion of hospital-acquired infections, especially among patients with indwelling devices (17). These infections are often caused by biofilm-producing strains which are difficult to eradicate and which may cause bacteremia and metastatic infections (30).
Telavancin is a new lipoglycopeptide antimicrobial agent with a core chemical structure similar to that of vancomycin yet modified to include a lipophilic side chain (15). Telavancin possesses a second mechanism of action that causes rapid depolarization and loss of the functional integrity of the bacterial membrane (1, 12). These two mechanisms of action may be implicated in the lower range of methicillin (meticillin)-resistant Staphylococcus aureus (MRSA) MICs observed with telavancin (MIC range, 0.06 to 1.0 μg/ml) compared to vancomycin (MIC range, 0.5 to 2 μg/ml) (8, 13).
Previous reports have suggested that telavancin is more effective than vancomycin against biofilm-forming S. aureus in a pharmacokinetic filter model (9). We compared telavancin and vancomycin activities by using previously described in vitro biofilm activity assays. The first assay evaluated each agent's activity in a preformed biofilm by using both a modified version and the standard version of the Calgary Biofilm Pin Lid Device (CBPD) (3, 14). The second assay evaluated activity in preventing biofilm formation by planktonic isolates (14).
(This work was presented in part at the 48th annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 24 to 28 October 2008.)
Telavancin and vancomycin analytical powder, freshly prepared each day, was obtained from Astellas Pharmaceuticals, Inc. (Audubon, PA), and Sigma-Aldrich (St. Louis, MO), respectively. Well-characterized biofilm-producing reference strains of S. aureus (ATCC 35556), Staphylococcus epidermidis (RP62A; ATCC 35984), and Enterococcus faecalis (ATCC 29212; vancomycin susceptible) were evaluated. A stable, slime-negative mutant, M7, from wild-type S. epidermidis RP62A served as a control (21). We also evaluated three randomly selected, biofilm-producing clinical isolates (methicillin-susceptible S. aureus [MSSA] L2, and MRSA L32 and L83) previously obtained from patients with catheter-related bloodstream infections at the Providence Veterans Affairs Medical Center.
The medium used for biofilm growth was Bacto tryptic soy broth (TSB; Becton Dickinson, Sparks, MD) plus 1% glucose and 2% NaCl (14). All assays were run in quadruplicate, and cultures were incubated at 35°C. Conventional MICs and minimal bactericidal concentrations (MBCs) were determined in duplicate by using Clinical and Laboratory Standards Institute (CLSI) guidelines (5, 6).
We used a modified version of the CBPD to determine the antimicrobial susceptibility of bacteria embedded in a 24-h biofilm to determine the minimum biofilm inhibitory concentration (MBIC) and the minimum biofilm eradication concentration (MBEC) (3). Briefly, a starting inoculum of 7 log10 CFU/ml was established by the direct colony suspension method from a 24-h tryptic soy agar (TSA; Becton Dickinson, Sparks, MD) plate. This inoculum was then standardized with McFarland standards and validated by determination of viable counts on TSA plates. Biofilms developed on the pin lid (Immuno TSP; Nunc, Roskilde, Denmark) submerged in the inoculated broth at 35°C for 24 h on a rocking table (Boekel Shake and Bake; Boekel Scientific; Feasterville, PA). The pin lid was then rinsed three times in 1× phosphate-buffered saline (PBS) to remove sessile bacteria before placement into fresh uninoculated broth containing serial dilutions of each antibiotic and incubated for 24 h at 35°C. The next day, the pin lid was removed and the MBIC was recorded and defined as the last well in which there is no visible growth after incubation in the presence of biofilm and antibiotic. Next, to obtain the MBEC, the pin lid was rinsed in 1× PBS and then sonication (10 min) was performed on a low-output sonicator (45 Hz) in order to disperse the bacteria from the pin surface. After sonication, the broth was vortexed for 30 s. The fluid was serially diluted and plated in duplicate on TSA, and the number of CFU per milliliter was determined. The limit of detection was 2.4 CFU/ml. Viable counts were also obtained by measuring the turbidity at 570 nm (A570) on a 96-well plate reader.
Quantification of biofilm formation in the presence of telavancin (0 to 16 μg/ml) and vancomycin (0 to 16 μg/ml) was conducted with a previously described colorimetric microtiter plate assay (4, 14, 24). Wells with sterile TSB alone served as negative controls, and the mean optical density (OD) values of these wells was subtracted from the OD values of the test wells.
Following incubation, the liquid was gently aspirated and replaced with sterile PBS (pH 7.3). Each well was rinsed three times and air dried. Adherent bacteria were then stained with crystal violet. The OD at 570 nm (OD570) of stained adherent bacterial films was read with a spectrophotometer (Synergy 2; Bio-Tek Instruments, Inc., Winooski, VT). The ODs of bacterial films were classified into nonadherent, weakly, moderately, and strongly adherent categories based on multiples of the OD readings as described by Stepanović et al. The test was carried out in quadruplicate. Results were averaged, and standard deviations were calculated (23).
Among planktonic bacteria, the ranges of telavancin MICs and MBCs were 0.03 to 0.25 and 0.125 to 1 μg/ml, respectively. The ranges of vancomycin MICs and MBCs were 1 to 2 and 2 to 16 μg/ml, respectively (Table 1). When evaluating the activity of sessile bacteria seeding the medium from formed biofilm, the MBICs of telavancin and vancomycin were 0.25 to 1 and 4 to 16 μg/ml, respectively (Table 1). Overall, the telavancin and vancomycin MBICs for each bacterial isolate were the same as the corresponding MICs or up to 3 dilutions higher.
TABLE 1.
Susceptibility testing results for planktonic and adherent (biofilm) strains
| Antibiotic and strain | Planktonic organisms tested by CLSI guidelines
|
MBIC (μg/ml)a for sessile bacteria seeding from formed biofilm | MBEC (μg/ml) based on A570b for CBPD activity in formed biofilm | |
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | |||
| Telavancin | ||||
| S. epidermidis M7 (non-BFc control) | 0.125 | 0.125 | 0.5 | 0.5 |
| S. epidermidis RP62A (ATCC 35984) | 0.125 | 0.125 | 0.5 | 1 |
| S. aureus ATCC 35556 | 0.06 | 0.25 | 0.25 | 0.125 |
| E. faecalis ATCC 29212 | 0.25 | 1 | 1 | 1 |
| MSSA L2 (clinical isolate) | 0.06 | 0.125 | 1 | 1 |
| MRSA L32 (clinical isolate) | 0.03 | 0.125 | 1 | 2 |
| MRSA L83 (clinical isolate) | 0.06 | 0.125 | 0.25 | 1 |
| Vancomycin | ||||
| S. epidermidis M7 (non-BF control) | 1 | 1 | 8 | 2 |
| S. epidermidis RP62A (ATCC 35984) | 2 | 2 | 4 | 4 |
| S. aureus ATCC 35556 | 1 | 2 | 16 | >256 |
| Enterococcus faecalis ATCC 29212 | 2 | 16 | 16 | >256 |
| MSSA L2 (clinical isolate) | 2 | 2 | 16 | >256 |
| MRSA L32 (clinical isolate) | 1 | 2 | 8 | >256 |
| MRSA L83 (clinical isolate) | 2 | 2 | 4 | >256 |
The values were obtained by determination of plate counts, and the limit of detection was 2.4 CFU/ml.
The values were obtained by measuring turbidity at 570 nm (A570) on a 96-well plate reader.
BF, biofilm.
As in previous reports, vancomycin demonstrated no activity against bacteria embedded in a biofilm produced by S. aureus (MBEC of ≥512 μg/ml; Table 1) (3). However, it is interesting that telavancin demonstrated activity against these bacteria with MBECs of 0.125 to 2 μg/ml. Thus, clinical doses of telavancin (93% of the drug is protein bound; the calculated maximum concentration of unbound drug in serum is 6.1 μg/ml) will exceed the MBIC and MBEC and should result in activity against bacteria embedded in a biofilm.
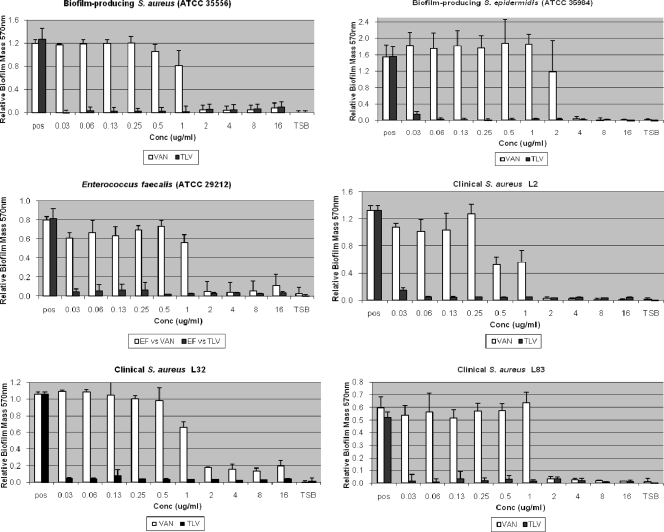
To quantify biofilm formation in the presence of each agent (Fig. 1), we first characterized the biofilm production of each isolate in the absence of any drug. Established biofilm producers MSSA (ATCC 35556) and S. epidermidis (ATCC 35984) demonstrated the most robust slime formation at an OD570 of 1.4 ± 0.23 (strongly adherent). The clinical isolates consistently produced biofilm (OD570 of 1.0 ± 0.41; moderately adherent), and the non-biofilm-forming control isolate (M7) did not produce biofilm (OD570 of 0.19 ± 0.008; nonadherent).
FIG. 1.
Effects of telavancin and vancomycin on biofilm formation. Biofilm-forming S. aureus, S. epidermidis, and E. faecalis strains were grown in 96-well polystyrene plates exposed to increasing concentrations of telavancin or vancomycin. Results are averages of four duplicates ± the standard error of the mean. TSB, negative control wells containing TSB only; pos, positive control wells (no drug).
Telavancin inhibited biofilm formation at concentrations below each isolate's respective MIC. In contrast, vancomycin inhibited biofilm formation at concentrations at or above each isolate's respective MIC.
At clinically achievable concentrations, telavancin was active against bacteria embedded in biofilm (MBEC) and bacteria seeding from a formed biofilm mass (MBIC) and inhibited biofilm development (Fig. 1). Vancomycin did not demonstrate this same activity in a formed biofilm at clinically achievable concentrations.
Telavancin's activity against bacteria embedded in biofilm may be explained by its chemical composition as a lipoglycopeptide (15), its multiple mechanisms of action including disruption of bacterial membrane function (12), and its activity against stationary bacteria (10).
Telavancin is a semisynthetic derivative of the glycopeptide vancomycin; however, it differs from vancomycin by additional hydrophobic and hydrophilic moieties (15). Specifically, telavancin contains a lipophilic decylaminoethyl side chain attached to the vancosamine sugar, as well as a hydrophilic [(phosphonomethyl)aminomethyl] group on the 4′ position of amino acid 7 (15). These substituents in the molecule classify this agent as a lipoglycopeptide. Telavancin has two proposed mechanisms of action. The first mechanism is similar to that established for vancomycin and involves telavancin binding in a highly specific, noncovalent fashion to the terminal d-Ala-d-Ala stem peptides. As a consequence of this binding, the cross-linking step in the cell wall is inhibited (12). The second proposed mechanism of action involves depolarization of the bacterial membrane. This results in disruption of the functional integrity of the bacterial membrane, causing rapid, concentration-dependent depolarization of the plasma membrane, increased permeability, and leakage of cellular ATP and K+ (12).
Bacteria embedded within a biofilm are difficult to eradicate due to a wide variation of nutrient gradients that slow or arrest bacterial growth, protein synthesis, and other physiologic activities (29). Slow-growing or nongrowing bacteria sequestered in biofilm are less susceptible to antibiotics by virtue of their reduced growth rates (2, 29). Although telavancin has been shown to have activity against stationary bacteria (10), the MBICs for sessile bacteria seeding the medium from an established biofilm required higher antimicrobial concentrations for inhibition compared to the MICs for planktonic bacteria.
Telavancin exerts concentration-dependent killing, and the pharmacodynamic index that best correlates with its antimicrobial effect is the area under the concentration-time curve over 24 h (AUC0-24) divided by the MIC (11). Maximal bacterial killing is observed at a maximal concentration of the drug in serum of 40 μg/ml and with an AUC0-24/MIC ratio of 404 (18). An achievable serum concentration of the clinically recommended intravenous telavancin dose of 10 mg/kg is 87.5 μg/ml, with an expected AUC0-24 of 604 ml/h/kg (22). Since 93% of the drug is protein bound, the calculated maximum concentration of unbound drug in serum is 6.1 μg/ml and the unbound-drug AUC is 42 ml/h/kg. Thus, clinical doses of telavancin (free drug) will exceed the MBIC and MBEC and should have activity against bacteria embedded in a biofilm.
Clinical data support the use of telavancin in the treatment of complicated skin and soft-tissue infections and hospital-acquired pneumonia infections (7, 25-27). Animal model data suggest efficacy in the treatment of bacteremia, endocarditis, meningitis, and pneumonia caused by gram-positive pathogens (1, 16, 19, 20, 28). Our findings suggest that future use of telavancin holds promise in treating infections caused by biofilm-producing staphylococci and enterococci and that it should be further evaluated for the treatment of biofilm-related infections such as salvaging colonized intravascular catheters with antibiotic lock therapy.
Acknowledgments
We thank Suzanne Woodmansee for technical assistance.
This research was supported in part by an unrestricted, investigator-initiated grant from Astellas Pharmaceuticals, Deerfield, IL.
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Attwood, R. J., and K. L. LaPlante. 2007. Telavancin: a novel lipoglycopeptide antimicrobial agent. Am. J. Health Syst. Pharm. 64:2335-2348. [DOI] [PubMed] [Google Scholar]
- 2.Brown, M. R., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-780. [DOI] [PubMed] [Google Scholar]
- 3.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Seventh edition: approved guideline M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing. Eighteenth edition: approved informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Corey, G. R. Studies of telavancin in cSSI. In Third International Symposium on Resistant Gram-Positive Infections. Niagara-on-the-Lake, Ontario, Canada; 10 October 2006.
- 8.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. Comparative surveillance study of telavancin activity against recently collected gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gander, S., A. Kinnaird, and R. Finch. 2005. Telavancin: in vitro activity against staphylococci in a biofilm model. J. Antimicrob. Chemother. 56:337-343. [DOI] [PubMed] [Google Scholar]
- 10.Hegde, S. S., N. Reyes, R. Skinner, and S. Difuntorum. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J. Antimicrob. Chemother. 61:169-172. [DOI] [PubMed] [Google Scholar]
- 11.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J. P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause, K. M., M. Renelli, S. Difuntorum, T. X. Wu, D. V. Debabov, and B. M. Benton. 2008. In vitro activity of telavancin against resistant gram-positive bacteria. Antimicrob. Agents Chemother. 52:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaPlante, K. L., and L. A. Mermel. 2007. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol. Dial. Transplant. 22:2239-2246. [DOI] [PubMed] [Google Scholar]
- 15.Leadbetter, M. R., S. M. Adams, B. Bazzini, P. R. Fatheree, D. E. Karr, K. M. Krause, B. M. Lam, M. S. Linsell, M. B. Nodwell, J. L. Pace, K. Quast, J. P. Shaw, E. Soriano, S. G. Trapp, J. D. Villena, T. X. Wu, B. G. Christensen, and J. K. Judice. 2004. Hydrophobic vancomycin derivatives with improved ADME properties: discovery of telavancin (TD-6424). J. Antibiot. (Tokyo) 57:326-336. [DOI] [PubMed] [Google Scholar]
- 16.Madrigal, A. G., L. Basuino, and H. F. Chambers. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3163-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 18.Odenholt, I., E. Lowdin, and O. Cars. 2007. Pharmacodynamic effects of telavancin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains in the presence of human albumin or serum and in an in vitro kinetic model. Antimicrob. Agents Chemother. 51:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes, N., R. Skinner, B. M. Benton, K. M. Krause, J. Shelton, G. P. Obedencio, and S. S. Hegde. 2006. Efficacy of telavancin in a murine model of bacteraemia induced by methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:462-465. [DOI] [PubMed] [Google Scholar]
- 20.Reyes, N., R. Skinner, K. Kaniga, K. M. Krause, J. Shelton, G. P. Obedencio, A. Gough, M. Conner, and S. S. Hegde. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher-Perdreau, F., C. Heilmann, G. Peters, F. Gotz, and G. Pulverer. 1994. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol. Lett. 117:71-78. [DOI] [PubMed] [Google Scholar]
- 22.Shaw, J. P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepanović, S., D. Vuković, I. Dakić, B. Savić, and M. Švabić-Vlahović. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175-179. [DOI] [PubMed] [Google Scholar]
- 24.Stepanović, S., D. Vuković, V. Hola, G. Di Bonaventura, S. Djukić, I. Cirković, and F. Ruzicka. 2007. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891-899. [DOI] [PubMed] [Google Scholar]
- 25.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallee, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]
- 27.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601-1607. [DOI] [PubMed] [Google Scholar]
- 28.Stucki, A., P. Gerber, F. Acosta, M. Cottagnoud, and P. Cottagnoud. 2006. Efficacy of telavancin against penicillin-resistant pneumococci and Staphylococcus aureus in a rabbit meningitis model and determination of kinetic parameters. Antimicrob. Agents Chemother. 50:770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, K. D., G. A. McFeters, and P. S. Stewart. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146(Pt. 3):547-549. [DOI] [PubMed] [Google Scholar]
- 30.Yarwood, J. M., D. J. Bartels, E. M. Volper, and E. P. Greenberg. 2004. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 186:1838-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]